Abstract
Candida parapsilosis is the second most common cause of candidemia in some geographical areas and in children in particular. Yet, the proportion among children varies, for example, from 10.4% in Denmark to 24.7% in Tehran, Iran. As this species is also known to cause hospital outbreaks, we explored if the relatively high number of C. parapsilosis pediatric cases in Tehran could in part be explained by undiscovered clonal outbreaks. Among 56 C. parapsilosis complex isolates, 50 C. parapsilosis were genotyped by Amplified Fragment Length Polymorphism (AFLP) fingerprinting and microsatellite typing and analyzed for nucleotide polymorphisms by FKS1 and ERG11 sequencing. AFLP fingerprinting grouped Iranian isolates in two main clusters. Microsatellite typing separated the isolates into five clonal lineages, of which four were shared with Danish isolates, and with no correlation to the AFLP patterns. ERG11 and FKS1 sequencing revealed few polymorphisms in ERG11 leading to amino-acid substitutions (D133Y, Q250K, I302T, and R398I), with no influence on azole-susceptibilities. Collectively, this study demonstrated that there were no clonal outbreaks at the Iranian pediatric ward. Although possible transmission of a diverse C. parapsilosis community within the hospital cannot be ruled out, the study also emphasizes the necessity of applying appropriately discriminatory methods for outbreak investigation.
1. Introduction
In a recent study of the epidemiology of pediatric candidemia in Tehran, Iran, Candida parapsilosis complex species isolates accounted for 24.7% of the cases [1]. C. parapsilosis is the second most common species in children although with some geographical variation [2,3]. However, C. parapsilosis is also the dominating Candida species on skin and therefore associated with outbreaks [4,5,6]. Fluconazole-resistant C. parapsilosis isolates have been increasingly reported in several countries, including but not limited to South Africa [7], India [8], South Korea [9], Kuwait [10], Mexico [11], Italy [12], and Finland [13], and has been associated with poor outcome and clinical failure [6,14,15]. Outbreaks involving fluconazole-resistant C. parapsilosis include azole-naïve patients and limit the use of fluconazole as the first-line agent in developing countries [3,15,16]. Outbreaks may be due to either a common reservoir or horizontal transmission of the pathogen. A recent study from Brazil found isogenic fluconazole-resistant C. parapsilosis isolates from the hands of healthcare workers, inanimate surfaces, and patient bloodstream infections [15].
Typing of isolates from patients and hospital environmental screening is essential in outbreak investigations. Amplified Fragment Length Polymorphism (AFLP) fingerprinting and microsatellite typing (Short Tandem Repeat, STRs) have been used as the most common typing tools. A recent study suggested that the latter genotyping method provided a better resolution compared to AFLP fingerprinting [17]. Nonetheless, further assessment is warranted to substantiate this finding. In addition, sequencing of drug target genes (primarily ERG11), have previously contributed to delineate clonal outbreaks [6,11,18]. We thus sequenced drug target genes (FKS1 and ERG11) and performed genotyping of the Iranian isolates using AFLP fingerprinting and STR typing.
In comparisons with other genetic markers, AFLP have led to inconsistent results and lack of reproducibility [19]. As AFLP uses dominant markers, the information from heterozygosity is lost, and in studies of outbreaks using AFLP markers, the grouping is often based on “unweighted pair group method with arithmetic mean” (UPGMA) as the clustering algorithm, which is insufficient for analyses of population structure. The codominant STR markers allow the use of F-statistics and similar approaches to reveal population structure. Codominant markers can also be analyzed with powerful Bayesian clustering methods, such as STRUCTURE [20], but they often rely on assumptions, such as Hardy–Weinberg equilibrium. These assumptions are clearly violated with organisms that have clonal reproduction. C. parapsilosis has been considered an asexual organism [21]. Although evidence has been presented that it may also recombine sexually or parasexually [22], it remains uncertain to what extent mating and recombination occur. Population structure of potential clonal or partly clonal populations can be evaluated by discriminant analysis of principal components (DAPC). The advantage of this approach is that it is based on a prior principal component analysis (PCA) that transforms allele information to uncorrelated variables. This allows analyses of populations in linkage disequilibrium where the markers potentially could be correlated [23].
The purpose of this study was to determine if the high percentage of C. parapsilosis belong to a single clonal lineage and are therefore likely part of a hospital-associated outbreak. This was achieved by applying DACP to SSR data. Furthermore, the study compared genotyping by STR and AFLP markers to clarify which of the two typing methods was more appropriate [14].
