Efficacy of Cordyceps militaris Extracts against Some Skin Pathogenic Bacteria and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of C. militaris Extract
2.2. Determination of Cordycepin and Adenosine in C. militaris Extracts by High Performance Liquid Chromatography (HPLC)
2.3. Determination of Antioxidant Activities of C. militaris Extracts
2.4. Total Phenolic Compound of C. militaris Extracts
2.5. Total Flavonoid Compound of C. militaris Extracts
2.6. Antibacterial Activity of C. militaris Extracts
2.7. Determination of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) of C. militaris Extracts
2.8. Time Killing Kinetics of C. militaris Extracts
2.9. Effect of C. militaris Extracts for Inhibition of Virulence Gene Expression by qRT-PCR
2.10. Statistical Analysis
3. Results
3.1. C. militaris Extraction
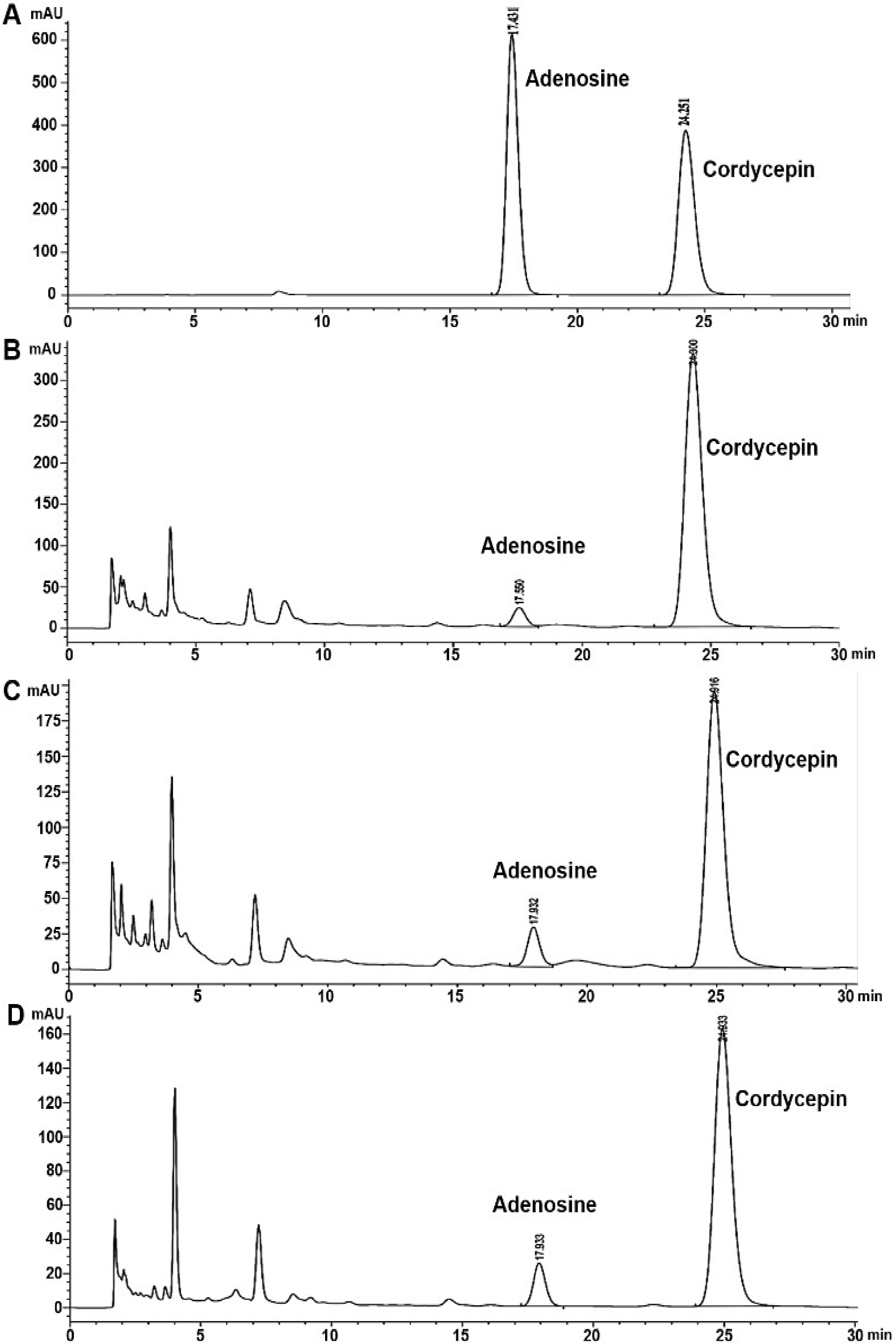
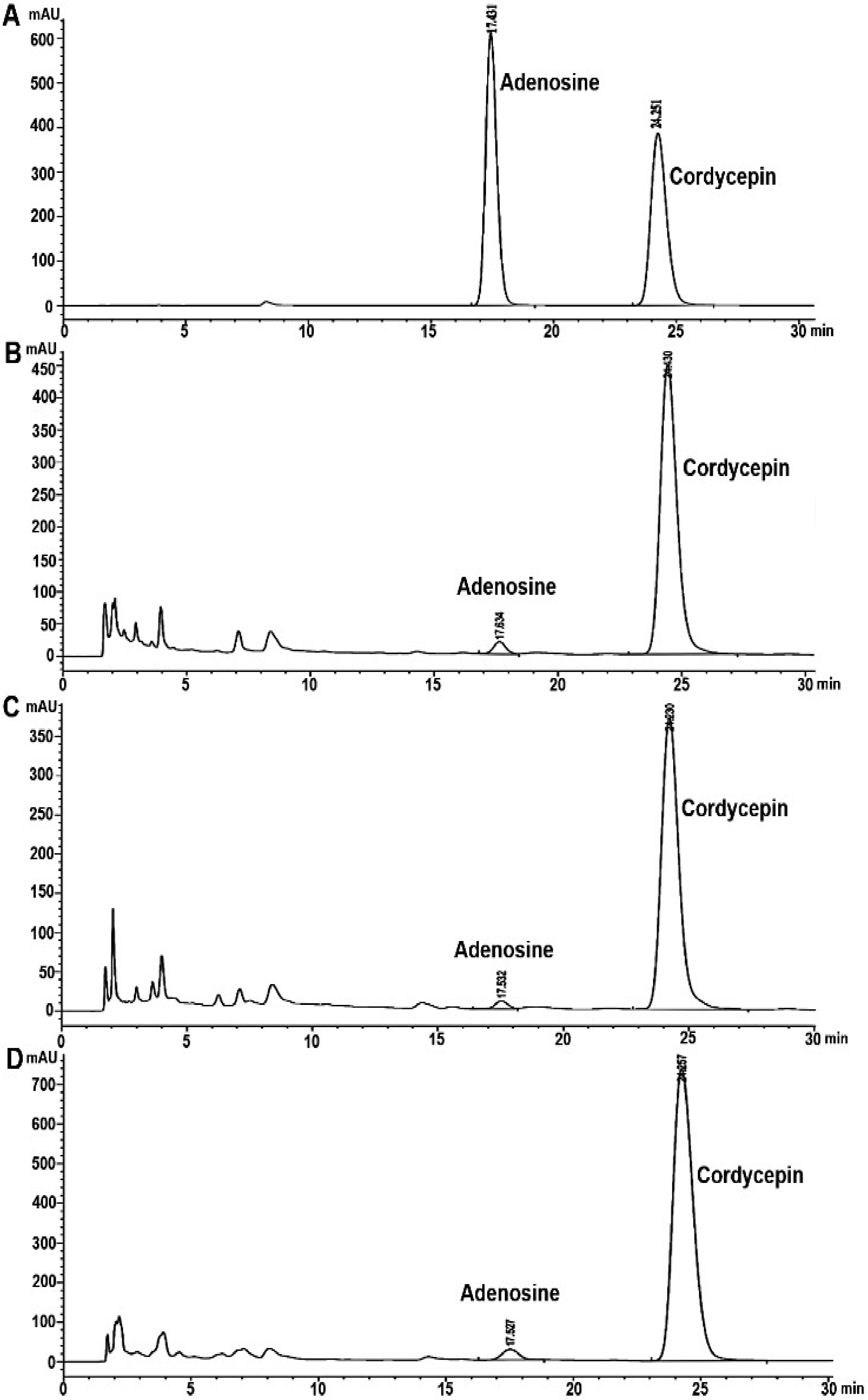
3.2. Analysis of Cordycepin and Adenosine in C. militaris Extracts by HPLC
3.3. Antioxidant Activities of C. militaris Extracts
3.4. Total Phenolic Compound of C. militaris Extracts
3.5. Total Flavonoid Compound of C. militaris Extracts
3.6. Antibacterial Activity of C. militaris Extracts by Agar Well Diffusion Method
3.7. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) of C. militaris Extracts
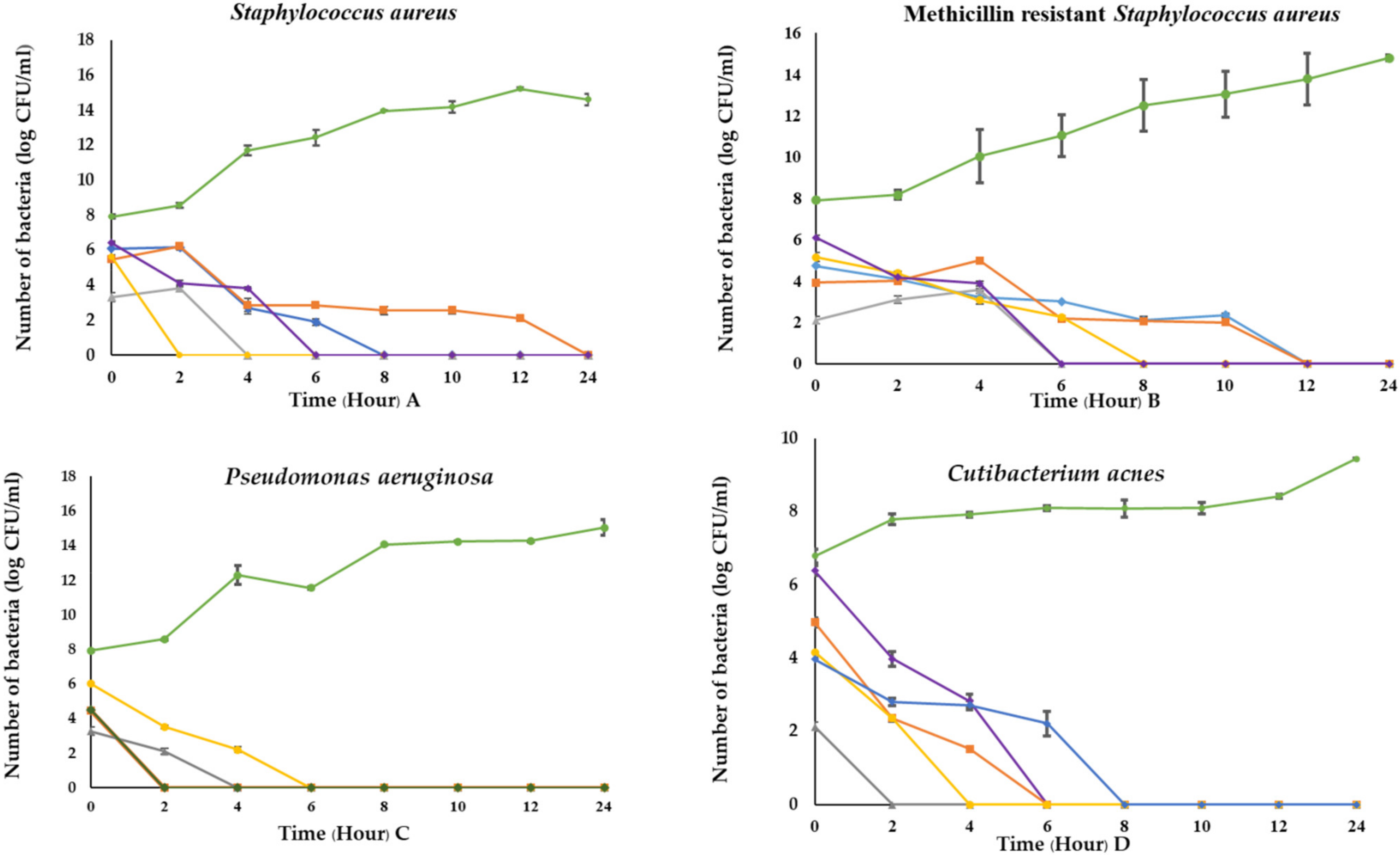
3.8. Time Killing Kinetics of C. militaris Extracts
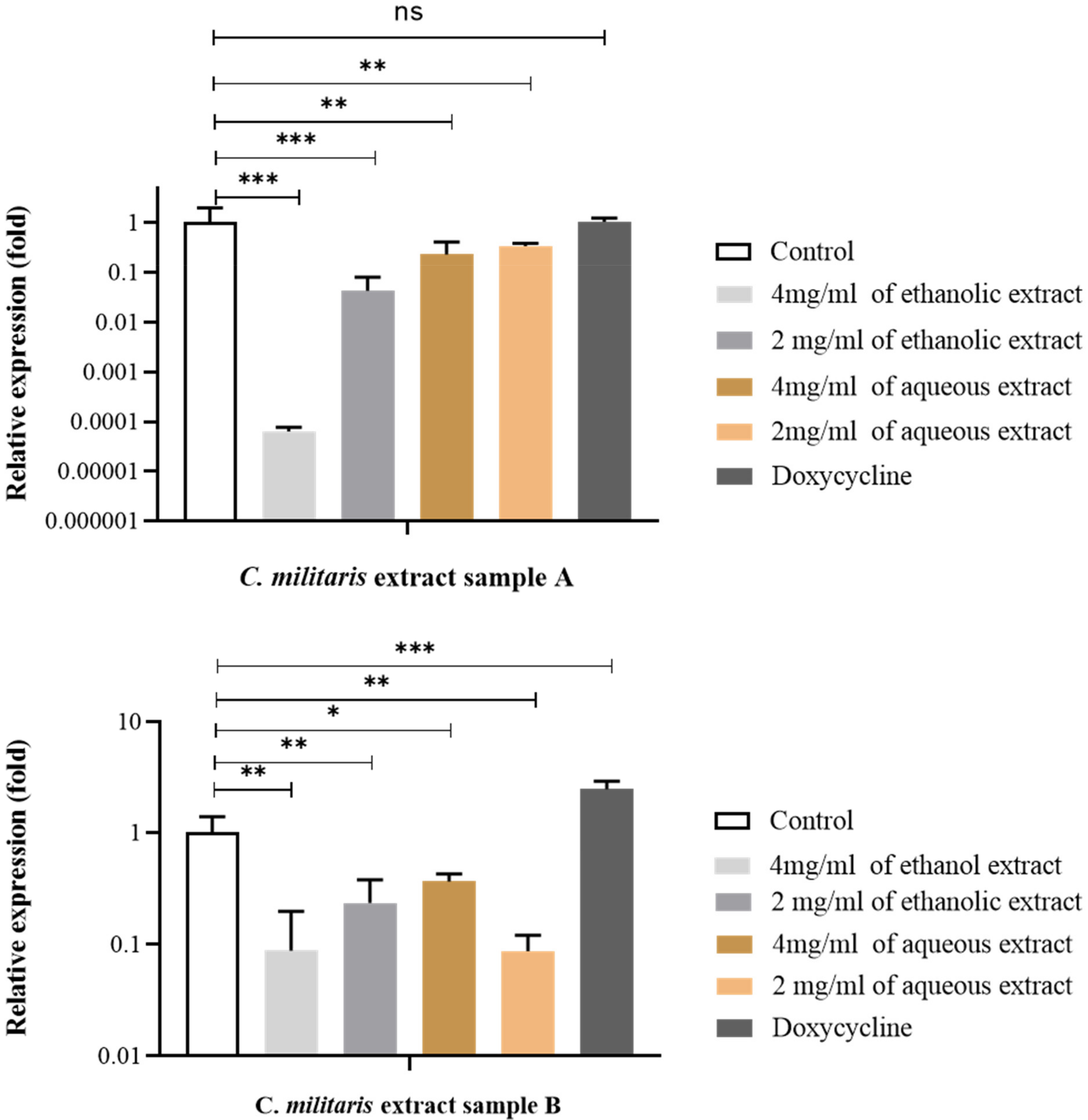
3.9. Effect of C. militaris Extracts on Inhibition of Virulence Gene Expression by qRT-PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasko, A.D.; Sperandio, A. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef]
- Sawanson, I.K. Antibiotic resistance of Propionibacterium acnes in acne vulgaris. Dermatol. Nurs. 2003, 5, 359–361. [Google Scholar]
