Arbuscular Mycorrhizal Fungi Are an Influential Factor in Improving the Phytoremediation of Arsenic, Cadmium, Lead, and Chromium
Abstract
1. Introduction
1.1. Metal(loid)s Pollution and Phytoremediation
1.2. Arbuscular Mycorrhizal Fungi (AMF)
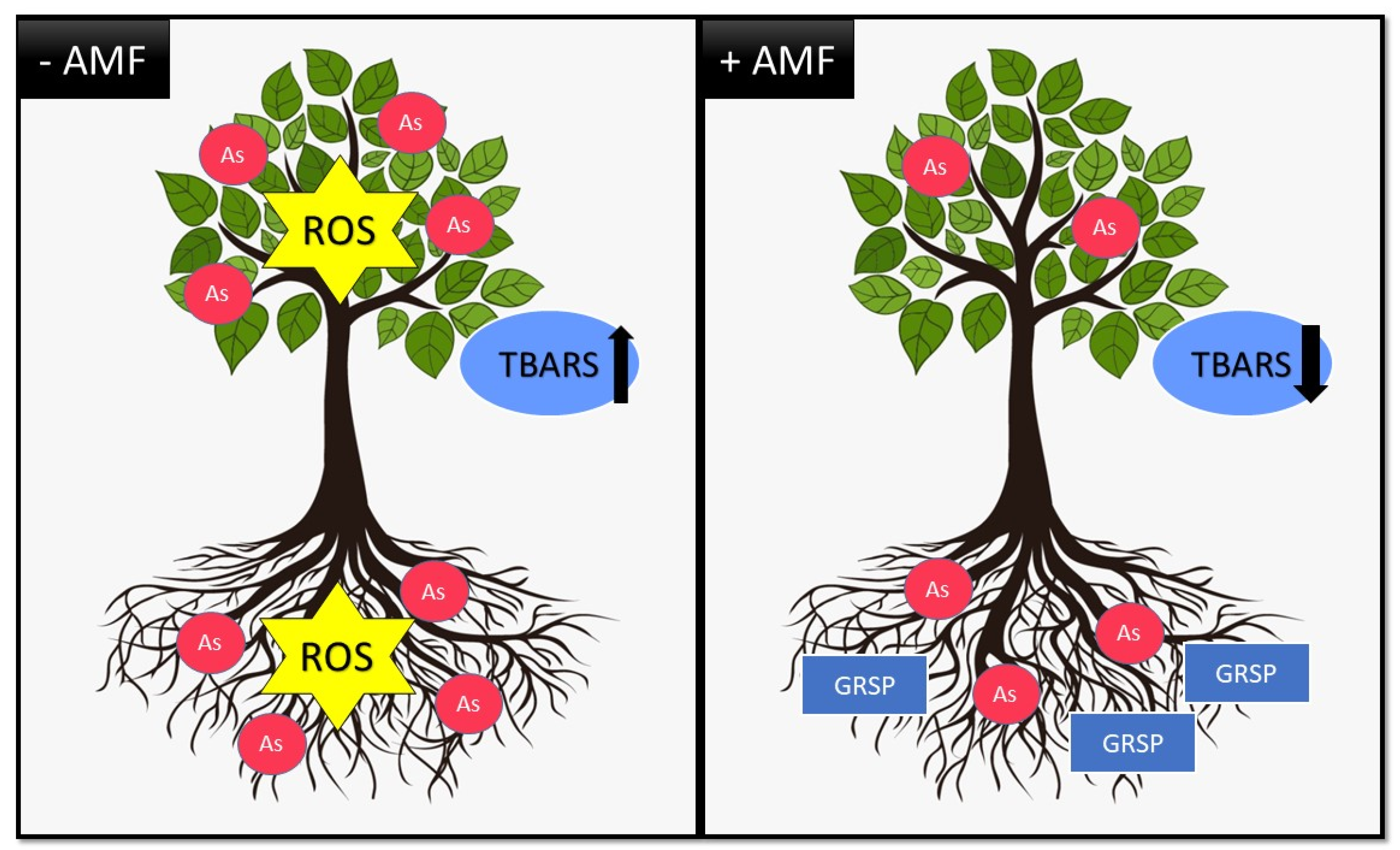
1.3. Arsenic (As)
1.4. Cadmium (Cd)

1.5. Lead (Pb)
1.6. Chromium (Cr)
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McLaughlin, M.J.; Hamon, R.; McLaren, R.; Speir, T.; Rogers, S. A bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand. Soil Res. 2000, 38, 1037–1086. [Google Scholar] [CrossRef]
- Swartjes, F.A. Dealing with Contaminated Sites: From Theory towards Practical Application; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.; Hijri, M.; St-Arnaud, M. Effect of arbuscular mycorrhizal fungi on trace metal uptake by sunflower plants grown on cadmium contaminated soil. New Biotechnol. 2013, 30, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-H.; Zhuo, F.; Jing, S.-H.; Li, X.; Yan, T.-X.; Lei, L.-L.; Lu, R.-R.; Zhang, X.-F.; Jing, Y.-X. Combined application of arbuscular mycorrhizal fungi and steel slag improves plant growth and reduces Cd, Pb accumulation in Zea mays. Int. J. Phytoremediat. 2019, 21, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Zhou, Q.-X.; Wei, S.-H.; Zhang, W.; Cao, L.; Ren, L.-P. Effects of exogenous chelators on phytoavailability and toxicity of Pb in Zinnia elegans Jacq. J. Hazard. Mater. 2007, 146, 341–346. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, İ.; Ünay, A.; Abdel-Daim, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Lourdes, G.-C.M.; Stéphane, D.; Maryline, C.-S. Impact of increasing chromium (VI) concentrations on growth, phosphorus and chromium uptake of maize plants associated to the mycorrhizal fungus Rhizophagus irregularis MUCL 41833. Heliyon 2021, 7, e05891. [Google Scholar] [CrossRef]
- Ogar, A.; Sobczyk, Ł.; Turnau, K. Effect of combined microbes on plant tolerance to Zn–Pb contaminations. Environ. Sci. Pollut. Res. 2015, 22, 19142–19156. [Google Scholar] [CrossRef]
- Li, Y.; Becquer, T.; Quantin, C.; Benedetti, M.; Lavelle, P.; Dai, J. Effects of heavy metals on microbial biomass and activity in subtropical paddy soil contaminated by acid mine drainage. Acta Ecol. Sin. 2004, 24, 2430–2436. [Google Scholar]
- Sharpley, A.N.; Weld, J.L.; Beegle, D.B.; Kleinman, P.J.; Gburek, W.; Moore, P.; Mullins, G. Development of phosphorus indices for nutrient management planning strategies in the United States. J. Soil Water Conserv. 2003, 58, 137–152. [Google Scholar]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.; Kim, C.-G.; Subramanian, P.; Kim, K.-Y.; Selvakumar, G.; Sa, T.-M. Arbuscular mycorrhizal fungi community structure, abundance and species richness changes in soil by different levels of heavy metal and metalloid concentration. PLoS ONE 2015, 10, e0128784. [Google Scholar] [CrossRef] [PubMed]
