Abstract
Yeast produced semiochemicals are increasingly used in pest management programs, however, little is known on which yeasts populate cherry fruits and no information is available on the volatiles that modify the behaviour of cherry pests including Rhagoletis cerasi flies. Eighty-two compounds were extracted from the headspaces of eleven yeast species associated with sweet and sour cherry fruits by solid phase micro extraction. Esters and alcohols were the most abundant volatiles released by yeasts. The multidimensional scaling analysis revealed that the odour blends emitted by yeasts were species-specific. Pichia kudriavzevii and Hanseniaspora uvarum yeasts released the most similar volatile blends while P. kluyveri and Cryptococcus wieringae yeasts produced the most different blends. Combined gas chromatographic and electroantennographic detection methods showed that 3-methybutyl acetate, 3-methylbutyl propionate, 2-methyl-1-butanol, and 3-methyl-1-butanol elicited antennal responses of both R. cerasi fruit fly sexes. The two-choice olfactometric tests revealed that R. cerasi flies preferred 3-methylbutyl propionate and 3-methyl-1-butanol but avoided 3-methybutyl acetate. Yeast-produced behaviourally active compounds indicated a potential for use in pest monitoring and control of R. cerasi fruit flies, an economically important pest of cherry fruits.
1. Introduction
Carposphere is a specific habitat populated by bacterial and fungal microorganisms including yeasts [1,2]. Berries and fruits are rich in carbohydrates and often bear the most diverse microbiome in a phyllosphere [2]. Carposphere microbiota are determined by a variety of factors such as environmental conditions, host genotype, berry developmental stage, and interactions with other organisms sharing a habitat [3,4,5,6,7]. Microorganisms associated with fruits and berries interact with insects that use these habitats for feeding and oviposition. Insects and yeasts could come into diverse relationships ranging from amensal to commensal and mutualistic [8]. Yeasts provide essential nutrients missing in sugar-rich berries that insects cannot produce while insects transfer yeasts from one substrate to another [1,9,10]. In addition to berry and fruit-related odours, yeast-produced volatiles are used by insects to acquire information about habitat quality and host choice [11,12,13,14]. The behaviour modifying effect of yeast volatiles has the potential for use in integrated pest management programs increasing the efficiency of attractive lures and serving as repellents in the push–pull pest control strategy [15].
The purpose of the study was to characterize volatile blends emitted by cultivable yeasts populating the fruit surface of sweet and sour cherries and to determine semiochemicals that modify the behaviour of R. cerasi fruit flies, the most important pest of cherry fruits.
2. Materials and Methods
2.1. Yeast Sampling, Culturing, and Identification
Yeast species have been isolated from sweet cherry (Prunus avium L.) and sour cherry (Prunus cerasus L.) (Rosales: Rosaceae) fruits collected during June–July of 2018–2020 from private plantations located in the Vilnius region (GPS coordinates: 54°45′08.2″ N, 25°17′10.0″ E; 54°46′14.4″ N 25°21′04.1″ E; 54°41′19.9″ N 25°26′20.6″ E), Klaipėda region (GPS coordinates: 55°36′1.66″ N 21°36′3.8″ E; 55°34′50.6″ N 21°14′58.1″ E) and Alytus region (GPS coordinates: 54°23′43.8″ N, 23°56′18.7″ E) of Lithuania. Isolation and culturing methods have been described in detail by Stanevičienė et al. [16]. Generally, cultivable yeasts are isolated by direct rinsing of fruits with MD medium (2% dextrose, 1% (NH4)2SO4, 0.09% KH2PO4, 0.05% MgSO4, 0.023% K2HPO4, 0.01% NaCl, 0.01% CaCl2) or by applying fermentation-based enrichment. After cultivation on YPD-agar plates (1% yeast extract, 1% peptone, 2% dextrose, 2% agar), morphologically distinct colonies proceeded molecular analysis. For taxonomic identification of the isolates, the ITS1-5.8S rDNA-ITS2 region or D1/D2 region of 26S rDNA were PCR-amplified as described in Stanevičienė et al. [16]. To assess their taxonomic position, the resolved sequences were compared with those available in the current version of the GenBank database at the National Centre for Biotechnology Information (NCBI) (Table A1).
2.2. Insects
European cherry fruit flies, Rhagoletis cerasi (L.) (Diptera: Tephritidae), were collected as pupae from soil under sweet and sour cherry trees in April of 2019–2021 at private orchards in Vilnius (GPS coordinates: 54°45′24.0″ N 25°03′02.4″ E) and Kaunas (GPS coordinates: 54°54′13.8″ N 23°48′07.9″ E) districts, Lithuania. Reactivation of the pupae took place in a climate chamber “Fitotron” under 20–24 °C, 16L:8D (light:dark) photoperiod, and 65–75% relative humidity. Each pupa was placed in an individual 14 mL glass vial bearing wet 3 cm2 filter paper inside and closed by foam stoppers. The filter paper was humidified periodically to keep the humidity up inside the vial. After emergence, the adults were kept within the same vials in the room under 18–20 °C, a natural daylight photoperiod, 50–60% relative humidity, and fed on 10% sugar solution in water. The flies possessing an ovipositor were attributed to females. After sexing, each individual was kept in a separate vial under the identical conditions as described above.