- Oumeish, I.; Oumeish, O.Y.; Bataineh, O. Acute Bacterial Skin Infections in children. Clin. Dermatol. 2001, 18, 667–678. [Google Scholar] [CrossRef]
- Sadick, N.S. Bacterial skin infections: Unapproved treatments. Clin. Dermatol. 2002, 20, 613–617. [Google Scholar] [CrossRef]
- Ventola, L.C. The antibiotic resistance crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Pathania, P.; Joshi, M.; Sugar, A. Morphological physiological and molecular studies on wildly collected Cordyceps militaris from North West Himalaya India. Eur. J. Biotechnol. Biosci. 2015, 3, 53–62. [Google Scholar]
- Wang, L.; Zhang, W.M.; Hu, B.; Chen, Y.Q.; Qu, L.H. Genetic variation of Cordyceps militaris and its allies based on phylogenetic analysis of rDNA ITS sequence data. Fungal Divers. 2008, 31, 147–155. [Google Scholar]
- Wang, L.; Zhang, W.M.; Hu, B.A.; Chen, Y.Q.; Qu, L.H. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J. Ethnopharmacol. 2004, 96, 555–561. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Van Griensven, L.J.; Soković, M.; Ferreira, I.C. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013, 62, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Landi, N.; Clemente, A.; Pedone, A.P.; Ragucci, S.; Maro, D.A. An Updated Review of Bioactive Peptides from Mushrooms in a Well-Defined Molecular Weight Range. Toxins 2022, 14, 84. [Google Scholar] [CrossRef]
- Dong, J.Z.; Wang, S.H.; Ai, X.R.; Yao, L.; Sun, Z.W.; Lei, C.; Wang, Y.; Wang, Q. Composition and characterization of cordyxanthins from Cordyceps militaris fruit bodies. J. Funct. Foods 2013, 5, 1450–1455. [Google Scholar] [CrossRef]
- Tang, H.B.; Chen, C.X.; Zou, Y.; Lou, H.W.; Zheng, Q.W.; Guo, L.Q.; Lin, J.F.; Ye, Z.W.; Yun, F. Purification and structural characterization of novel natural pigment cordycepin from edible and medicinal mushroom Cordyceps militaris. Apply Microbiol. Biotechnol. 2019, 103, 7943–7952. [Google Scholar] [CrossRef]