2. Materials and Methods
Isolates. A set of 50 C. parapsilosis and six C. orthopsilosis isolates from 42 and five candidemic pediatric patients, respectively, hospitalized in Tehran during July 2014–December 2017 were included. The clinical data (Table S1) and antifungal susceptibility data for these isolates have been described previously [1,24]. Six isolates from the previous study [1] were not stored and omitted for this study. Consecutive isolates from the same species and patient recovered more than 30 days apart were considered as separate episodes occurring in that patient. In addition, 34 isolates from 33 Danish (DK) patients were included as comparators in the microsatellite typing.
Antifungal susceptibility testing was previously performed using the EUCAST E.Def 7.3 method as described in Mirhendi et al. [1].
AFLP fingerprint analysis. The genotypic diversity of isolates was investigated by AFLP as previously described [25]. AFLP data were analyzed by BioNumerics software v7.6 (Applied Math, Sint Martems-Latum, Belgium). The reference and type strains of C. parapsilosis (CBS 604, CBS 1818, CBS 1954, CBS 2195, and CBS 2917), C. metapsilosis (CBS 2315, CBS 2916, and CBS 10907), and C. orthopsilosis (CBS 10906) were included.
Microsatellite typing. Six short tandem repeat (STR) markers were used for microsatellite typing of C. parapsilosis sensu stricto as previously described [26]. Two multiplex PCR reactions were run to amplify the trinucleotide (3A, 3B, and 3C) and hexanucleotide markers (6A, 6B, and 6C), respectively. Forward amplification primers were 5′ labelled with FAM, HEX, and TAMRA as described [26], and all primers were acquired from TAG Copenhagen A/S, Copenhagen, Denmark. Following modifications were applied. PCR was run in 25-µL reaction volumes with 2 µL genomic DNA, 0.4-µM primer concentrations, and 1 × Extract-N-Amp™ PCR ReadyMix (from Sigma (now Merck), Søborg, Denmark). PCR amplification was run with 35 cycles and TM at 54 °C. Amplicons were diluted 35× with distilled H2O and 1 µL diluted amplicons mixed with 12.2 µL distilled H2O and 0.8 µL GeneScan™ 500 ROX™ size standard (Thermo Fischer Scientific, Roskilde, Denmark). DNA fragments were denatured at 95 °C for 1 min, followed by rapid cooling on ice before injection in the genetic analyzer ABI3500xL Genetic Analyzer (Thermo Fischer Scientific (Applied Biosystems), Nærum, Denmark). For all six markers, at least three samples with different sizes (except 6B, which only covered two fragment sizes) were amplified with unlabeled forward primers and subjected to Sanger sequencing (Macrogen, Amsterdam, Holland) in order to correlate fragment size to repeat numbers.
ERG11 and FKS1 sequencing of the C. parapsilosis complex isolates were done as previously described [17].
Statistical analysis. The STR dataset was analyzed using the package poppr v. 2.9.3 [27] in R v. 4.0.5 (R Core Team, Vienna, Austria). A genotype accumulation curve was plotted to ensure that the number of loci captured the variation present in the two populations. The mlg.filter function was activated to determine the true number of multi-locus genotypes (MLGs) by using a genetic distance threshold determined using the cutoff predictor tool, which finds a gap in the distance distribution. The analyses carried out in poppr included a summary of diversity measures, Bruvo’s genetic distance [28], and minimum spanning tree based on the distance matric.
The index of association (IA) was calculated in poppr to test for linkage disequilibrium based on a clone-corrected dataset. The modification of IA that removes the bias of sample size d [29] was also calculated.
F-statistics were calculated using the GenAlEx. Overall FST, FIS, and Ht (gene diversity) were calculated as well as population differentiation RST, which is based on a stepwise mutation model [30]. The significance of the RST structure between populations was tested using 1000 permutations.
DAPC was run using the adegenet package 2.1.15 (updated 2020) [23]) in R. DAPC analyses were only conducted with de novo grouping, as a priori groupings based on insignificant RST values between the two geographical samplings made little sense. Instead, the find.clusters() function was used to determine the number of groups (K) de novo, with optimal K selected as that with the lowest BIC value. The optimal number of PCs to use in the DAPC was determined using the optim.a.score() and xvalDapc() commands and 1000 replicates. Root mean squared error by number of PC of PCA s (RMSE) was chosen as the most important criterion for selecting the number of PC’s.
Ethical considerations. This study was reviewed by ethical committee members of Tehran University of Medical Sciences and granted with the approval ethical code (IR NIMAD REC 1396 245). Written consent forms were obtained from patients involved in the candidemia project, and patients were assigned with numerical codes for anonymity purposes.