2.3. Sampling and Analysis of Volatiles Produced by Yeasts
For sampling of volatile organic compounds, yeasts were selected based on isolation frequency over the different ripening stages of cherries (Figure A1). The methods have been described in detail by Lukša et al. [7]. In summary, overnight grown yeast cells (50 µL) at the concentration of about 3–5 × 107 cells/mL were placed on the surface of YPD-agar medium and cultivated for two days at 25 °C. The solid-phase micro-extraction (SPME) technique was used to sample the headspace volatiles produced by yeasts. For sampling background volatiles, YPD-agar plates without yeast were used as control samples. The SPME needle was placed above the yeast culture through a small hole drilled in a Petri dish; the purified fibre coated with a polydimethylsiloxane-divinylbenzene absorbent (65 mm coating layer thickness) was exposed to the headspace for 60 min at room temperature. The volatiles collected on the fibre were desorbed for 2 min in the injection liner of a gas chromatograph (GC).
GC and mass spectrometer (MS) were used to analyse the collected volatiles. The compounds were separated by a DB-Wax column under the subsequent temperature program: isothermal at 40 °C for 1 min and afterwards gradually increased to 200 °C at a rate of 5 °C/min, then to 240 °C at a rate of 10 °C/min, and maintained isothermally for 11 min. The GC injector was run isothermally at 240 °C. Helium served as a carrier gas. The relative amount of each of the compounds was determined based on the area of the chromatographic peak. The volatile compounds were identified by comparing their mass spectra and retention indexes with those presented in a NIST version 2.0 mass spectral library and those of the available synthetic standards. C8–C28 n-alkanes were used to calculate the retention indexes of the volatiles.
2.4. Gas Chromatography-Electroantennogram Detection
Gas chromatographic and electroantennogram detection (EAD) techniques were applied to determine yeasts produced olfactory active volatiles to R. cerasi flies. A detailed description of the GC-EAD setup as well as the procedure has been published by Būda et al. [17]. Briefly, the GC was set up with a polar DB-Wax column. The injector and the detector were run at 240 °C. The oven temperature was maintained at 40 °C for 1 min; afterwards, it was raised to 240 °C at a rate of 10 °C/min, then maintained isothermally for 13 min. Hydrogen, at a flow rate of 1.5 mL/min, was used as a carrier gas. At the end of the GC column, a splitter divided an eluent into two equal parts, allowing simultaneous flame ionisation (FID) and EAD detection of the separated volatiles. A nitrogen make-up gas at 5 mL/min flow rate was added to increase FID sensitivity. The part of an eluent allocated to EAD was mixed with charcoal filtered and humidified air flowing at 0.5 m/s through a glass tube over antenna preparation. Glass capillary electrodes were used. The EAD and the FID signals were registered simultaneously, saved, and analysed. Before EAD recording, the antenna was stimulated with 1 µg of 3-methyl-1-butanol to check sensitivity. Four to seven days old flies were used in the tests. Each antenna tested was from a different fly. In total, 21 antennae of males and 18 antennae of females were used.
2.5. Electroantennogram Dose-Response
The same electrophysiological recording setup and the antennal preparation technique were used to record electroantennogram (EAG) dose–responses of male and female flies to the synthetic EAD active compounds: 3-methylbutyl acetate; 3-methylbutyl propionate; 2-methyl-1-butanol; and 3-methyl-1-butanol.
The compounds were tested at the doses of 10−5, 10−4, 10−3, 10−2, and 10−1 mg applied in 10 µL hexane on filter paper (5 × 45 mm). The compounds were selected randomly, and five doses of each compound were tested in ascending order. A solvent blank (10 µL of hexane after evaporation) was tested as a control stimulus both at the beginning and the end of stimulation with each compound. Each EAD test was replicated 13 times, and each antenna used was from a different fly. The EAG response (R) to the EAD-active compound dose was calculated according to the formula R = RA − (RC1 + RC2)/2, where RA is the EAG response to the EAD active compound, and RC1 and RC2 are EAG responses to the first and the second control stimuli, respectively.
2.6. Behavioural Assay
To test the behavioural choice of the flies to the synthetic EAD active compounds versus the control, a Y-tube olfactometer [18] (25 cm main tube, 17 cm arms, 110° branching angle, the inner diameter of each arm and main tube 5 cm) was used. The olfactometer was placed in a fume cupboard. Four T8/840, Colourlux plus, 18 W tube type lamps (NARVA Lichtquellen GmbH + Co. KG, Brand-Erbisdorf, Germany) covered with a white, mat, plastic shield (65 cm length, 42 cm width) at a distance of 23 cm were placed in front of the Y tube of the olfactometer. For the fruit flies, positive phototaxis is characteristic, and the light slightly stimulated the insects to move towards the light source. Each arm of the olfactometer was connected to a glass tube that contained either the stimulus or control. A purified air delivery system CADS-4CPP (Sigma Scientific LLC, Micanopy, FL, USA) was used to push air at a rate of 0.5 L/min through each arm.
The synthetic 3-methylbutyl propionate, 2-methyl-1-butanol, and 3-methyl-1-butanol were dissolved in hexane while paraffin oil was used to dissolve 3-methylbutyl acetate and the four component mixture. Behaviour modifying effect was assessed at the few doses by dispensing the 10 µL of the solution on a filter paper strip (5 × 40 mm). The proportion of EAD active components in the mixture used in the bioassay was based on the proportion of EAD active compounds determined in the sample of H. uvarum yeasts. The synthetic mixture consisted of 3-methylbutyl acetate 0.55 mg, 3-methylbutyl propionate 0.05 mg, 2-methyl-1-butanol 0.012 mg, and 3-methyl-1-butanol 0.28 mg per 10 µL of paraffin oil. After 0.5 min of solvent evaporation (only applicable for samples that were dissolved in hexane and no evaporation was carried out when paraffin oil was used), the filter paper strip was placed in the glass tube connected to one arm of the olfactometer. The same size filter paper was treated either with 10 µL of hexane or with paraffin oil and was placed in the other arm serving as the control. After each test, the olfactometer was taken apart and the glassware was cleaned with hexane, soaked overnight in distilled water, and dried for 2 h in an oven, raising the temperature to 200 °C. Silicone parts of the Y-tube olfactometer were cleaned with hexane, soaked overnight in distilled water, and air-dried or replaced between the tests.