- Li, H.C.; Sun, P.; Feng, C.Q. The research of cordycepin as an active component in Cordyceps. Nat. Sci. 2010, 31, 93–96. [Google Scholar]
- Wen, T.C.; Lei, B.X.; Kang, J.C.; Li, G.R.; He, J. Enhanced production of mycelial culture using additives and cordycepin by submerged in Cordyceps militaris. Food Ferment. Ind. 2009, 35, 49–53. [Google Scholar]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris current state and prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Teng, M.; Zhang, S.; Yin, M.; Lu, J.; Liu, Y.; Lee, J.R.; Wang, D.; Teng, L. Cordyceps militaris induces tumor cell death via the caspase-dependent mitochondrial pathway in HepG2 and MCF-7 cells. Mol. Med. Rep. 2016, 13, 5132–5140. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Li, Y.; Chen, Y.; Wang, X.; Zhou, X. Determination and analysis of cordycepin and adenosine in the products of Cordyceps spp. Afr. J. Microbiol. Res. 2009, 3, 957–961. [Google Scholar] [CrossRef]
- Hou, W.C.; Chen, Y.C.; Chen, H.J.; Lin, Y.H.; Yang, L.L.; Lee, M.H. Antioxidant activities of trypsin inhibitor, a33 kDa root storage protein of sweet potato. J. Agric. Food Chem. 2001, 49, 2978–2981. [Google Scholar] [CrossRef]
- Deori, M.; Devi, D.; Devi, R. Nutrient composition and antioxidant activities of muga and eri silkworm pupae. Int. J. Sci. Nat. 2014, 5, 636–640. [Google Scholar]
- Jahangirian, H.; Haron, M.; Ismail, M.; Moghaddam, R.; Hejri, L.; Abdollahi, Y.; Rezayi, M.; Vafaei, N. Well diffusion method for evaluation of antibacterial activity of copper phenyl fatty hydroxamate synthesized from canola and palm kernel oils. Dig. J. Nanomater. Biobtructures 2013, 8, 1263–1270. [Google Scholar]