- Jadia, C.D.; Fulekar, M. Phytoremediation of heavy metals: Recent techniques. Afr. J. Biotechnol. 2009, 8, 921–928. [Google Scholar]
- Gjorgieva Ackova, D. Heavy metals and their general toxicity on plants. Plant Sci. Today 2018, 5, 15–19. [Google Scholar] [CrossRef]
- Chang, Q.; Diao, F.-W.; Wang, Q.-F.; Pan, L.; Dang, Z.-H.; Guo, W. Effects of arbuscular mycorrhizal symbiosis on growth, nutrient and metal uptake by maize seedlings (Zea mays L.) grown in soils spiked with Lanthanum and Cadmium. Environ. Pollut. 2018, 241, 607–615. [Google Scholar] [CrossRef]
- Basta, N.; McGowen, S. Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ. Pollut. 2004, 127, 73–82. [Google Scholar] [CrossRef]
- Navarro, C.; Díaz, M.; Villa-García, M.A. Physico-chemical characterization of steel slag. Study of its behavior under simulated environmental conditions. Environ. Sci. Technol. 2010, 44, 5383–5388. [Google Scholar] [CrossRef]
- Khan, Z.; Doty, S. Endophyte-assisted phytoremediation. Plant Biol. 2011, 12, 97–105. [Google Scholar]
- Xu, P.; Chen, M.; Zeng, G.; Huang, D.; Lai, C.; Wang, Z.; Yan, M.; Huang, Z.; Gong, X.; Song, B. Effects of multi-walled carbon nanotubes on metal transformation and natural organic matters in riverine sediment. J. Hazard. Mater. 2019, 374, 459–468. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, W.; Yan, J.; Han, L.; Gao, W.; Liu, R.; Chen, M. Effective removal of heavy metal by biochar colloids under different pyrolysis temperatures. Bioresour. Technol. 2016, 206, 217–224. [Google Scholar] [CrossRef]
- Kumar, A.; Tsechansky, L.; Lew, B.; Raveh, E.; Frenkel, O.; Graber, E.R. Biochar alleviates phytotoxicity in Ficus elastica grown in Zn-contaminated soil. Sci. Total Environ. 2018, 618, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, F.; Zhang, X.-F.; Lei, L.-L.; Yan, T.-X.; Lu, R.-R.; Hu, Z.-H.; Jing, Y.-X. The effect of arbuscular mycorrhizal fungi and biochar on the growth and Cd/Pb accumulation in Zea mays. Int. J. Phytoremediat. 2020, 22, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef]
- Vejvodová, K.; Száková, J.; García-Sánchez, M.; Praus, L.; Romera, I.G.; Tlustoš, P. Effect of dry olive residue–based biochar and arbuscular mycorrhizal fungi inoculation on the nutrient status and trace element contents in wheat grown in the As-, Cd-, Pb-, and Zn-contaminated soils. J. Soil Sci. Plant Nutr. 2020, 20, 1067–1079. [Google Scholar] [CrossRef]
- Mahar, A.; Ping, W.; Ronghua, L.; Zhang, Z. Immobilization of lead and cadmium in contaminated soil using amendments: A review. Pedosphere 2015, 25, 555–568. [Google Scholar] [CrossRef]
- Deng, L.; Li, Z.; Wang, J.; Liu, H.; Li, N.; Wu, L.; Hu, P.; Luo, Y.; Christie, P. Long-term field phytoextraction of zinc/cadmium contaminated soil by Sedum plumbizincicola under different agronomic strategies. Int. J. Phytoremediat. 2016, 18, 134–140. [Google Scholar] [CrossRef]