A single fly was released into the Y olfactometer at the end of the main tube. The duration within which a fly must have reached the branch point was set to 15 min. A fly was considered to have made a choice when it reached the distal end of the glass tube containing either a stimulus or a control (solvent after evaporation), irrespectively of whether the fly switched arms or not before reaching the odour source. The fly was considered as not making a choice if none of the arms was chosen within 15 min. After every five tests, the positions of the two Y-tube arms were reversed. All insects were observed individually and used in a bioassay only once. The tests were carried out at 23 ± 2 °C, 60% RH, between 10 h AM and 5 h PM local time.
2.7. Statistical Analysis
A nonparametric Mann–Whitney U test was applied to evaluate differences in the volatile amounts between the yeast and control samples. To assess and visualise the associations between odour blends of eleven yeast species and volatile compounds, a multidimensional scaling (MDS) analysis with a Bray–Curtis index was performed on absolute amounts expressed as areas under chromatographic peaks using R (version 4.0.2) and Rstudio (version 1.3.959), with the metaMDS function in the vegan package (version 2.5–6), and the results were visualised using ggplot2 (version 3.3.2). Prior to analysis, the data were log-transformed. Dendrogram of the odour blends was obtained by cluster analysis based on Euclidean distance using the same way transformed data as in the MDS analysis. The clustering was carried out based on the average of quantified volatile compounds from three different isolates per species. The different clusters were identified by visually evaluating the clustering. Paired t test was applied to compare EAG amplitudes of R. cerasi antennae of males versus females at each dose tested. To evaluate the choices of flies to the synthetic EAD active compounds versus the control, the total number of flies that made a choice was analysed with a χ2 test (observed vs. expected). All the analysis except MDS was performed using Statistica 6.0 software (StatSoft, Inc., Tulsa, OK, USA).
3. Results
3.1. Composition of Yeast Produced Volatile Blends
Analysis of yeast produced volatiles revealed 82 compounds that were exclusively present in the headspace of eleven yeast species (Table A1) or occurred at significantly larger amounts compared to those of the control samples. The esters represented by 41 compounds were accounted as the most abounded group of volatiles released by yeasts followed by 18 alcohols, nine compounds bearing aromatic moiety, eight ketones, six fatty acids, four terpenoids, three lactones, and one each of isothiocyanate, furane, and sulphide functional group was detected once (Table 1). Compounds bearing ester, alcohol, aromatic, ketone, and fatty acid moiety were detected in the volatile blends of all yeast species while terpenoid, lactone, isothiocyanoate, furane, and sulphide type volatiles were emitted by the yeast of single or few species (Figure 1).

Table 1.
Odour blends of eleven yeast species and controls sampled from a headspace by the SPME technique.
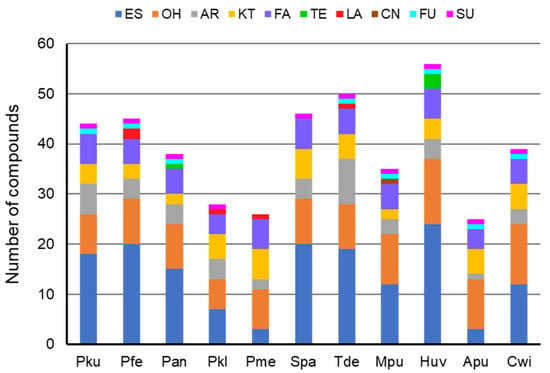
Figure 1.
Chemical diversity of the volatile blends produced by yeasts. Pku–Pichia kudriavzevii, Pfe–P. fermentans, Pan-P. anomala, Pkl-P. kluyveri, Pme-P. membranifaciens, Spa-Saccharomyces paradoxus, Tde-Torulaspora delbrueckii, Mpu-Metschnikowia pulcherrima, Huv-Hanseniaspora uvarum, Apu-Aureobasidium pullulans, Cwi-Cryptococcus wieringae. Functional group of volatiles: ES–ester; OH–alcohol; AR–aromatic; KT–ketone; FA–fatty acid; TE–terpenoid; LA–lactone; CN–isothiocyanoate; FU—furane; SU–sulphide.
Nonmetric multidimensional scaling analysis showed that volatile blends of all eleven species grouped in a species-specific manner (Figure 2).
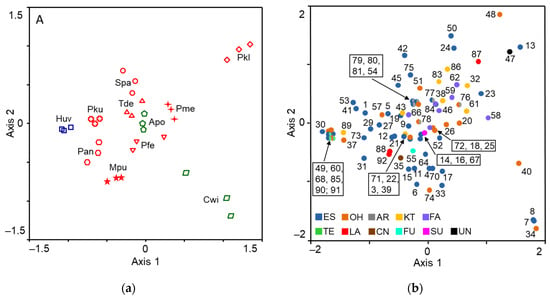
Figure 2.
