Arbuscular Mycorrhizal Fungi and Endophytic Fungi Activate Leaf Antioxidant Defense System of Lane Late Navel Orange
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Inoculums
2.2. Field Inoculation of Fungi
2.3. Experimental Design
2.4. Sample Collection
2.5. Determination of Root Fungal Colonization and Soil Hyphal Length
2.6. Determination of Leaf Antioxidant Enzyme Activity
2.7. Determination of Leaf Non-Enzymatic Antioxidant Concentration
2.8. Determination of Degree of Membrane Lipid Peroxidation
2.9. Determination of Leaf ROS Levels
2.10. Determination of Leaf Antioxidant Enzyme Gene Expressions
2.11. Data Analysis
3. Results
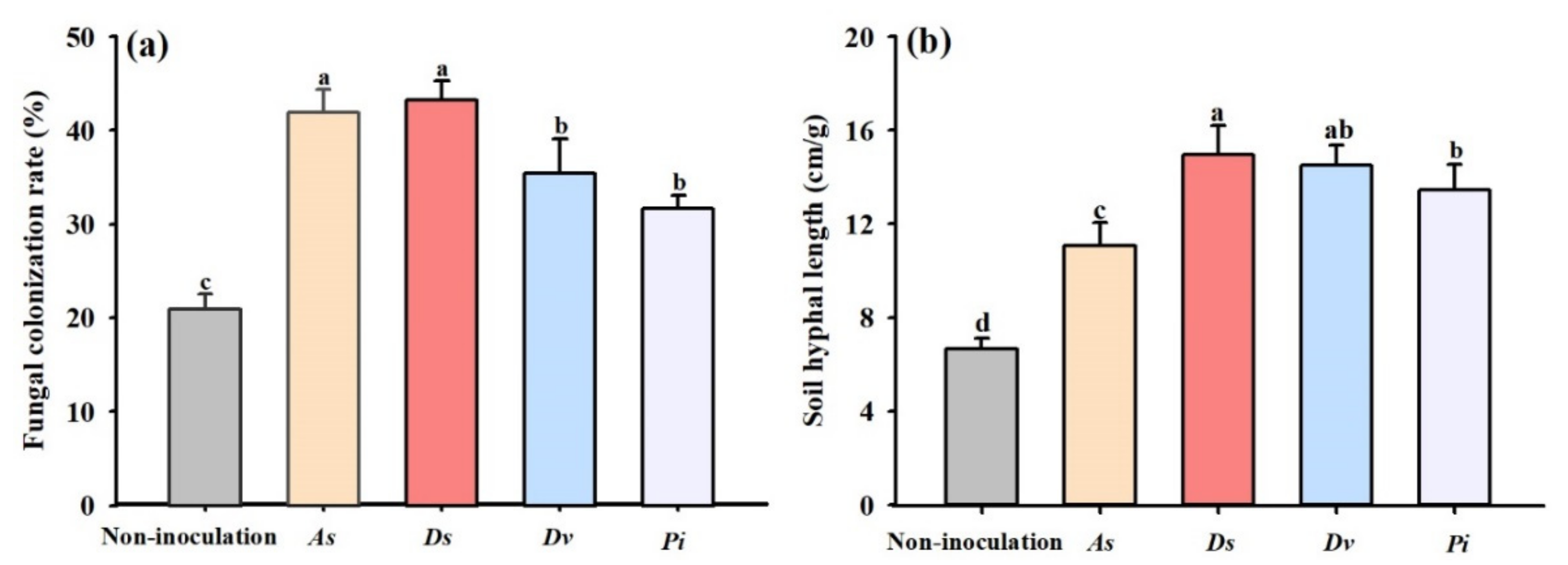
3.1. Changes in Root Fungal Colonization and Soil Hyphal Length
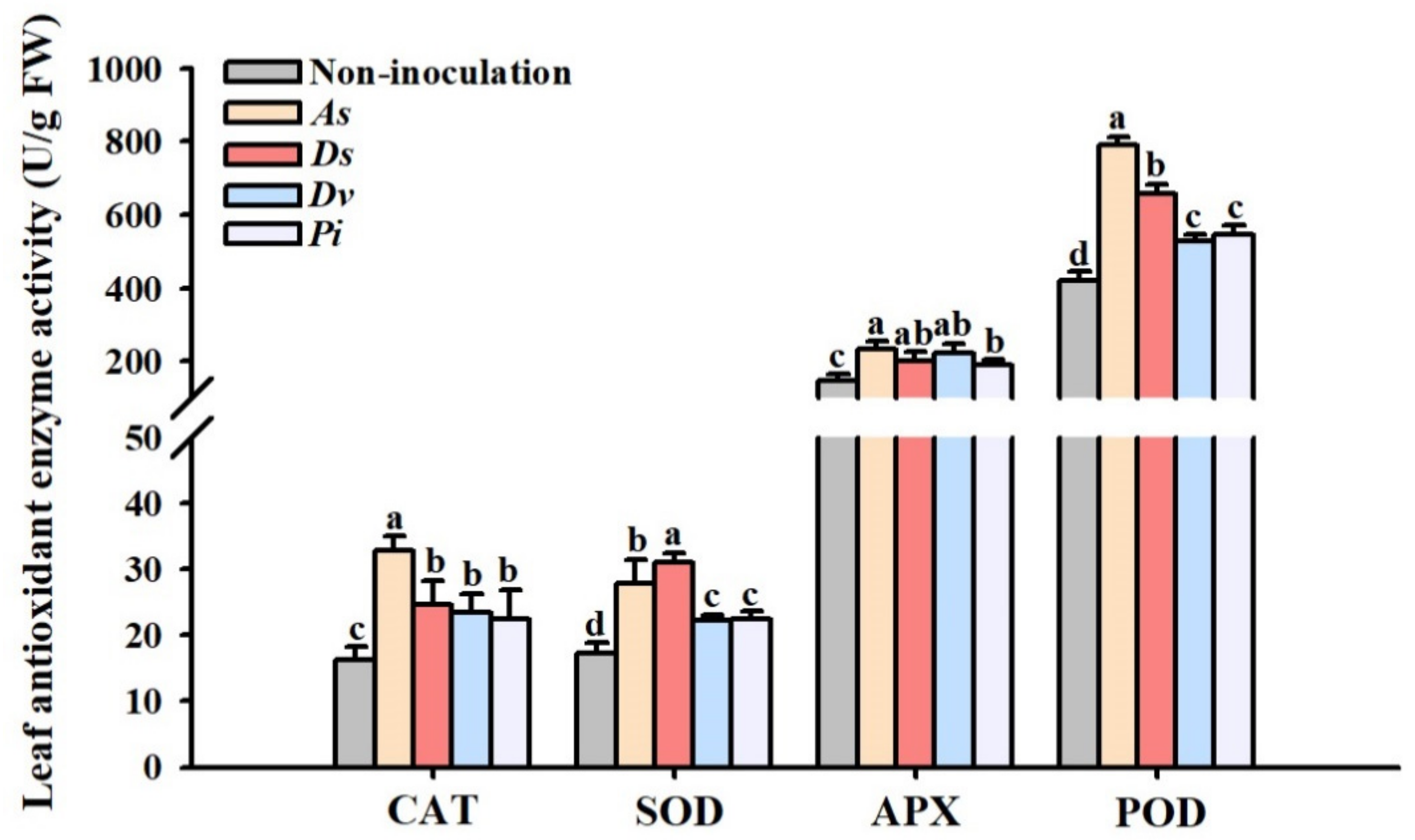
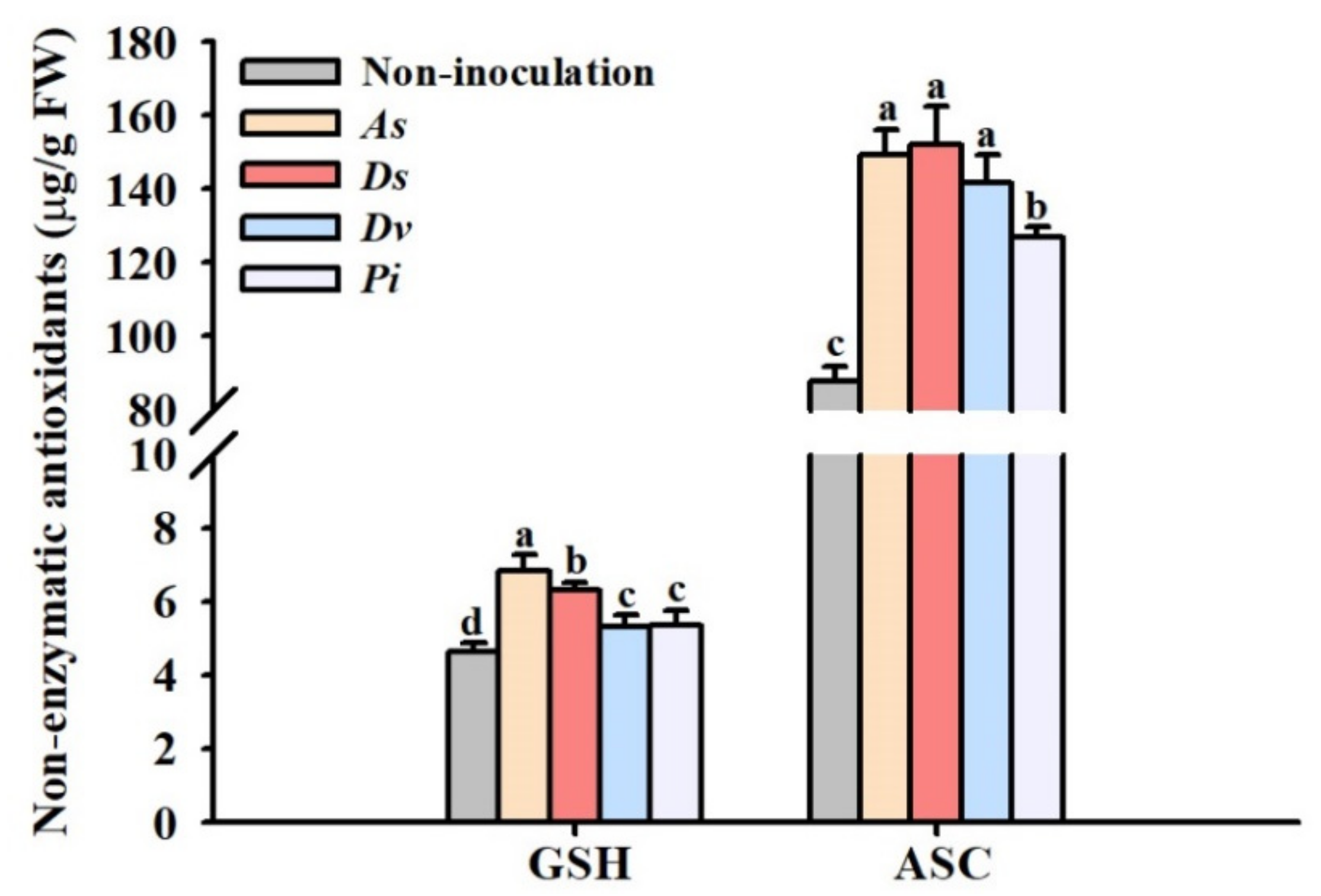
3.2. Changes in Leaf Antioxidant Enzyme Activity
3.3. Changes in Sugar Concentrations of Leaves and Roots
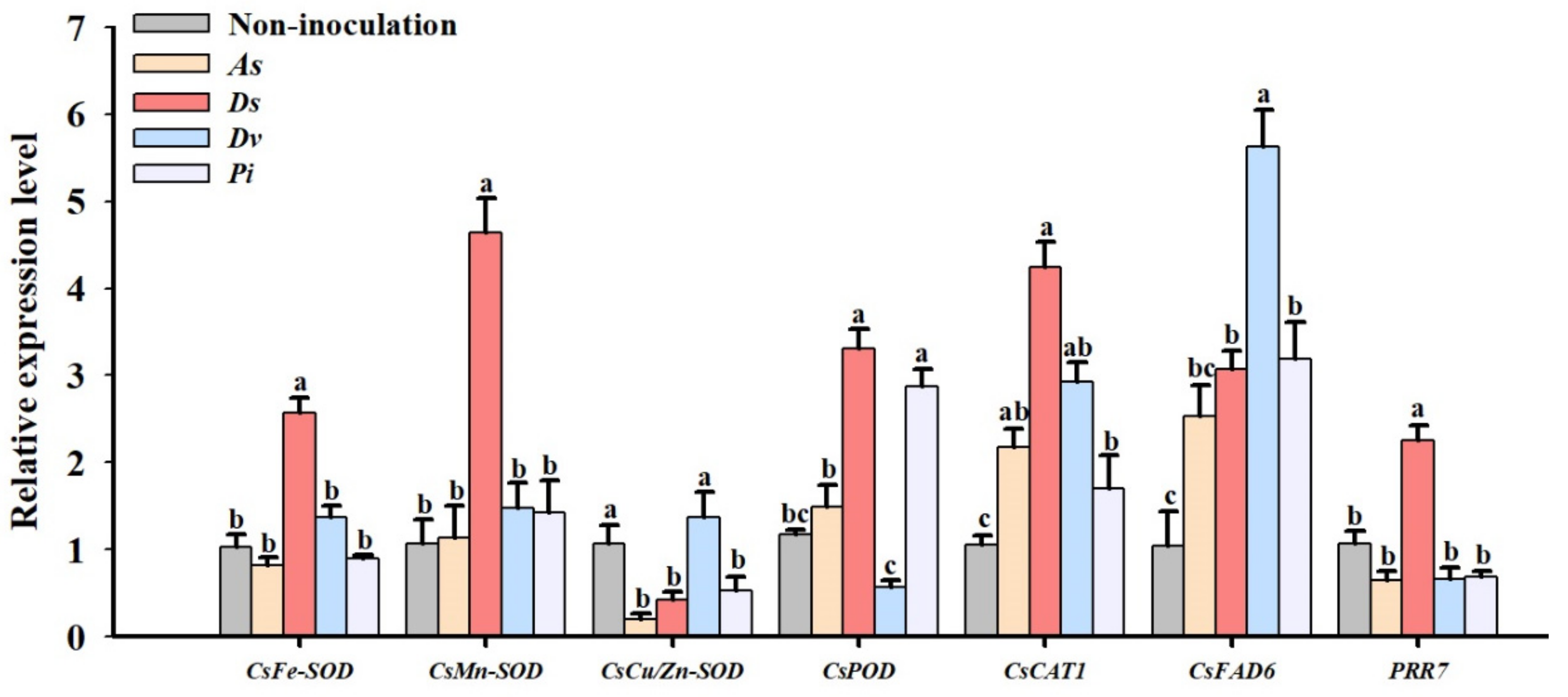
3.4. Changes in Expression Levels of Leaf Antioxidant Enzyme Protein Genes
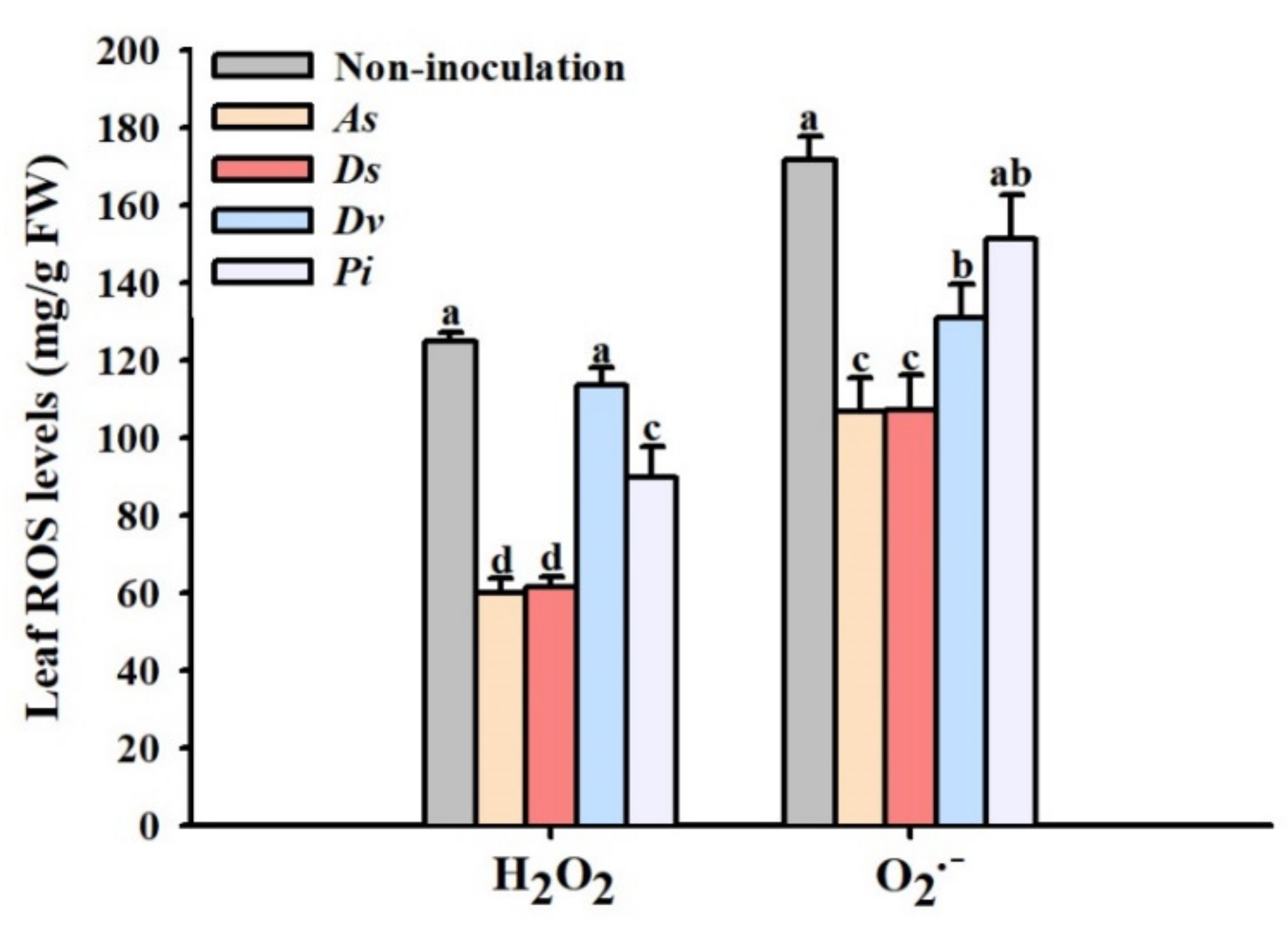
3.5. Changes in Leaf ROS Levels
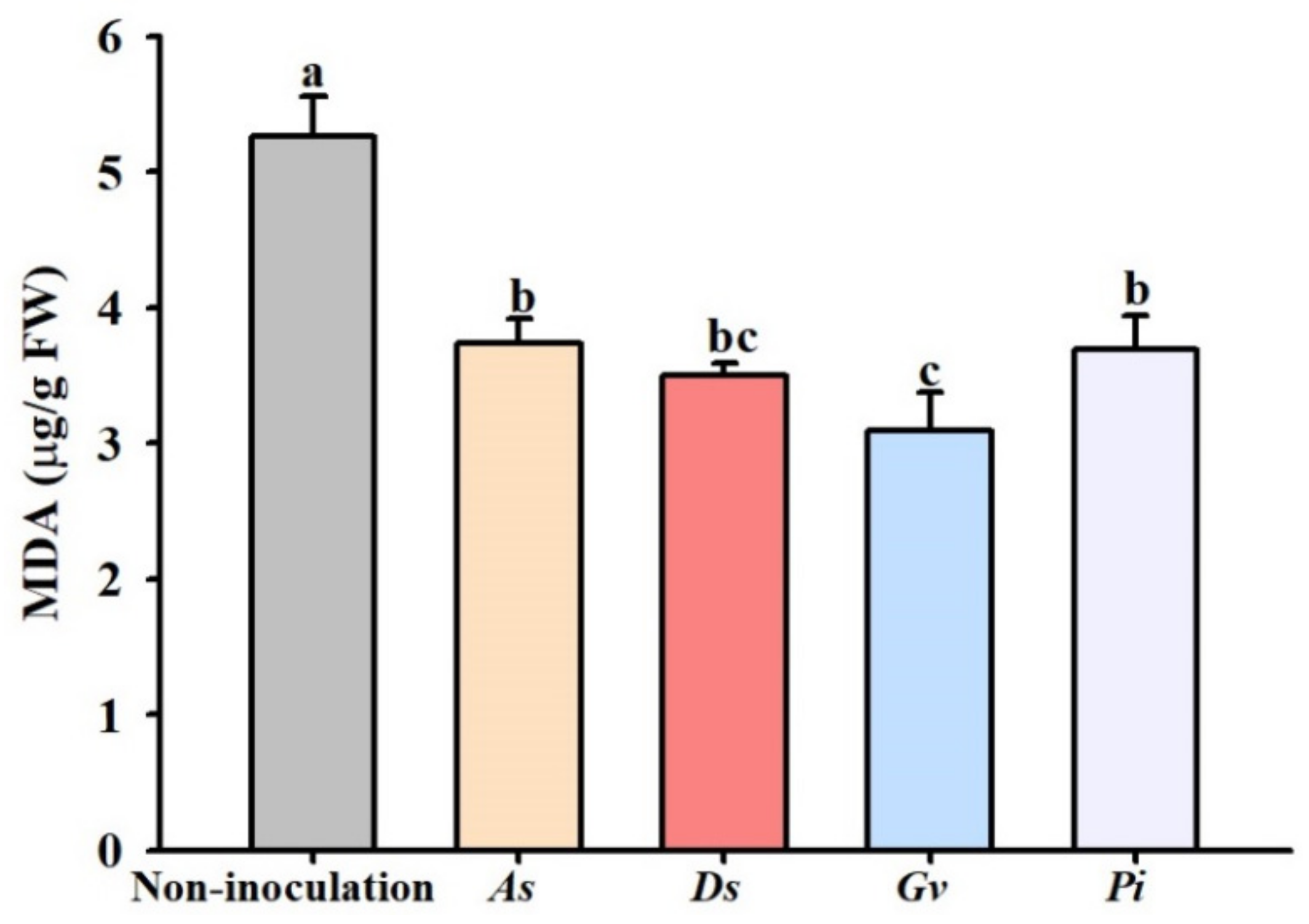
3.6. Changes in Leaf MDA Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ollitrault, P.; Curkb, F.; Krueger, R. Citrus taxonomy. In The Genus Citrus; Talon, M., Caruso, M., Fred, G., Gmitter, F.G., Eds.; Woodhead Publishing Elsevier: London, UK, 2020; pp. 57–81. [Google Scholar]
- Sina, N.; Morteza, G.; Abdoolnabi, B.; Assunta, B. Citrus industry: Phytoplasma-associated diseases and related challenges for Asia, America and Africa. Crop Prot. 2021, 152, 105822. [Google Scholar]
- Shafqat, W.; Naqvi, S.A.; Maqbool, R.; Haider, M.S.; Jaskani, M.J.; Khan, I.A. Climate Change and Citrus; IntechOpen: London, UK, 2021; pp. 1–12. [Google Scholar]