- Dos Santos, J.V.; Varón-López, M.; Soares, C.R.F.S.; Leal, P.L.; Siqueira, J.O.; de Souza Moreira, F.M. Biological attributes of rehabilitated soils contaminated with heavy metals. Environ. Sci. Pollut. Res. 2016, 23, 6735–6748. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Mosca, G. Field crops for phytoremediation of metal-contaminated land. A review. Environ. Chem. Lett. 2010, 8, 1–17. [Google Scholar] [CrossRef]
- Malaviya, P.; Singh, A.; Anderson, T.A. Aquatic phytoremediation strategies for chromium removal. Rev. Environ. Sci. Bio/Technol. 2020, 19, 897–944. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Yu, X.; Kang, X.; Li, Y.; Cui, Y.; Tu, W.; Shen, T.; Yan, M.; Gu, Y.; Zou, L.; Ma, M. Rhizobia population was favoured during in situ phytoremediation of vanadium-titanium magnetite mine tailings dam using Pongamia pinnata. Environ. Pollut. 2019, 255, 113167. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.G.; Kuek, C.; Chaudhry, T.; Khoo, C.S.; Hayes, W.J. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000, 41, 197–207. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Q.; Liu, W.; Liu, X.; Hou, J.; Teng, Y.; Luo, Y.; Christie, P. Biosurfactant-producing microorganism Pseudomonas sp. SB assists the phytoremediation of DDT-contaminated soil by two grass species. Chemosphere 2017, 182, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Pajević, S.; Borišev, M.; Nikolić, N.; Arsenov, D.D.; Orlović, S.; Župunski, M. Phytoextraction of heavy metals by fast-growing trees: A review. Phytoremediation 2016, 2016, 29–64. [Google Scholar]
- Souri, Z.; Karimi, N.; Sandalio, L.M. Arsenic hyperaccumulation strategies: An overview. Front. Cell Dev. Biol. 2017, 5, 67. [Google Scholar] [CrossRef]
- Pedroso, D.d.F.; Barbosa, M.V.; dos Santos, J.V.; Pinto, F.A.; Siqueira, J.O.; Carneiro, M. Arbuscular mycorrhizal fungi favor the initial growth of Acacia mangium, Sorghum bicolor, and Urochloa brizantha in soil contaminated with Zn, Cu, Pb, and Cd. Bull. Environ. Contam. Toxicol. 2018, 101, 386–391. [Google Scholar] [CrossRef]
- Patra, D.K.; Pradhan, C.; Patra, H.K. Chromium bioaccumulation, oxidative stress metabolism and oil content in lemon grass Cymbopogon flexuosus (Nees ex Steud.) W. Watson grown in chromium rich over burden soil of Sukinda chromite mine, India. Chemosphere 2019, 218, 1082–1088. [Google Scholar] [CrossRef]
- Rosa, C.; Sierra, M.; Radetski, C. Use of plant tests in the evaluation of textile effluent toxicity. Ecotoxicol. Environ. Res. 1999, 2, 56–61. [Google Scholar]
- Gomes, M.; Carvalho, M.; Carvalho, G.; Marques, T.; Garcia, Q.; Guilherme, L.; Soares, A. Phosphorus improves arsenic phytoremediation by Anadenanthera peregrina by alleviating induced oxidative stress. Int. J. Phytoremediat. 2013, 15, 633–646. [Google Scholar] [CrossRef]