Multidimensional scaling plots: (a) eleven yeast species (each represented by three different isolates); (b) volatile compounds produced by yeasts. Volatiles were sampled from the headspace by SPME. Pku—Pichia kudriavzevii, Pfe—P. fermentans, Pan—P. anomala, Pkl—P. kluyveri, Pme—P. membranifaciens, Spa—Saccharomyces paradoxus, Tde—Torulaspora delbrueckii, Mpu—Metschnikowia pulcherrima, Huv—Hanseniaspora uvarum, Apu—Aureobasidium pullulans, Cwi—Cryptococcus wieringae. Apu and Cwi yeasts are more common on unripe fruits and their symbols are coloured green, Huv yeasts are the most common on medium-ripe and ripe fruits and are indicated by the blue colour, and the red colour represents yeasts, the most common on ripe fruits. The name of volatiles indicated by numbers are listed in Table 1. Functional group of volatiles: ES—ester; OH—alcohol; AR—aromatic; KT—ketone; FA—fatty acid; TE—terpenoid; LA—lactone; CN—isothiocyanoate; FU—furane; SU—sulphide; UN—unidentified.
P. kudriavzevii and H. uvarum yeasts released the most similar volatile blends and together with P. anomala as well as M. pulcherrima yeasts formed a distinct cluster. Yeasts of P. fermentans, P. membranifaciens, A. pullulans, T. delbrueckii, and S. paradoxus clustered in another group. The most different blends were produced by P. kluyveri and C. wieringae yeasts (Figure 3).
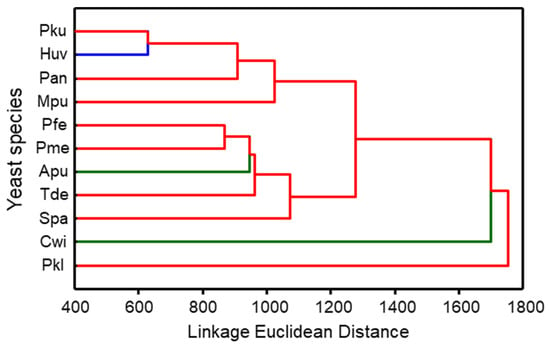
Figure 3.
Dendrogram of odour blends sampled by SPME from the headspace of eleven yeast species. The dendrogram was obtained by cluster analysis based on Euclidean distance. The clustering was carried out based on the average of quantified volatile compounds from three different isolates per species. The different clusters were identified by visually evaluating the clustering. Pku—Pichia kudriavzevii, Pfe—P. fermentans, Pan—P. anomala, Pkl—P. kluyveri, Pme—P. membranifaciens, Spa—Saccharomyces paradoxus, Tde—Torulaspora delbrueckii, Mpu—Metschnikowia pulcherrima, Huv—Hanseniaspora uvarum, Apu—Aureobasidium pullulans, Cwi—Cryptococcus wieringae. Apu and Cwi yeasts are more common on unripe fruits and are represented by the green colour, Huv yeasts are the most common on medium-ripe and ripe fruits and are indicated by the blue colour, and the red colour represents yeasts, the most common on ripe fruits.
3.2. EAD Active Compounds
GC–EAD analyses of the headspace collections from five yeast species representing three fruit ripening stages showed that antennae of R. cerasi flies, a pest of cherry fruits, responded to 3-methybutyl acetate, 3-methylbutyl propionate, 2-methyl-1-butanol, and 3-methyl-1-butanol (Figure 4, Table 2). Antennae of both R. cerasi sexes responded to all four EAD active compounds (Table 2).
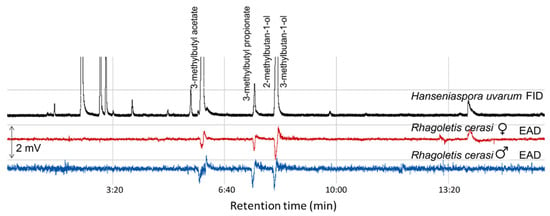
Figure 4.
GC-EAD response of male and female Rhagoletis cerasi to headspace volatiles of Hanseniaspora uvarum yeast. FID, flame ionisation detector; EAD, electroantennographic detector; DB-Wax capillary column (30 m × 0.25 mm × 0.25 µm; Agilent Technologies, Santa Clara, CA, USA.)

Table 2.
Electroantennographic responses of R. cerasi flies to volatiles present in the headspace of five yeast species.
Unripe fruits associated with yeast-like fungi, A. pullulans, and C. wieringae yeasts produced two alcohols, 2-methyl-1-butanol and 3-methyl-1-butanol, which elicited antennae responses of R. cerasi flies. H. uvarum, the most common yeast species inhabiting medium-ripe fruits as well P. kudriavzevii attributed to microbiota of ripe fruits in addition to two EAD active alcohols produced two esters, 3-methybutyl acetate and 3-methylbutyl propionate, which evoked antennae responses. Ripe fruits associated yeasts, M. pulcherrima released three EAD active volatiles 3-methybutyl acetate, 2-methyl-1-butanol, and 3-methyl-1-butanol (Table 2).
3.3. EAG Dose–Response
The antennographic responses of females to 3-methybutyl acetate and 3-methylbutyl propionate at the moderate and the higher doses were of significantly higher amplitudes compared to the responses of males (Figure 5a,b). Females showed a higher sensitivity to 2-methyl-1-butanol than males at all doses tested, and significant differences were revealed at the doses 10−5, 10−3, and 10−2 mg (Figure 5c). Significant stronger responses of females than males were recorded to 3-methyl-1-butanol at the dose 10−4 mg, while antennae stimulation with the higher doses revealed stronger responses of males compared to the responses of females (Figure 5d).