- Petrus, E.M.; Tinakamuri, S.; Chai, L.C.; Ubong, A.; Tanung, R.; Elexon, N.; Chai, L.F.; Son, R. A study on the minimum inhibitory concentration and minimum bactericidal concentration of nano colloidal silver on food-borne pathogens. Int. Food Res. J. 2011, 18, 55–66. [Google Scholar]
- Rukholm, G.; Mugabe, C.; Azghani, A.O.; Omri, A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: A time–kill study. Int. J. Antimicrob. Agents 2006, 27, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Korting, H.C.; Schafer, W.; Bastert, J.; Chen, W.C.; Hube, B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol. Microbiol. 1999, 34, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Y.; Zhang, L.; Guo, F.; Ren, L.; Yang, R.; Li, Y.; Lou, H. In vivo inhibitory effect on the biofilm formation of Candida albicans by liverwort derived riccardin D. PLoS ONE 2012, 7, e35543. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F. Practice guidelines for the diagnosis and management of skin and soft tissue infections: Update by the infectious diseases society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, K. Skin and Soft Tissue Infections. Am. Fam. Physician 2015, 92, 474–483. [Google Scholar]
- Eron, L.J. Managing skin and soft tissue infections. J. Antimicrob. Chemother. 2003, 52, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Soltani, M.; Malex, A.R.; Ware, I.; Ramli, S.; Elsayed, A.E.; Aziz, R.; Ensshasy, E. Optimization of Cordycerin Extraction from Cordyceps militaris Fermentation Broth. J. Sci. Ind. Res. 2017, 76, 355–361. [Google Scholar]
- Guo, M.; Guo, S.; Huaijun, Y.; Bu, N.; Dong, C.-H. Comparison of Major Bioactive Compounds of the Caterpillar Medicinal Mushroom, Cordyceps militaris (Ascomycetes) Fruiting Bodies Cultured on Wheat Substrate and Pupae. Int. J. Med. Mushrooms 2016, 18, 327–336. [Google Scholar] [CrossRef]