3. Results
A total of 50 C. parapsilosis and 6 C. orthopsilosis isolates from 47 candidemic pediatric patients were included. The median age was 10.5 months (range 2 days to 10 years), and predisposing factors included exposure to vancomycin and third-generation cephalosporins, N = 47 (100%); admission at intensive care unit (ICU), N = 34 (74%); central venous catheter (CVC), N = 41 (89%); total parental nutrition (TPN), N = 26 (57%); and underlying conditions, such as prematurity, N = 12 (26%); cancer, N = 8 (17%); and metabolic disease, N = 5 (11%) (Table S1). All five patients with C. orthopsilosis infections survived, while 46% (19/41) of C. parapsilosis infected patients succumbed (p = 0.067).
AFLP fingerprint was first performed and provided two clusters of C. parapsilosis (Table 1 and Figure S1): G1-P covered 44 isolates from 36 patients, and G2-P covered 6 isolates from five patients. Likewise, two clusters were defined for C. orthopsilosis: G1-Orth and G2-Orth covering three isolates each from three and two patients, respectively. Four patients (IR-Pt-2, IR-Pt-29, IR-Pt-32, and IR-Pt-39) had two or more isolates that belonged to the same AFLP groups (G1-P, G2-P, G1-P, and G1-Orth, respectively). Eight reference strains were included in the AFLP fingerprint testing, including seven C. parapsilosis and one C. orthopsilosis. Of these, three C. parapsilosis (CBS 1818, CBS 1954, and CBS 604) belonged to G1-P, four C. parapsilosis (CBS 2197, CBS 2915, CBS 2194, and CBS 2196) belonged to G2-P, and finally, one C. orthopsilosis (CBS 10906) differed from the included Iranian C. orthopsilosis isolates.

Table 1.
Overview of the number and origin of C. parapsilosis complex isolates in each AFLP fingerprint cluster (further details are provided in Supplementary Figure S1).
Microsatellite typing revealed 29 unique genotypes (MLGs) among the Danish isolates of C. parapsilosis and 41 (MLGs) among the Iranian isolates (Figure 1 and Table S2). A genotype accumulation (saturation) curve of MLGs showed sufficient STR markers (six loci) had been included (Figure S2). The calculated D value according to Simpson’s index of diversity was 0.992. No apparent correlation was observed between AFLP fingerprinting and microsatellite typing.
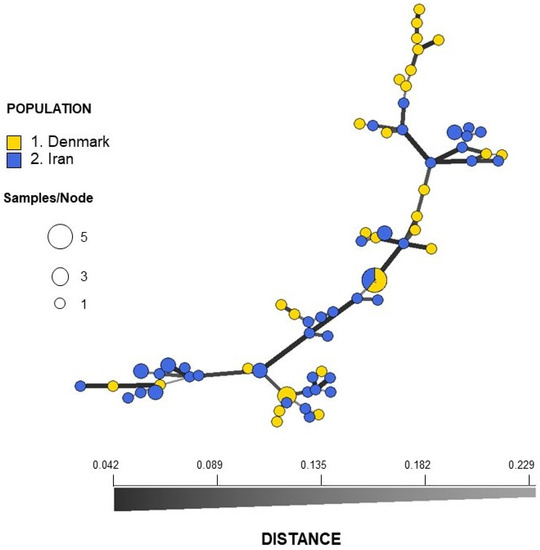
Figure 1.
Minimum spanning network (MSNs) inferred using STRs from Danish (blue) and Iranian (yellow) isolates of Candida parapsilosis. The network is based on Bruvo’s distance matrix. Each node represents a multi-locus genotype (MLG), with variable size depending on the number of individuals within that MLG. The distance between the nodes represents the genetic distance between MLGs.
Ten isolates were in three clusters each, consisting of 2–5 repeat isolates from the same patient (Figure S3). Twelve isolates were in six clusters (IR-C1 to IR-C6) (16.2%), each consisting of two isolates from different patients. Thus, 12 out of 43 isolates from different patients were in clusters (27.9%). Of note, one Iranian cluster involved patients hospitalized in the same ward and during the same time period. In comparison, the 34 Danish C. parapsilosis isolates represented 29 unique genotypes, of which three were clusters: one with two isolates from the same patient and two (6.9%) consisting of three isolates from different patients. Neither the number of clusters among unique genotypes nor the number of isolates (from different patients) in clusters differed between Danish and Iranian isolates (p = 0.45 and p = 0.42, respectively).