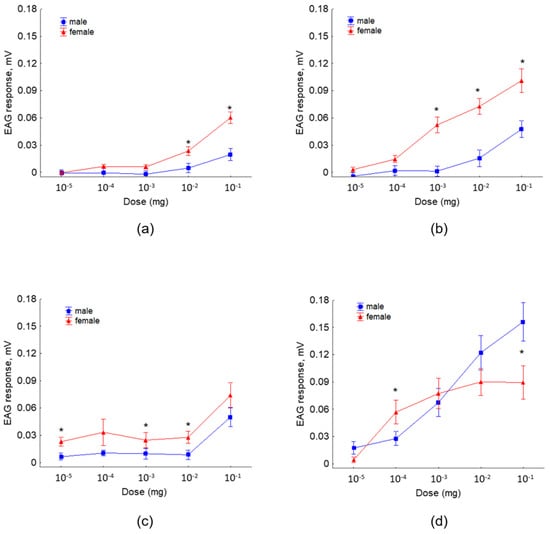
Figure 5.
EAG responses (mean amplitude ± standard error (SE), mV) of R. cerasi male and female antennae to different doses (10−5 to 10−1 mg) of synthetic: (a) 3-methylbutyl acetate, (b) 3-methylbutyl propionate, (c) 2-methyl-1-butanol, and (d) 3-methyl-1-butanol. The asterisk denotes significant differences in EAG responses between sexes (paired t test, p < 0.05); each EAD test was replicated 13 times and each antenna used was from a different fly.
3.4. Behavioural Tests in Olfactometer
In the two-choice tests, R. cerasi males showed no preference to 3-methylbutyl acetate at the dose 10−2 mg, while females significantly avoided the olfactometer arm bearing 3-methylbutyl acetate at the dose 10−3 mg. Fruit flies of both sexes significantly preferred 3-methylbutyl propionate provided at the dose of 10−2 mg for males and at the dose 10−3 mg for females (Figure 6). Flies of both sexes do not discriminate between olfactory arms bearing 2-methyl-1-butanol. Males chose 3-methyl-1-butanol at the dose 10−2 mg and showed no preference to a 10 times lower dose. Females preferred the alcohol at the dose of 10−4 mg, while a 10 times higher dose obliterated the preference.
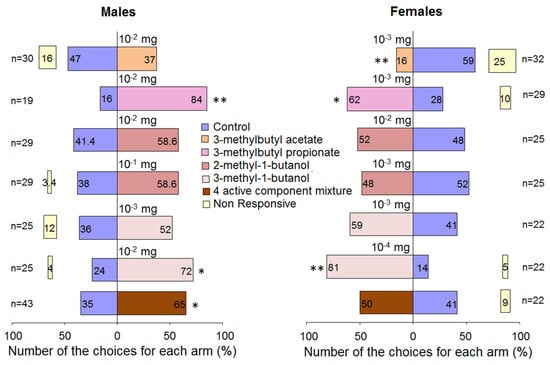
Figure 6.
Behavioural responses of Rhagoletis cerasi flies in Y-tube olfactometer to EAD active volatiles. The mixture consisted of 3-methylbutyl acetate 0.55 mg, 3-methylbutyl propionate 0.05 mg, 2-methyl-1-butanol 0.012 mg, and 3-methyl-1-butanol 0.28 mg. n–number of flies tested. Stars denote significant differences in behavioural responses of flies between stimulus and control: * p < 0.05 and ** p < 0.01 by χ2 test.
4. Discussion
Yeasts produce a wide range of volatile compounds [19]. Common volatiles at large amounts emitted by different yeast species originate from primary metabolism pathways and these compounds are considered as side products or waste compounds [20]. Ethanol and ethyl acetate are the best-known examples of such volatile metabolites. Our analysis showed that yeasts of S. paradoxus species released high amounts of ethanol while P. anomala, P. kudriavzevii, H. uvarum, and M. pulcherrima yeasts produced the largest amounts of ethyl acetate, an ester derived from ethanol. Ethanol or ethyl acetate were the major components in the volatile bouquets of these yeast species. Interestingly, we did not detect ethanol and ethyl acetate in the headspaces of P. kluyveri and P. membranifaciens yeasts. More structurally diverse volatiles released by yeasts are derived from amino or fatty acid synthesis and degradation as well as from terpene biosynthetic pathways [19,20]. Analysis of volatile profiles of eleven yeast species isolated from sweet and sour cherry fruits showed that esters and alcohols were the most numerous groups of volatiles.
The multidimensional scaling analysis revealed that volatile blends of all yeast species were clearly separated from each other, therefore, the amount and composition of volatiles characterised yeast species. Our results are consistent with previous findings that volatile profiles of microorganisms are species-specific, reflecting specific metabolic activities of the particular microorganisms [19].
The ecological role of microbial volatiles falls in two major groups: they are important as semiochemicals mediating information flow at intra- and inter-specific levels; and function as promoters or inhibitors of microbial growth [21,22,23]. Yeast volatiles play an essential role in distribution of yeast from one habitat to another by attracting vector insects. Our data showed that 3-methybutyl acetate, 3-methylbutyl propionate, 2-methyl-1-butanol, and 3-methyl-1-butanol emitted by yeasts populating P. avium and P. cerasus fruits elicited electroantennographic responses in both sexes of R. cerasi fruit flies, a common pest of cherry fruits. Recently, it was reported that ten volatiles emitted by P. kudriavzevii yeast species including 3-methybutyl acetate, 3-methylbutyl propionate, and 3-methyl-1-butanolelicited antennal responses in males and females of closely related R. batava flies while 2-methyl-1-butanol was not EAD active [24].