- Bawadekji, A.; Ali Al, K.; Ali Al, M. A review of the bioactive compound and medicinal value of Cordyceps militaris. J. North Basic Appl. Sci. 2016, 1, 69–76. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. 2010, 112, 6646–6652. [Google Scholar] [CrossRef]
- Yu, H.M.; Wang, B.S.; Huang, S.C.; Duh, P.D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2006, 54, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Dong, C.-H.; Yao, Y.-J. Antioxidant activities of aqueous extract from cultivated fruit-bodies of Cordyceps militaris (L.) Link in vitro. J. Integr. Plant Biol. 2006, 48, 1365–1370. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Xie, Y.; Xu, Y.; Li, J. Extraction and antioxidant property in vitro of cordycepin in artificially cultivated Cordyceps militaris. Adv. Mater. Res. 2013, 750, 1593–1596. [Google Scholar] [CrossRef]
- Kontogiannatos, D.; Koutrotsio, G.; Xekalaki, S.; Zervakis, I.G. Biomass and Cordycepin Production by the Medical Mushroom Cordyceps militaris-A review of Various Aspects and Recent Trends towards the Exploitation of a Valuable Fungus. J. Fungi 2021, 7, 986. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J. Funct. Food 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, L.S.; Khan, N.; Ghani, L.; Poulson, G.B.; Emwas, A.; Jaremko, J. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Pan, H.; Fan, L.; Soccol, C.R.; Pandey, A. Production of Powerful Antioxidant Supplement via Solid-State Fermentation of Wheat (Triticum aestivum Linn.) by Cordyceps militaris. Food Technol. Biotechnol. 2011, 50, 32–39. [Google Scholar]
- Cotelle, N. Role of Flavonoids in Oxidative Stress. Med. Chem. 2001, 1, 569–590. [Google Scholar] [CrossRef]
- Yu, R.; Yang, W.; Song, L.; Yan, C.; Zhang, Z.; Zhao, Y. Structural characterization and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps militaris. Carbohydr. Polym. 2007, 70, 430–436. [Google Scholar] [CrossRef]