- Chaer, G.M.; Resende, A.S.; Campello, E.F.C.; de Faria, S.M.; Boddey, R.M. Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol. 2011, 31, 139–149. [Google Scholar] [CrossRef]
- Marques, T.C.L.L.d.S.; Moreira, F.M.d.S.; Siqueira, J.O. Growth and metal concentration of seedlings of woody species in a heavy metal contaminated soil. Pesqui. Agropecu. Bras. 2000, 35, 121–132. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L.; Švehla, J.; Chrastný, V.; Štíchová, J. Trace elements in Phragmites australis growing in constructed wetlands for treatment of municipal wastewater. Ecol. Eng. 2009, 35, 303–309. [Google Scholar] [CrossRef]
- Schneider, J.; Bundschuh, J.; de Melo Rangel, W.; Guilherme, L.R.G. Potential of different AM fungi (native from As-contaminated and uncontaminated soils) for supporting Leucaena leucocephala growth in As-contaminated soil. Environ. Pollut. 2017, 224, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Min, H.; Cai, S.; Fu, Y.; Sha, S.; Xie, K.; Du, K. Subcellular distribution and toxicity of cadmium in Potamogeton crispus L. Chemosphere 2012, 89, 114–120. [Google Scholar] [CrossRef]
- Zhu, X.F.; Lei, G.J.; Jiang, T.; Liu, Y.; Li, G.X.; Zheng, S.J. Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 2012, 236, 989–997. [Google Scholar] [CrossRef]
- Ralph, J.; Bunzel, M.; Marita, J.M.; Hatfield, R.D.; Lu, F.; Kim, H.; Schatz, P.F.; Grabber, J.H.; Steinhart, H. Peroxidase-dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochem. Rev. 2004, 3, 79–96. [Google Scholar] [CrossRef]
- He, Y.-M.; Yang, R.; Lei, G.; Li, B.; Jiang, M.; Yan, K.; Zu, Y.-Q.; Zhan, F.-D.; Li, Y. Arbuscular mycorrhizal fungi reduce cadmium leaching from polluted soils under simulated heavy rainfall. Environ. Pollut. 2020, 263, 114406. [Google Scholar] [CrossRef]
- Jiang, X.J.; Liu, W.; Chen, C.; Liu, J.; Yuan, Z.-Q.; Jin, B.; Yu, X. Effects of three morphometric features of roots on soil water flow behavior in three sites in China. Geoderma 2018, 320, 161–171. [Google Scholar] [CrossRef]
- Krämer, U. Phytoremediation: Novel approaches to cleaning up polluted soils. Curr. Opin. Biotechnol. 2005, 16, 133–141. [Google Scholar] [CrossRef]
- Nasiri, K.; Babaeinejad, T.; Ghanavati, N.; Mohsenifar, K. Arbuscular mycorrhizal fungi affecting the growth, nutrient uptake, and phytoremediation potential of different plants in cadmium-polluted soil. Biometals 2022, 35, 1243–1253. [Google Scholar] [CrossRef]
- Quintella, C.M.; Mata, A.M.; Lima, L.C. Overview of bioremediation with technology assessment and emphasis on fungal bioremediation of oil contaminated soils. J. Environ. Manag. 2019, 241, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Trinh, N.N.; Le, C.M.T.; Nguyen, T.T.; Tran, T.V.; Thai, B.V.; Le, T.V. The interactive effects of biochar and cow manure on rice growth and selected properties of salt-affected soil. Arch. Agron. Soil Sci. 2018, 64, 1744–1758. [Google Scholar] [CrossRef]