Analyses of STR data revealed no population differentiation between the Danish and the Iranian isolates (RST = 0.011, p = 0.14), and analysis of molecular variance (AMOVA) showed 99% variation within populations and only 1% among populations (Table 2). The lack of differentiation was also illustrated in the minimum spanning network (MSN) based on Bruvo’s distance, where no structure that could separate the two samplings (DK and Iran) was seen (Figure 1). The network showed no reticulations indicating lack of recombination. This was also revealed by index of association IA and the unbiased index of association d, which gave values significantly different from what is expected of a freely recombining population (p < 0.001) (Figure S4).

Table 2.
Summary AMOVA table. Probability, P(rand ≥ data), for RST is based on standard permutation across the full data set. Pops, populations; dF, degrees of freedom; SS, sum of square; MS, mean squares; Est. Var., estimated variance. p-value shows no genetic differentiation between the Danish and Iranian isolates. Furthermore, 99% of the variation lies within the two populations.
The Danish and the Iranian isolates had similar genetic diversities. Bruvo’s distance was 0.3898 for the Danish population and 0.3706 for the Iranian. Both values differed significantly from randomized data, again supporting that clonal reproduction is dominating.
The find.clusters in adegenets DAPC revealed K = 5 without a priori groupings, and eight principle components (PCs) were retained for analysis based on the lowest root mean squared error (RMSE). The scatter plot assigned all individuals to the five clusters with high certainty (Figure 2). Most isolates were found in two clusters: cluster 2 and cluster 5, which contained both Danish and Iranian isolates. One cluster (cluster 3) was only found in Iran (Figure 3).
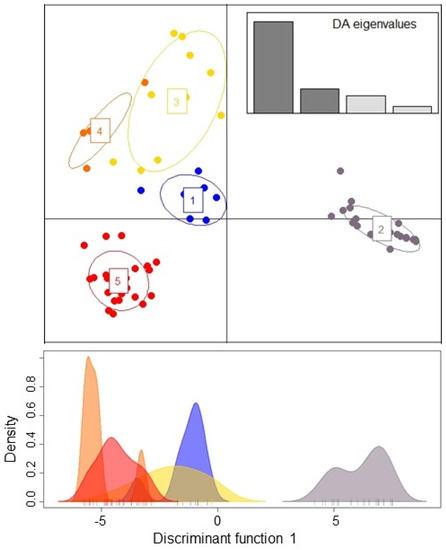
Figure 2.
DAPC of Danish and Iranian individuals of Candida parapsilosis based on STR data. Scatter plot represents the distribution of individuals (dots) among the five clusters (top). A single discriminant function plotted along the x-axis (bottom).
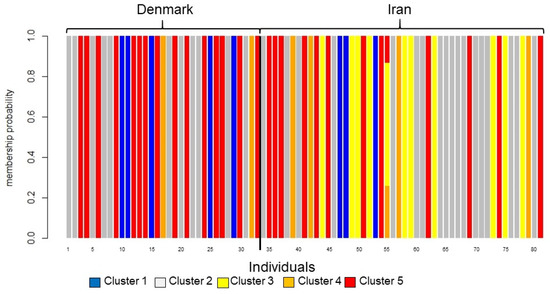
Figure 3.
Graphical translation of membership probabilities to the five clusters. The colors of the bars correspond to the clusters in Figure 1. The vertical axis gives membership probability from 0 to 1. All isolates except isolate 55 are assigned with 100% certainty. The black vertical line separates Danish and Iranian isolates. The clusters are almost evenly distributed between Denmark and Iran except cluster 3 (yellow), which is only found in Iran.
All isolates were susceptible to azoles, echinocandins, and amphotericin B and were FKS1 (hot-spot) wild-type (Table S3). Thirty-eight isolates were ERG11 wild-type, and twelve isolates (24%) had one or two homozygous mutations leading to the following amino acid changes: D133Y, Q250K, I302T, and R398I. All six C. orthopsilosis isolates were azole and echinocandin susceptible and displayed wild-type ERG11 and FKS1 (hot-spots) sequences (Table S3).
4. Discussion
Previous studies have reported a relatively high incidence of C. parapsilosis infections in the children’s ICU at Teheran University of Medical Sciences [1,24]. Since this opportunistic pathogen is known to cause hospital-associated outbreaks [31], this study applied genotyping to determine the extent of clonality among the isolates and help to clarify the possible occurrence of outbreaks in an Iranian pediatric setting. Indeed, AFLP fingerprinting initially suggested two potential clonal clusters, namely G1-P and G2-P, which could have prompted presumptive conclusions possibly due to poor infection control and a critical nosocomial clonal spread.