- Dong, C.H.; Yang, T.; Lian, T.A. A Comparative Study of the Antimicrobial, Antioxidant, and Cytotoxic Activities of Methanol Extracts from Fruit Bodies and Fermented Mycelia of Caterpillar Medicinal Mushroom Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2014, 16, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Sandhu, S.S.; Kashyap, D.; Sharma, A.K. Optimization of Extraction Conditions and Antimicrobial Potential of a Bioactive Metabolite, Cordycepin from Cordyceps militaris 3636. Semant. Sch. 2014, 3, 1525–1535. [Google Scholar]
- Joshi, M.; Sagar, A.; Kanwar, S.; Shamsher, S.S. Anticancer antibacterial and antioxidant activities of Cordyceps militaris. Indian J. Exp. Biol. 2017, 57, 15–20. [Google Scholar]
- Pathania, P.; Sagar, A. Studies on antibacterial activity of Cordyceps militaris (L.) link. Int. J. Pharm. Bio. Sci. 2014, 5, 61–68. [Google Scholar]
- Shorr, F.A. Epidemiology of staphylococcal resistance. Clin. Infect. Dis. 2007, 3, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. MRSA virulence and spread. Cell Microbiol. 2012, 14, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- Chambers, H.F. Methicillin-resistant Staphylococcus aureus. mechanisms of resistance and implications for treatment. Postgrad. Med. 2001, 109, 43–50. [Google Scholar] [CrossRef]
- Jang, S. Multidrug efflux pump in Staphylococus aureus and their clinical implications. J. Microbiol. 2016, 54, 1–8. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Burt, S. Essential oils; their antibacterial properties and potential applications in food a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]

| Genes | Sequence of PCR Primers (5′–3′) |
|---|---|
| mecA | Forward: GGCTATCGTGTCACAATCGTTGACG Reverse: CAAATCCGGTACTGCAGAAC |