- Meng, L.L.; Liang, S.M.; Srivastava, A.K.; Li, Y.; Liu, C.Y.; Zou, Y.N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Easily extractable glomalin-related soil protein as foliar spray improves nutritional qualities of late ripening sweet oranges. Horticulturae 2021, 7, 228. [Google Scholar] [CrossRef]
- Legua, P.; Bellver, R.; Forner, J.B.; Giner, M.A. Trifoliata hybrids rootstocks for ‘Lane Late’ navel orange in Spain. Sci. Agric. 2011, 68, 548–553. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.P.; Robles, J.M.; Botia, P. Influence of deficit irrigation in phase III of fruit growth on fruit quality in ‘lane late’ sweet orange. Agric. Water Manag. 2009, 96, 969–974. [Google Scholar] [CrossRef]
- He, L.G.; Jiang, Y.C.; Wang, Z.J.; Wu, L.M.; Tong, Z.; Xu, M.; Sun, Z.H. Effects of cold and fungicide treatments on fruit quality of Late navel orange from Three Gorges Reservior region during storage. Hubei Agric. Sci. 2017, 56, 4116–4118. [Google Scholar]
- Li, Y.; Peng, D.L.; Luo, Q.W.; Liu, B.Y. Development status and suggestions of Xingshan late-ripening citrus industry in Hubei Province. China Fruit Indust. Inform. 2020, 37, 29–31. [Google Scholar]
- Wang, P.; Shu, B.; Wang, Y.; Zhang, D.J.; Liu, J.F.; Xia, R.X. Diversity of arbuscular mycorrhizal fungi in red tangerine (Citrus reticulata Blanco) rootstock rhizospheric soils from hillside citrus orchards. Pedobiologia 2013, 56, 161–167. [Google Scholar] [CrossRef]
- Cheng, X.F.; Xie, M.M.; Li, Y.; Liu, B.Y.; Liu, C.Y.; Wu, Q.S.; Kuča, K. Effects of field inoculation with arbuscular mycorrhizal fungi and endophytic fungi on fruit quality and soil properties of Newhall navel orange. Appl. Soil. Ecol. 2022, 170, 104308. [Google Scholar] [CrossRef]