- Rajkumar, M.; Sandhya, S.; Prasad, M.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Rani, R.; Chandra, A.; Varjani, S.J.; Kumar, V. Effectiveness of plant growth-promoting Rhizobacteria in phytoremediation of chromium stressed soils. In Waste Bioremediation; Springer: Singapore, 2018; pp. 301–312. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, UK, 2010. [Google Scholar]
- Parvin, S.; Van Geel, M.; Yeasmin, T.; Lievens, B.; Honnay, O. Variation in arbuscular mycorrhizal fungal communities associated with lowland rice (Oryza sativa) along a gradient of soil salinity and arsenic contamination in Bangladesh. Sci. Total Environ. 2019, 686, 546–554. [Google Scholar] [CrossRef]
- Nelson, L.W.; Cheeke, T.E.; Cifizzari, K. An inquiry-based lab activity to investigate potential effects of arbuscular mycorrhizal fungi on seed germination. Am. Biol. Teach. 2021, 83, 537–541. [Google Scholar] [CrossRef]
- Gil-Cardeza, M.L.; Müller, D.; Amaya-Martin, S.M.; Viassolo, R.; Gómez, E. Differential responses to high soil chromium of two arbuscular mycorrhizal fungi communities isolated from Cr-polluted and non-polluted rhizospheres of Ricinus communis. Sci. Total Environ. 2018, 625, 1113–1121. [Google Scholar] [CrossRef]
- Hu, S.; Hu, B.; Chen, Z.; Vosátka, M.; Vymazal, J. Arbuscular mycorrhizal fungi modulate the chromium distribution and bioavailability in semi-aquatic habitats. Chem. Eng. J. 2021, 420, 129925. [Google Scholar] [CrossRef]
- Vallino, M.; Fiorilli, V.; Bonfante, P. Rice flooding negatively impacts root branching and arbuscular mycorrhizal colonization, but not fungal viability. Plant Cell Environ. 2013, 37, 557–572. [Google Scholar] [CrossRef]
- Lumini, E.; Vallino, M.; Alguacil, M.M.; Romani, M.; Bianciotto, V. Different farming and water regimes in Italian rice fields affect arbuscular mycorrhizal fungal soil communities. Ecol. Appl. 2011, 21, 1696–1707. [Google Scholar] [CrossRef]
- Miransari, M. Arbuscular mycorrhizal fungi and heavy metal tolerance in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; pp. 147–161. [Google Scholar]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, UK, 2008. [Google Scholar]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. mMycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Steinberg, P.D. Glomalin production by an arbuscular mycorrhizal fungus: A mechanism of habitat modification? Soil Biol. Biochem. 2002, 34, 1371–1374. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Xiao, Z.; Ban, Y.; Belvett, N. Positive effects of Funneliformis mosseae inoculation on reed seedlings under water and TiO2 nanoparticles stresses. World J. Microbiol. Biotechnol. 2019, 35, 1–13. [Google Scholar] [CrossRef]