| 16s RNA | Forward: GAGTTTTAACCTTGCGGCC Reverse: CCAGGTGTAGCGGTGAAAT |
| C. militaris | Extraction Yields (%) | |
|---|---|---|
| Aqueous Extract | Ethanolic Extract | |
| Sample A | 16.4 | 3.6 |
| Sample B | 12.6 | 6.2 |
| Sample C | 19.9 | 5.9 |
| C. militaris | C. militaris Extracts (mg/g Extract) | |||
|---|---|---|---|---|
| Aqueous Extract | Ethanolic Extract | |||
| Cordycepin | Adenosine | Cordycepin | Adenosine | |
| Sample A | 23.98 ± 0.39 b | 2.86 ± 0.01 a | 32.08 ± 0.10 b | 3.78 ± 0.05 a |
| Sample B | 25.66 ± 0.11 a | 2.77 ± 1.25 b | 57.42 ± 0.27 a | 0.28 ± 0.01 c |
| Sample C | 17.15 ± 0.16 c | 1.16 ± 0.06 c | 25.26 ± 0.22 c | 0.49 ± 0.01 b |
| Aqueous Extracts | Antioxidant Activity (mg GAE/g Extract) | Total Phenolic Content (mg GAE/g Extract) | Total Flavonoid Content (mg QAE/g Extract) |
|---|---|---|---|
| Sample A Sample B Sample C | 9.12 ± 0.21 a 9.42 ± 0.75 a 3.72 ± 0.26 b | 49.04 ± 0.42 a 45.52 ± 0.56 a 40.03 ± 3.06 b | 7.45 ± 0.19 c 9.10 ± 0.26 b 11.31 ± 0.64 a |
| Ethanolic Extracts | Antioxidant Activity (mg GAE/g Extract) | Total Phenolic Content (mg GAE/g Extract) | Total Flavonoid Content (mg QAE/g Extract) |
| Sample A Sample B Sample C | 9.50 ± 0.51 a 4.53 ± 0.15 b 3.76 ± 0.27 c | 28.87 ± 1.14 a 23.71 ± 1.85 a 24.62 ± 1.34 a | 10.59 ± 1.98 a 2.85 ± 0.27 b 2.83 ± 0.15 b |
| Aqueous Extracts | Diameter of Inhibition Zone on Tested Bacteria (mm) | |||
|---|---|---|---|---|
| MRSA | P. aeruginosa | C. acnes | S. aureus | |
| Sample A | 20.50 ± 2.00 a | 15.50 ± 0.50 b | 14.17 ± 0.29 b | 18.83 ± 1.26 a |
| Sample B | 19.83 ± 0.76 a | 15.33 ± 1.04 b | 16.67 ± 1.61 b | 18.00 ± 0.87 ab |
| Sample C | 17.00 ± 1.00 a | 12.83 ± 0.76 b | 0 c | 14.17 ± 0.29 b |
| Ethanolic Extracts | Diameter of Inhibition Zone on Tested Bacteria (mm) | |||