- Cheng, S.; Zou, Y.N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Elucidating the mechanisms underlying enhanced drought tolerance in plants mediated by arbuscular mycorrhizal fungi. Front. Microbiol. 2021, 12, 809473. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.C.; Xiao, Z.Y.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Mycorrhizal fungal diversity and its relationship with soil properties in Camellia oleifera. Agriculture 2021, 11, 470. [Google Scholar] [CrossRef]
- Alotaibi, M.O.; Saleh, A.M.; Sobrinho, R.L.; Sheteiwy, M.S.; El-Sawah, A.M.; Mohammed, A.E.; AbdElgawad, H. Arbuscular mycorrhizae mitigate aluminum toxicity and regulate proline metabolism in plants grown in acidic soil. J. Fungi 2021, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Elgawad, H.A.; Xiong, Y.C.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Hamoud, Y.A.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant. 2021, 172, 2153–2169. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with arbuscular mycorrhizal fungi and bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Wu, Q.S.; Kuča, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ. Exp. Bot. 2020, 171, 103962. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X. Effects of AM fungi on drought tolerance of citrus grafting seedling trifoliate orange/cara. Chin. J. Appl. Ecol. 2005, 16, 865–869. [Google Scholar]
- Wu, Q.S.; Gao, W.Q.; Srivastava, A.K.; Zhang, F.; Zou, Y.N. Nutrient acquisition and fruit quality of Ponkan mandarin in response to AMF inoculation. Ind. J. Agric. Sci. 2020, 90, 1563–1567. [Google Scholar]