The results of the microsatellite analyses showed that the Iranian outbreak, which was considered as two clones when assayed with AFLP markers, consisted of five clusters. This can be explained by the low resolution of the current format of the AFLP that introduce null-alleles resulting in binary data. Moreover, the mutation model behind AFLP markers is based on frequency of mutations in restriction sites, whereas microsatellites have a much higher mutation rate, and a stepwise mutation model that allows inclusion of repeat length. This conclusion was supported by a previous study [14], which found an insufficient resolution by AFLP and suggested that STR should be the preferred method for genetic analysis to uncover outbreaks. Nevertheless, it remains to be understood if inclusion of additional primers targeting more restriction recognition sites and/or use an electrophoretic system capable of providing higher-resolution gel pictures would improve the performance of AFLP.
Four out of five clusters were shared by Denmark and Iran. This is in agreement with other studies showing no geographic differentiation of C. parapsilosis between France and Uruguay [32] and illustrates the dispersal potential of the fungus. One cluster was only found in Iran, indicating that new clusters may still occur locally.
The clonal nature of the pathogen is obvious from the analyses of IA and d. Though the DACP analysis cannot distinguish clonal population structures from recombining, the minor overlap between clusters and little uncertainty in cluster assignment indicate clonality. The clonal diploid reproduction of the fungus will tend to increase the genetic differentiation among populations compared to the parent population [21,33], as known from parasite populations. This does not mean that the fungus is not able to undergo recombination. The five clusters may well represent the outcome of genomic recombination, which have also been shown by genomic studies where the genomes of C. parapsilosis strains showed signs of recombination events [22].
We previously documented that an amino acid substitution in the HS1 of Fks1 was found in an echinocandin-susceptible C. glabrata isolate [34] and later identified fluconazole-susceptible C. parapsilosis isolate harboring Y132F in Erg11 (unpublished data). Therefore, despite the lack of fluconazole and echinocandin resistance in the current study, sequencing of ERG11 and FKS1 was included in order to further delineate molecular correlation between the isolates and to map the genes in susceptible strains. FKS1 profiles were highly conserved, while ERG11 showed common variants in more than one-sixth of the isolates, which did not influence azole susceptibilities [1]. Furthermore, these variations (instead of a single variant) did not support the hypothesis of a clonal outbreak as opposed to the previously described Brazilian outbreak with a fluconazole-resistant Erg11 Y132F mutant strain [31]. Microsatellite typing was introduced, and in contrast to the AFLP data, the results showed a high genotypic diversity among Iranian isolates. More clusters were found in the Iranian population compared to the Danish, but this difference did not reach statistical significance. Thus, no clear evidence for a clonal outbreak was found as cause of the high C. parapsilosis proportion in the Iranian study population compared to pediatric candidemia in Denmark. Different levels of (hand) hygiene could be significant for the incidence of C. parapsilosis infections, and nosocomial infections remain a threat but not necessarily with the same strain. Additional sampling of other patients, hospital workers, and units combined with genotyping of C. parapsilosis positive samples could have aided a more detailed evaluation of potential transmission routes. Still, the source of candidemia is primarily regarded as a patient’s own microbiota underlining the key role of hygiene to avoid infection. Moreover, geographical differences in the Candida species distribution of the normal colonizing mycobiota are poorly investigated. Therefore, it remains unknown whether the difference in the C. parapsilosis proportion in Iranian and Danish pediatric candidemia is due to differences in colonization of healthy people or due to differences in infection control practices.
This study is associated with limitations. As the study was based on a single hospital, the data may not be fully representative of C. parapsilosis blood stream infections in the Iranian pediatric population. Moreover, the number of isolates in both groups was limited. The Danish isolates were from the entire country and thus included several hospitals and patients without any likely risk of transmission. Nevertheless, we found that two major clusters were shared between Denmark and Iran. It is likely that if whole genome sequence-based analysis had been adopted, some of these apparent clusters might consist of unique isolates although this would not have altered the main conclusions of this study.
In conclusion, our study rejected the hypothesis of an outbreak at the Teheran pediatric ICU department caused by two clones and underlined that AFLP fingerprinting alone can potentially lead to inaccurate conclusions of clonal outbreaks and should always be supported by other more discriminatory methods.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof8020183/s1, Table S1, Clinical data of included patients. Table S2, Microsatellite data. Table S3, Antifungal susceptibility data and Fks1 and Erg11 profiles. Figure S1, AFLP fingerprint data. Figure S2, Genotype accumulation curve. Figure S3. Minimum spanning tree. Figure S4, Test for linkage disequilibrium.