| MRSA | P. aeruginosa | C. acnes | S. aureus | |
| Sample A | 18.83 ± 1.04 a | 12.17 ± 0.76 c | 0 d | 15.17 ± 1.26 b |
| Sample B | 17.17 ± 0.29 a | 13.33 ± 0.29 b | 0 c | 13.33 ± 1.15 b |
| Sample C | 18.00 ± 1.73 a | 12.50 ± 0.00 b | 0 c | 17.67 ± 2.08 a |
| Gentamycin | 0 | 28.02 ± 0.55 | 32.70 ± 2.22 | 27.67 ± 1.63 |
| Doxycycline | 25.67± 0.58 | ND | ND | ND |
| Extracts | Samples | MIC/MBC of C. militaris Extracts (mg/mL) | |||
|---|---|---|---|---|---|
| MRSA | P. aeruginosa | C. acnes | S. aureus | ||
| Aqueous extracts | A | 15.62 | 15.62 | 7.81 | 31.25 |
| B | 7.81 | 15.62 | 7.81 | 31.25 | |
| C | 15.62 | 31.25 | 7.81 | 15.62 | |
| Ethanolic extracts | A | 3.91 | 15.62 | 7.81 | 31.25 |
| B | 3.91 | 31.25 | 7.81 | 15.62 | |
| C | 7.81 | 31.25 | 7.81 | 15.62 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiamthaworn, K.; Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of Cordyceps militaris Extracts against Some Skin Pathogenic Bacteria and Antioxidant Activity. J. Fungi 2022, 8, 327. https://doi.org/10.3390/jof8040327
Eiamthaworn K, Kaewkod T, Bovonsombut S, Tragoolpua Y. Efficacy of Cordyceps militaris Extracts against Some Skin Pathogenic Bacteria and Antioxidant Activity. Journal of Fungi. 2022; 8(4):327. https://doi.org/10.3390/jof8040327
Chicago/Turabian StyleEiamthaworn, Kiratiya, Thida Kaewkod, Sakunnee Bovonsombut, and Yingmanee Tragoolpua. 2022. "Efficacy of Cordyceps militaris Extracts against Some Skin Pathogenic Bacteria and Antioxidant Activity" Journal of Fungi 8, no. 4: 327. https://doi.org/10.3390/jof8040327
APA StyleEiamthaworn, K., Kaewkod, T., Bovonsombut, S., & Tragoolpua, Y. (2022). Efficacy of Cordyceps militaris Extracts against Some Skin Pathogenic Bacteria and Antioxidant Activity. Journal of Fungi, 8(4), 327. https://doi.org/10.3390/jof8040327