Females showed the higher sensitivity to 3-methybutyl acetate, 3-methylbutyl propionate, and 2-methyl-1-butanol than males and the more pronounced difference in antennal sensitivity was recorded at higher doses of both esters. As far as we know, higher antennae sensitivity of females to microbial volatiles has not been reported in other tephritid fruit flies.
Two EAD active compounds 3-methylbutyl propionate and 3-methyl-1-butanol stimulated both sexes of R. cerasi flies to choose the olfactometer arm bearing these volatiles versus control without stimulus, while 2-methyl-1-butanol did not significantly affect the choice of flies. 3-Methyl-1-butanol is the end product of degradation of the amino acid leucine [20] and is found in volatile bouquets of many yeast species [20,25]. In our samplings, 3-methyl-1-butanol at various amounts was isolated from the emissions of all eleven yeast species. As an attractant, 3-methyl-1-butanol functions in five dipteran species [26] including one tephritid species [25]. Attractiveness of 3-methylbutyl propionate has not been reported for any dipteran species.
The two-choice test showed that R. cerasi females avoided the olfactometer hand bearing 3-methybutyl acetate at the dose 10−2 mg, while males did not significantly discriminate stimulus versus the control at even higher 10−1 mg dose. R. cerasi females prefer to lay eggs in cherries at the stage of colour change from green to yellow [27] (i.e., just at the beginning of ripening). At the early maturation stage of fruits, yeast-like fungi from Aureobasidium genus, yeasts from Cryptococcus, Taphrina, Cladosporium, and some other genus are more prevalent compared to yeast from Pichia, Metschnikowia, Saccharomyces, and Torulaspora genus inhabiting ripe fruits [3,7,28]. Analysis of chromatographic profiles of yeast emitted volatiles revealed that unripe fruits associated with yeast-like fungi, Aureobasidium pullulans, and Cryptococcus wieringae yeasts produced none or very low amounts of 3-methybutyl acetate, acting as a repellent to R. cerasi females. 3-Methybutyl acetate is a ubiquitous volatile in the odour bouquets of ripe and fermenting fruit-yeast complexes [29], which is in agreement with our data showing that fermenting yeasts emitted large amounts of this acetate. Contrary to R. cerasi, many Drosophila species oviposit in ripe fruits and berries and 3-methybutyl acetate released by ripe fruits associated yeasts functions as an attractant [29,30].
Ammonium acetate is reported as the most efficient food attractant for R. cerasi flies [31]. The number of yeast-based commercially available attractive lures such as brewer’s yeast waste, baker’s yeast (Saccharomyces cerevisiae), and Torula yeast (Candida utilis) have been used in tephritid pest control programs [32], however, none of the formulations tested showed a potential to control R. cerasi fruit flies. A possible explanation is that all lures released high amounts of 3-methybutyl acetate, the repellent to R. cerasi fruit flies. Our data suggest that yeast species selected for an efficient lure to target R. cerasi pests should release high amounts of 3-methylbutyl propionate and/or 3-methyl-1-butanol and do not emit 3-methybutyl acetate. Moreover, our data provide a background for the application of behaviour modifying semiochemicals in push-pull and other integrated pest management techniques to control R. cerasi fruit flies.
5. Conclusions
The odour blends emitted by yeasts were species-specific. 3-Methybutyl acetate, 3-methylbutyl propionate, 3-methylbutanol, and 2-methyl-1-butanol released by yeasts populating P. avium and P. cerasus fruits elicited electroantennographic responses and modulated behaviour of R. cerasi fruit flies, a common pest of cherry fruits. Therefore, these olfactory and behaviourally active compounds show potential for use in integrated pest management techniques to control R. cerasi fruit flies.
Author Contributions
Conceptualisation, R.M., E.S. and V.B.; Methodology, D.A., S.R., V.A. and R.S.; Software, R.M., S.R. and L.B.-Č.; Validation, V.A., S.R., L.B.-Č., E.S. and R.M.; Formal analysis, V.A., S.R., J.B., L.B.-Č., E.S. and R.M.; Investigation, D.A., S.R., V.A., R.S. and L.B.-Č.; Resources, R.M., V.B. and E.S.; Data curation, V.A., S.R., L.B.-Č. and E.S.; writing—original draft preparation, R.M.; writing—review and editing, R.M., S.R., L.B.-Č., V.B. and E.S.; visualisation, R.M., S.R. and L.B.-Č.; Supervision, R.M. and V.B.; Project administration, R.M. and L.B.-Č.; Funding acquisition, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EUROPEAN SOCIAL FUND, grant number 09.3.3-LMT-K-712-01-0099 under grant agreement with the Research Council of Lithuania (LMTLT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank A. Amšiejus and L. Levulytė for the kind permission to collect puparia in their cherry orchards. Thanks are also given to I. Vepštaitė-Monstavičė and J. Lukša for providing cherry fruits.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
List of yeast isolates analysed in this study.
Table A1.