- Min, J.; Ling, C.; Xin, H.L.; Zheng, C.J.; Khalid, R.; Han, T.; Khalid, L.P. A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front. Microbiol. 2016, 7, 906. [Google Scholar]
- Rai, M.; Varma, A. Arbuscular mycorrhiza-like biotechnological potential of Piriformospora indica, which promotes the growth of Adhatoda vasica Nees. Electron. J. Biotechn. 2005, 8, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Cao, J.L.; Zou, Y.N.; Wu, Q.S. Piriformospora indica: A root endophytic fungus and its roles in plants. Not. Bot. Horti. Agrobo. 2020, 48, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Gill, R.; Trivedi, D.K.; Anjum, N.A.; Sharma, K.K.; Ansari, M.W.; Ansari, A.A.; Johri, A.K.; Prasad, R.; Pereira, E.; et al. Piriformospora indica: Potential and significance in plant stress tolerance. Front Microbiol. 2020, 7, 332. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.L.; Liu, R.C.; Yang, L.; Zou, Y.N.; Srivastava, A.K.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Giri, B.; Wu, Q.S. The change in fatty acids and sugars reveals the association between trifoliate orange and endophytic fungi. J. Fungi 2021, 7, 716. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Ansari, M.W.; Bains, G.; Shukla, A.; Pant, R.C.; Tuteja, N. Low temperature stress ethylene and not Fusarium might be responsible for mango malformation. Plant Physiol. Bioch. 2013, 69, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Y.N.; Tian, Z.H.; Wu, Q.S.; Kuča, K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 2021, 277, 109815. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J.; Ames, R.N. Comparison of two methods for quantifying extraradical mycelium of vesicular-arbuscular mycorrhizal fungi. Soil Sci. Soc. Am. J. 1987, 51, 125–134. [Google Scholar] [CrossRef]