Author Contributions
Conceptualization, H.M., A.A. and R.K.H.; data curation, A.A., F.D., H.M., A.C., H.E. and R.K.H.; formal analysis, A.A., F.D., S.R. and R.K.H.; funding acquisition, H.M.; investigation, A.A., F.D., H.M., A.C., H.E. and R.K.H.; methodology, A.A., F.D. and R.K.H.; project administration, H.M., A.C. and R.K.H.; resources, H.M.; software, S.R. and R.K.H.; supervision, A.A., T.B., H.M. and M.C.A.; validation, A.A. and F.D.; visualization, F.H., S.R. and R.K.H.; writing—original draft preparation, A.A., R.K.H. and M.C.A.; writing—review and editing, R.K.H., A.A., S.R., A.C., F.D., H.E., H.M., T.B., F.H. and M.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 642095; Iranian National Institute for Medical Research Development (NIMAD), Grant No. 958686.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the study was reviewed by ethical committee members of Tehran University of Medical Sciences and granted with the approval ethical code (IR NIMAD REC 1396 245).
Informed Consent Statement
Written consent forms were obtained from patients involved in the candidemia project, and patients were assigned with numerical codes for anonymity purposes.
Data Availability Statement
Deposition of isolates and sequence data. Six C. orthopsilosis isolates obtained from this study were deposited in the Westerdijk Fungal Biodiversity Institute culture collection, Utrecht, the Netherlands, with the accession numbers CBS 15856–15861. Sequences obtained for ERG11 (MK521929–MK521979) and HS1 and HS2 of FKS1 (MK522274–MK522386) were deposited in NCBI GenBank.
Acknowledgments
The entire Statens Serum Institute mycology laboratory and Nissrine Abou-Chakra specifically are acknowledged for their involvement in Candida parapsilosis microsatellite typing.
Conflicts of Interest
All authors declare no conflicts of interest in relation to this study. R.K.H. has over the past 5 years received an unrestricted research grant and a travel grant from Gilead. M.C.A. has, over the past 5 years, received research grants/contract work (paid to the SSI) from Amplyx, Basilea, Cidara, F2G, Gilead, Novabiotics, and Scynexis, and speaker honoraria (personal fee) from Astellas, Chiesi, Gilead, MSD, and SEGES. She is the current chairman of the EUCAST-AFST.
References
- Mirhendi, H.; Charsizadeh, A.; Eshaghi, H.; Nikmanesh, B.; Arendrup, M.C. Species distribution and antifungal susceptibility profile of Candida isolates from blood and other normally sterile foci from pediatric ICU patients in Tehran, Iran. Med. Mycol. 2019, 58, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Pana, Z.-D.; Oletto, A.; Lundin, R.; Castagnola, E.; Lehrnbecher, T.; Groll, A.H.; Roilides, E. Etiology and Outcome of Candidemia in Neonates and Children in Europe. Pediatr. Infect. Dis. J. 2020, 39, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Roilides, E.; Berman, D.; Hoffman, J.A.; Groll, A.H.; Bin-Hussain, I.; Palazzi, D.L.; Castagnola, E.; Halasa, N.; Velegraki, A.; et al. Results From a Prospective, International, Epidemiologic Study of Invasive Candidiasis in Children and Neonates. Pediatr. Infect. Dis. J. 2012, 31, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Pinhati, H.M.S.; Casulari, L.A.; Souza, A.C.R.; Siqueira, R.A.; Damasceno, C.M.G.; Colombo, A.L. Outbreak of candidemia caused by fluconazole resistant Candida parapsilosis strains in an intensive care unit. BMC Infect. Dis. 2016, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Castro, R.; Arroyo-Escalante, S.; Carrillo-Casas, E.M.; Moncada-Barrón, D.; Álvarez-Verona, E.; Hernández-Delgado, L.; Torres-Narváez, P.; Lavalle-Villalobos, A. Outbreak of Candida parapsilosis in a neonatal intensive care unit: A health care workers source. Eur. J. Pediatr. 2010, 169, 783–787. [Google Scholar] [CrossRef]