List of yeast isolates analysed in this study.
| Yeast Species | Strain | GenBank Reference | Identity (%) |
|---|---|---|---|
| Aureobasidium pullulans | PA-4-13 | MN400109 | 100 |
| PC-5-28 | HQ909088 | 99.6 | |
| PC-4-8 | MW361317 | 99.8 | |
| Cryptococcus wieringae | PC-4-6 | KF981864 | 100 |
| PC-4-9 | KF981864 | 100 | |
| PA-5-41 | KF981864 | 100 | |
| Metschnikowia pulcherrima | PC-4-5 | MF574308 | 100 |
| PA-5-13 | KT029787 | 99.7 | |
| PA-5-47 | MK352050 | 100 | |
| Hanseniaspora uvarum | PA-5-27 | MF062209 | 100 |
| PC-3-8 | MK352020 | 99.9 | |
| PC-5-12 | KY103573 | 100 | |
| Pichia kudriavzevii | PA-1-15 | MF685423 | 100 |
| PC-4-25 | MF685411 | 100 | |
| PC-4-19.3 | MG015972 | 99.6 | |
| Pichia fermentans | PC-5-47 | FJ713081 | 100 |
| PC-4-19.1 | MF462777 | 100 | |
| PA-4-39 | KY104537 | 100 | |
| Pichia kluyveri | PA-4-30 | JX103190 | 100 |
| PA-4-34 | KY108823 | 99.8 | |
| PC-4-35 | KC510043 | 100 | |
| Pichia membranifaciens | PC-3-36 | JX188207 | 100 |
| PC-2-71 | FJ231461 | 100 | |
| PA-2-55 | JX188207 | 99.6 | |
| Saccharomyces paradoxus | PA-5-14 | FJ713072 | 100 |
| PC-3-33 | FJ713072 | 100 | |
| PC-3-59 | KY105204 | 99.9 | |
| Torulaspora delbrueckii | PA-4-16 | KY105641 | 100 |
| PA-5-17 | MN371902 | 99.8 | |
| PC-2-42 | MK352012 | 100 | |
| Pichia anomala | PC-5-5 | KJ527050 | 100 |
| PA-5-18 | MH248067 | 100 | |
| PC-5-24 | MK343437 | 100 |
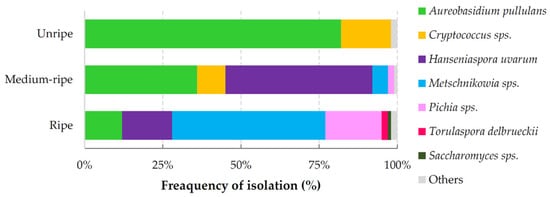
Figure A1.
Frequency of yeast isolation among cherry samples at different ripening stages. C. wieringae dominated among Cryptococcus genus species; M. pulcherrima frequently occurred among Metschnikowia yeasts; Pichia genus were represented by P. anomala, P. kudriavzevii, P. kluyveri, P. fermentans, and P. membranifaciens yeasts; S. paradoxus occurred among Saccharomyces yeasts; others represented yeasts occurred in low frequency.
References
- Starmer, W.T.; Lachance, M.-A. Chapter 6-Yeast Ecology. In The Yeasts, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: London, UK, 2011; pp. 65–83. [Google Scholar] [CrossRef]
- Allard, S.M.; Ottesen, A.R.; Brown, E.W.; Micallef, S.A. Insect exclusion limits variation in bacterial microbiomes of tomato flowers and fruit. J. Appl. Microbiol. 2018, 125, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gobbi, A.; Santoni, S.; Hansen, L.H.; This, P.; Peros, J.P. Assessing the impact of plant genetic diversity in shaping the microbial community structure of Vitis vinifera phyllosphere in the Mediterranean. Front. Life Sci. 2018, 11, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Vepstaite-Monstavic, I.; Luksa, J.; Staneviclene, R.; Strazdaite-Zieliene, Z.; Yurchenko, V.; Serva, S.; Serviene, E. Distribution of apple and blackcurrant microbiota in Lithuania and the Czech Republic. Microbiol. Res. 2018, 206, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kioroglou, D.; Kraeva-Deloire, E.; Schmidtke, L.M.; Mas, A.; Portillo, M.C. Geographical origin has a greater impact on grape berry fungal community than grape variety and maturation state. Microorganisms 2019, 7, 669. [Google Scholar] [CrossRef] [Green Version]
- Luksa, J.; Vepstaite-Monstavice, I.; Apsegaite, V.; Blazyte-Cereskiene, L.; Staneviciene, R.; Strazdaite-Zieliene, Z.; Ravoityte, B.; Aleknavicius, D.; Buda, V.; Mozuraitis, R.; et al. Fungal microbiota of sea buckthorn berries at two ripening stages and volatile profiling of potential biocontrol yeasts. Microorganisms 2020, 8, 456. [Google Scholar] [CrossRef] [Green Version]
- Stefanini, I. Yeast-insect associations: It takes guts. Yeast 2018, 35, 315–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackwell, M. Made for each other: Ascomycete yeasts and insects. Microbiol. Spectr. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Janson, E.M.; Stireman, J.O.; Singer, M.S.; Abbot, P. Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution 2008, 62, 997–1012. [Google Scholar] [CrossRef] [Green Version]
- Dobzhansky, T.; Cooper, D.M.; Phaff, H.J.; Knapp, E.P.; Carson, H.L. Studies on the ecology of Drosophila in the Yosemite region of California Differential attraction of species of Drosophila to different species of yeasts. Ecology 1956, 37, 544–550. [Google Scholar] [CrossRef]