- Wu, Q.S. Experimental Guideline in Plant Physiology; China Agricultural Press: Beijing, China, 2019. [Google Scholar]
- Yang, L.F.; Pang, J.; Peng, X.L.; Yan, J.J. Measurement of catalase activity in plants by ultraviolet spectrophotometry. Mod. Agric. Sci. Technol. 2009, 20, 364–366. [Google Scholar]
- Li, Z.G.; Gong, M. Improvement of determination of plant peroxidase activity by guaiacol method. Plant Physiol. Commun. 2008, 2, 323–324. [Google Scholar]
- Chen, J.X.; Wang, X.F. Experimental Guideline in Plant Physiology; South China Polytechnic University Press: Guangzhou, China, 2002. [Google Scholar]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzymes efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Wang, A.H.; Luo, G.H. Quantitative relation between superoxide anion radicals and the reation of hydroxylamine in plants. Plant Physiol. Commun. 1990, 26, 55–57. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N.; Malhotra, S.K. Mycorrhizas in citrus: Beyond soil fertility and plant nutrition. Ind. J. Agric. Sci. 2017, 87, 427–443. [Google Scholar]
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Gange, A.C.; Ayres, R.L. On the relation between arbuscular mycorrhizal colonization and plant benefit. Oikos 1999, 87, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, J.; Xu, L.; Wang, A.; Oelmüller, R. Drought stress responses in maize are diminished by Piriformospora indica. Plant Signal. Behav. 2018, 13, e1414121. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, M.; Bruno, L.B.; Banu, J.R. Alleviation of environmental stress in plants: The role of beneficial Pseudomonas spp. Crit. Rev. Env. Sci. Tec. 2017, 47, 372–407. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepahvand, T.; Etemad, V.; Matinizadeh, M.; Shirvany, A.; Press, C.C. Symbiosis of AMF with growth modulation and antioxidant capacity of Caucasian Hackberry (Celtis caucasica L.) seedlings under drought stress. Central Asian J. Technol. Innov. 2021, 1, 20–35. [Google Scholar]