- Arastehfar, A.; Hilmioğlu-Polat, S.; Daneshnia, F.; Pan, W.; Hafez, A.; Fang, W.; Liao, W.; Şahbudak-Bal, Z.; Metin, D.Y.; De Almeida, J.N., Jr.; et al. Clonal Candidemia Outbreak by Candida parapsilosis Carrying Y132F in Turkey: Evolution of a Persisting Challenge. Front. Cell Infect. Microbiol. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Govender, N.P.; Patel, J.; Magobo, R.E.; Naicker, S.; Wadula, J.; Whitelaw, A.; Coovadia, Y.; Kularatne, R.; Govind, C.; Lockhart, S.R.; et al. Emergence of azole-resistant Candida parapsilosis causing bloodstream infection: Results from laboratory-based sentinel surveillance in South Africa. J. Antimicrob. Chemother. 2016, 71, 1994–2004. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.K.; de Groot, T.; Kumar, A.; Mathur, P.; Tarai, B.; Sachdeva, N.; Upadhyaya, G.; Sarma, S.; Meis, J.F.; et al. Emergence of clonal fluconazole-resistant Candida parapsilosis clinical isolates in a multicentre laboratory-based surveillance study in India. J. Antimicrob. Chemother. 2019, 74, 1260–1268. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, Y.-J.; Yong, D.; Byun, J.; Kim, T.S.; Chang, Y.S.; Choi, M.J.; Byeon, S.A.; Won, E.J.; Kim, S.H.; et al. Fluconazole-Resistant Candida parapsilosis Bloodstream Isolates with Y132F Mutation in ERG11 Gene, South Korea. Emerg. Infect. Dis. 2018, 24, 1768–1770. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Ahmad, S.; Al-Sweih, N.; Khan, Z. Epidemiology and Molecular Basis of Resistance to Fluconazole Among Clinical Candida parapsilosis Isolates in Kuwait. Microb. Drug Resist. 2017, 23, 966–972. [Google Scholar] [CrossRef]
- Corzo-Leon, D.E.; Peacock, M.; Rodriguez-Zulueta, P.; Salazar-Tamayo, G.J.; MacCallum, D.M. General hospital outbreak of invasive candidiasis due to azole-resistant Candida parapsilosis associated with an Erg11 Y132F mutation. Med. Mycol. 2021, 59, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Deshpande, L.M.; Messer, S.A.; Rhomberg, P.R.; Pfaller, M.A. Analysis of global antifungal surveillance results reveals predominance of Erg11 Y132F alteration among azole-resistant Candida parapsilosis and Candida tropicalis and country-specific isolate dissemination. Int. J. Antimicrob. Agents 2020, 55, 105799. [Google Scholar] [CrossRef] [PubMed]
- Sarvikivi, E.; Lyytikäinen, O.; Soll, D.R.; Pujol, C.; Pfaller, M.A.; Richardson, M.; Koukila-Kähkölä, P.; Luukkainen, P.; Saxén, H. Emergence of Fluconazole Resistance in a Candida parapsilosis Strain That Caused Infections in a Neonatal Intensive Care Unit. J. Clin. Microbiol. 2005, 43, 2729–2735. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Hilmioğlu-Polat, S.; Fang, W.; Yaşar, M.; Polat, F.; Metin, D.Y.; Rigole, P.; Coenye, T.; Ilkit, M.; et al. First Report of Candidemia Clonal Outbreak Caused by Emerging Fluconazole-Resistant Candida parapsilosis Isolates Harboring Y132F and/or Y132F+K143R in Turkey. Antimicrob. Agents Chemother. 2020, 64, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, D.Y.; de Almeida, J.N.; Sejas, O.N.E.; Del Negro, G.M.B.; Carvalho, G.O.M.H.; Gimenes, V.M.F.; de Souza, M.; Arastehfar, A.; Camargo, C.; Motta, A.; et al. Environmental Clonal Spread of Azole-Resistant Candida parapsilosis with Erg11-Y132F Mutation Causing a Large Candidemia Outbreak in a Brazilian Cancer Referral Center. J. Fungi 2021, 7, 259. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sood, P.; Rudramurthy, S.M.; Chen, S.; Kaur, H.; Capoor, M.; Chhina, D.; Rao, R.; Eshwara, V.K.; Xess, I.; et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015, 41, 285–295. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Najafzadeh, M.J.; Hagen, F.; Mahmoudi, S.; Salehi, M.; Zarrinfar, H.; Namvar, Z.; Zareshahrabadi, Z.; Khodavaisy, S.; et al. Evaluation of Molecular Epidemiology, Clinical Characteristics, Antifungal Susceptibility Profiles, and Molecular Mechanisms of Antifungal Resistance of Iranian Candida parapsilosis Species Complex Blood Isolates. Front. Cell Infect. Microbiol. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Magobo, R.E.; Lockhart, S.R.; Govender, N.P.; Wadula, J.; Rensburg, V.; van Rensburg, C.J.; Whitelaw, A.; Zietsman, I.; Miller, N.; Smith, P.; et al. Fluconazole-resistant Candida parapsilosis strains with a Y132F substitution in the ERG11 gene causing invasive infections in a neonatal unit, South Africa. Mycoses 2020, 63, 471–477. [Google Scholar] [CrossRef]