- Date, P.; Dweck, H.K.M.; Stensmyr, M.C.; Shann, J.; Hansson, B.S.; Rollmann, S.M. Divergence in olfactory host plant preference in D-mojavensis in response to cactus host use. PLoS ONE 2013, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keesey, I.W.; Knaden, M.; Hansson, B.S. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 2015, 41, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Scheidler, N.H.; Liu, C.; Hamby, K.A.; Zalom, F.G.; Syed, Z. Volatile codes: Correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci. Rep. 2015, 5, 14059. [Google Scholar] [CrossRef] [Green Version]
- Holighaus, G.; Rohlfs, M. Fungal allelochemicals in insect pest management. Appl. Microbiol. Biotechnol. 2016, 100, 5681–5689. [Google Scholar] [CrossRef]
- Staneviciene, R.; Luksa, J.; Strazdaite-Zieliene, Z.; Ravoityte, B.; Losinska-Siciuniene, R.; Mozuraitis, R.; Serviene, E. Mycobiota in the carposphere of sour and sweet cherries and antagonistic features of potential biocontrol yeasts. Microorganisms 2021, 9, 1423. [Google Scholar] [CrossRef] [PubMed]
- Buda, V.; Blazyte-Cereskiene, L.; Radziute, S.; Apsegaite, V.; Stamm, P.; Schulz, S.; Aleknavicius, D.; Mozuraitis, R. Male-produced (-)-delta-heptalactone, pheromone of fruit fly Rhagoletis batava (Diptera: Tephritidae), a sea buckthorn berries pest. Insects 2020, 11, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, D.J. Bioassay methods with terrestrial invertebrates. In Methods in Chemical Ecology: Bioassay Methods; Millar, J.G., Haynes, K.F., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 2000; pp. 212–270. [Google Scholar]
- Lemfack, M.C.; Nickel, J.; Dunkel, M.; Preissner, R.; Piechulla, B. mVOC: A database of microbial volatiles. Nucleic Acids Res. 2014, 42, D744–D748. [Google Scholar] [CrossRef] [PubMed]
- Ebert, B.E.; Halbfeld, C.; Blank, L.M. Exploration and Exploitation of the Yeast Volatilome. Curr. Metab. 2017, 5, 102–118. [Google Scholar] [CrossRef]
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [Green Version]
- Becher, P.G.; Hagman, A.; Verschut, V.; Chakraborty, A.; Rozpedowska, E.; Lebreton, S.; Bengtsson, M.; Flick, G.; Witzgall, P.; Piskur, J. Chemical signaling and insect attraction is a conserved trait in yeasts. Ecol. Evol. 2018, 8, 2962–2974. [Google Scholar] [CrossRef] [Green Version]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Mozuraitis, R.; Aleknavicius, D.; Vepstaite-Monstavice, I.; Staneviciene, R.; Emami, S.N.; Apsegaite, V.; Radziute, S.; Blazyte-Cereskiene, L.; Serviene, E.; Buda, V. Hippophae rhamnoides berry related Pichia kudriavzevii yeast volatiles modify behaviour of Rhagoletis batava flies. J. Adv. Res. 2020, 21, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Vitanovic, E.; Aldrich, J.R.; Boundy-Mills, K.; Cagalj, M.; Ebeler, S.E.; Burrack, H.; Zalom, F.G. Olive Fruit Fly, Bactrocera oleae (Diptera: Tephritidae), attraction to volatile compounds produced by host and insect-associated yeast strains. J. Econ. Entomol. 2020, 113, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S.; Landolt, P.J. A survey of insect assemblages responding to volatiles from a ubiquitous fungus in an agricultural landscape. J. Chem. Ecol. 2013, 39, 860–868. [Google Scholar] [CrossRef]
- Daniel, C.; Grunder, J. Integrated management of European cherry fruit fly Rhagoletis cerasi (L.): Situation in Switzerland and Europe. Insects 2012, 3, 956–988. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarzadeh, B.; Sampiao, J.P.; Arzanlou, M. Grape maturity significantly influences yeast community on grape berries: Basidiomycetous yeasts are dominant colonizers of immature grape berries in northwestern Iran. Nova Hedwig. 2021, 113, 191–206. [Google Scholar] [CrossRef]
- Revadi, S.; Vitagliano, S.; Stacconi, M.V.R.; Ramasamy, S.; Mansourian, S.; Carlin, S.; Vrhovsek, U.; Becher, P.G.; Mazzoni, V.; Rota-Stabelli, O.; et al. Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol. Entomol. 2015, 40, 54–64. [Google Scholar] [CrossRef]
- Cha, D.H.; Gill, M.A.; Epsky, N.D.; Werle, C.T.; Adamczyk, J.J.; Landolt, P.J. From a non-target to a target: Identification of a fermentation volatile blend attractive to Zaprionus indianus. J. Appl. Entomol. 2015, 139, 114–122. [Google Scholar] [CrossRef]
- Katsoyannos, B.I.; Papadopoulos, N.T.; Stavridis, D. Evaluation of trap types and food attractants for Rhagoletis cerasi (Diptera: Tephritidae). J. Econ. Entomol. 2000, 93, 1005–1010. [Google Scholar] [CrossRef]
- Biasazin, T.D.; Chernet, H.T.; Herrera, S.L.; Bengtsson, M.; Karlsson, M.F.; Lemmen-Lechelt, J.K.; Dekker, T. Detection of volatile constituents from food lures by Tephritid fruit flies. Insects 2018, 9, 119. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).