- Dabral, S.; Yashaswee; Varma, A.; Choudhary, D.K.; Nath, M. Biopriming with Piriformospora indica ameliorates cadmium stress in rice by lowering oxidative stress and cell death in root cells. Ecotox. Environ. Saf. 2019, 186, 109741. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Zou, Y.N.; Wu, Q.S. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 2017, 7, 42335. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Huang, Y.M.; Wu, Q.S.; He, X.H. Mycorrhiza-induced lower oxidative burst is related with higher antioxidant enzyme activities, net H2O2 effluxes, and Ca2+influxes in trifoliate orange roots under drought stress. Mycorrhiza 2015, 25, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Shao, K.H.; Chan, M.T.; Cheng, C.P.; Wang, S.J. Piriformospora indica symbiosis improves water stress tolerance of rice through regulating stomata behavior and ROS scavenging systems. Plant Signal. Behav. 2020, 15, 1722447. [Google Scholar] [CrossRef]
- Jangir, P.; Shekhawat, P.K.; Bishnoi, A.; Ram, H.; Soni, P. Role of Serendipita indica in enhancing drought tolerance in crops. Physiol. Mol. Plant Pathol. 2021, 116, 1101691. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X.; Zou, Y.N. Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J. Plant Physiol. 2006, 163, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticumae stivum L.). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.Q.; Yu, D.; Yang, S.; Wan, Q. Effects of arbuscular mycorrhizal fungi on drought resistance of grape seedlings. China Fruits 2018, 2, 8–12. [Google Scholar]