- Vatanshenassan, M.; Boekhout, T.; Mauder, N.; Robert, V.; Maier, T.; Meis, J.F.; Berman, J.; Then, E.; Kostrzewa, M.; Hagen, F. Evaluation of microsatellite typing, ITS sequencing, AFLP fingerprinting, MALDI-TOF MS, and Fourier-Transform Infrared Spectroscopy Analysis of Candida auris. J. Fungi 2020, 6, 146. [Google Scholar] [CrossRef]
- Hubisz, M.J.; Falush, D.; Stephens, M.; Pritchard, J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009, 9, 1322–1332. [Google Scholar] [CrossRef]
- Butler, G.; Rasmussen, M.D.; Lin, M.F.; Santos, M.A.S.; Sakthikumar, S.; Munro, C.A.; Rheinbay, E.; Grabherr, M.; Forche, A.; Reedy, J.L.; et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 2009, 459, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Pryszcz, L.P.; Németh, T.; Gácser, A.; Gabaldón, T. Unexpected genomic variability in clinical and environmental strains of the pathogenic yeast Candida parapsilosis. Genome Biol. Evol. 2013, 5, 2382–2392. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T.; Bateman, A. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Charsizadeh, A.; Mirhendi, H.; Nikmanesh, B.; Eshaghi, H.; Makimura, K. Microbial epidemiology of candidaemia in neonatal and paediatric intensive care units at the Children’s Medical Center, Tehran. Mycoses 2018, 61, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Khodavaisy, S.; Daneshnia, F.; Najafzadeh, M.-J.; Mahmoudi, S.; Charsizadeh, A.; Salehi, M.-R.; Zarrinfar, H.; Raeisabadi, A.; Dolatabadi, S.; et al. Molecular Identification, Genotypic Diversity, Antifungal Susceptibility, and Clinical Outcomes of Infections Caused by Clinically Underrated Yeasts, Candida orthopsilosis, and Candida metapsilosis: An Iranian Multicenter Study (2014–2019). Front. Cell. Infect. Microbiol. 2019, 9. [Google Scholar] [CrossRef]
- Diab-Elschahawi, M.; Forstner, C.; Hagen, F.; Meis, J.F.; Lassnig, A.M.; Presterl, E.; Klaassen, C.H.W. Microsatellite genotyping clarified conspicuous accumulation of Candida parapsilosis at a cardio-thoracic surgery intensive care unit. J. Clin. Microbiol. 2012, 50, 3422–3426. [Google Scholar] [CrossRef][Green Version]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Bruvo, R.; Michiels, N.K.; D’Souza, T.G.; Schulenburg, H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol. Ecol. 2004, 13, 2101–2106. [Google Scholar] [CrossRef]
- Agapow, P.-M.; Burt, A. Indices of multilocus linkage disequilibrium. Mol. Ecol. Notes 2001, 1, 101–102. [Google Scholar] [CrossRef]
- Slatkin, M. A measure of population subdivision based on microsatellite allele frequencies. Genetics 1995, 139, 457–462. [Google Scholar] [CrossRef]
- Fekkar, A.; Blaize, M.; Bouglé, A.; Normand, A.-C.; Raoelina, A.; Kornblum, D.; Kamus, L.; Piarroux, R.; Imbert, S. Hospital Outbreak of Fluconazole-Resistant Candida parapsilosis: Arguments for Clonal Transmission and Long-Term Persistence. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Desnos-Ollivier, M.; Bórmida, V.; Poirier, P.; Nourrisson, C.; Pan, D.; Bretagne, S.; Puime, A.; Dromer, F.; Uruguayan Invasive Fungal Infection Network. Population Structure of Candida parapsilosis: No Genetic Difference Between French and Uruguayan Isolates Using Microsatellite Length Polymorphism. Mycopathologia 2018, 183, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Prugnolle, F.; De Meeûs, T. The impact of clonality on parasite population genetic structure. Parasite 2008, 15, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Daneshnia, F.; Salehi, M.; Yaşar, M.; Hoşbul, T.; Ilkit, M.; Pan, W.; Hagen, F.; Arslan, N.; Türk-Dağı, H.; et al. Low level of antifungal resistance of Candida glabrata blood isolates in Turkey: Fluconazole minimum inhibitory concentration and FKS mutations can predict therapeutic failure. Mycoses 2020, 63, 911–920. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).