- Tian, Q.; Wang, D.; Zhang, W.L.; Duan, N.B.; Ding, H.F. Effects of aging treatment on seed vigor and ASA-GSH cycle in soybean. Plant Physiol. J. 2016, 52, 543–550. [Google Scholar]
- Wu, H.H.; Zou, Y.N.; Wu, Q.S. Effects of AMF on growth and active oxygen metabolism of potted Immature Poncirus aurantii seedlings under drought stress. South China Fruits 2018, 47, 36–38. [Google Scholar]
- Abdelaziz, M.E.; Kim, D.; Ali, S.; Fedoroff, N.V.; Babili, S.A. The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 2017, 263, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, F.Q.; Liu, J.; Gao, Q.K.; Lou, B.G. Effects of Piriformospora indica on drought resistance of tobacco. Tob. Sci. 2017, 50, 1–7. [Google Scholar]
- He, J.D.; Zou, Y.N.; Wu, Q.S. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci. Hortic. 2019, 262, 108745. [Google Scholar] [CrossRef]
- Ding, Y.E.; Zou, Y.N.; Kuča, K. Mycorrhizal fungi regulate daily rhythm of circadian clock in trifoliate orange under drought stress. Tree Physiol. 2021, tpab132. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, X.; Bao, E.; Cao, K.; Zou, Z. Effects of arbuscular mycorrhizal fungi on watermelon growth, elemental uptake, antioxidant, and photosystem II activities and stress-response gene expressions under salinity-alkalinity stresses. Front. Plant Sci. 2019, 10, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.S.; He, J.D.; Srivastava, A.K.; Zou, Y.N.; Kuča, K. Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiol. 2019, 39, 1149–1158. [Google Scholar] [CrossRef]
| Gene Name | Gene ID | Primer Sequences (5′→3′) |
|---|---|---|
| CsFe-SOD | Cs7g19250 | F: AGTAAGGAGCGGCGAGTA |
| R: GTGGCTAATGCGGTGAAT | ||
| CsMn-SOD | Cs7g29850 | F: GGCGAGCCACCACATAGT |
| R: CACCCTCAGCATTCATCTTTT | ||
| CsCu/Zn-SOD | Cs3g12080 | F: GGACCAGCATGGACTACAAGACC |
| R: GGATGCCGGTGGAAGTGTTACC | ||
| CsPOD | Cs1g18600 | F: GGCTCAACTTGTCCACCTC |
| R: TATCGTCGCCCTGTCTG | ||
| CsCAT1 | Cs3g27280 | F: TAACAGTGGAGGAGCGAACA |
| R: GGAGCCAGTGCTAAGGGT | ||
| CsFAD6 | Cs8g17450 | F: CTGCACGGAGATACAGCTTGGC |
| R: GGAATGTGAGGAGCCGTATGATGC | ||
| CsPRR7 | Cs6g03960.1 | F: TAGGAGCACACAAGAGCAGC |
| R: TTGTGGAACAGCTTCAGCCA | ||
| β-Actin | Cs1g05000 | F: CCGACCGTATGAGCAAGGAAA |
| R: TTCCTGTGGACAATGGATGGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.-S.; Xie, Y.-C.; Rahman, M.M.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Arbuscular Mycorrhizal Fungi and Endophytic Fungi Activate Leaf Antioxidant Defense System of Lane Late Navel Orange. J. Fungi 2022, 8, 282. https://doi.org/10.3390/jof8030282
Li Q-S, Xie Y-C, Rahman MM, Hashem A, Abd_Allah EF, Wu Q-S. Arbuscular Mycorrhizal Fungi and Endophytic Fungi Activate Leaf Antioxidant Defense System of Lane Late Navel Orange. Journal of Fungi. 2022; 8(3):282. https://doi.org/10.3390/jof8030282
Chicago/Turabian StyleLi, Qiu-Shuang, Ya-Chao Xie, Mohammed Mahabubur Rahman, Abeer Hashem, Elsayed Fathi Abd_Allah, and Qiang-Sheng Wu. 2022. "Arbuscular Mycorrhizal Fungi and Endophytic Fungi Activate Leaf Antioxidant Defense System of Lane Late Navel Orange" Journal of Fungi 8, no. 3: 282. https://doi.org/10.3390/jof8030282
APA StyleLi, Q.-S., Xie, Y.-C., Rahman, M. M., Hashem, A., Abd_Allah, E. F., & Wu, Q.-S. (2022). Arbuscular Mycorrhizal Fungi and Endophytic Fungi Activate Leaf Antioxidant Defense System of Lane Late Navel Orange. Journal of Fungi, 8(3), 282. https://doi.org/10.3390/jof8030282