- Gu, H.-H.; Zhou, Z.; Gao, Y.-Q.; Yuan, X.-T.; Ai, Y.-J.; Zhang, J.-Y.; Zuo, W.-Z.; Taylor, A.A.; Nan, S.-Q.; Li, F.-P. The influences of arbuscular mycorrhizal fungus on phytostabilization of lead/zinc tailings using four plant species. Int. J. Phytoremediat. 2017, 19, 739–745. [Google Scholar] [CrossRef]
- Solís-Domínguez, F.A.; Valentín-Vargas, A.; Chorover, J.; Maier, R.M. Effect of arbuscular mycorrhizal fungi on plant biomass and the rhizosphere microbial community structure of mesquite grown in acidic lead/zinc mine tailings. Sci. Total Environ. 2011, 409, 1009–1016. [Google Scholar] [CrossRef]
- Carrasco, L.; Azcón, R.; Kohler, J.; Roldán, A.; Caravaca, F. Comparative effects of native filamentous and arbuscular mycorrhizal fungi in the establishment of an autochthonous, leguminous shrub growing in a metal-contaminated soil. Sci. Total Environ. 2011, 409, 1205–1209. [Google Scholar] [CrossRef]
- Akhtar, O.; Kehri, H.K.; Zoomi, I. Arbuscular mycorrhiza and Aspergillus terreus inoculation along with compost amendment enhance the phytoremediation of Cr-rich technosol by Solanum lycopersicum under field conditions. Ecotoxicol. Environ. Saf. 2020, 201, 110869. [Google Scholar] [CrossRef]
- Ismail, Y.; Hijri, M. Arbuscular mycorrhisation with Glomus irregulare induces expression of potato PR homologues genes in response to infection by Fusarium sambucinum. Funct. Plant Biol. 2012, 39, 236–245. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Jiang, Q.-Y.; Zhuo, F.; Liu, H.; Wang, Y.-T.; Li, S.-S.; Ye, Z.-H.; Jing, Y.-X. Effect of inoculation with Glomus versiforme on cadmium accumulation, antioxidant activities and phytochelatins of Solanum photeinocarpum. PLoS ONE 2015, 10, e0132347. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Genet. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, Y.-G.; Smith, F.A.; Wang, Y.; Chen, B. Arbuscular mycorrhiza enhanced arsenic resistance of both white clover (Trifolium repens Linn.) and ryegrass (Lolium perenne L.) plants in an arsenic-contaminated soil. Environ. Pollut. 2008, 155, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.A.; Smith, S.E. How harmonious are arbuscular mycorrhizal symbioses? Inconsistent concepts reflect different mindsets as well as results. New Phytol. 2015, 205, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Karandashov, V.; Bucher, M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 2005, 10, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Campos-Soriano, L.; García-Garrido, J.M.; Segundo, B.S. Activation of basal defense mechanisms of rice plants by Glomus intraradices does not affect the arbuscular mycorrhizal symbiosis. New Phytol. 2010, 188, 597–614. [Google Scholar] [CrossRef]
- Wu, S.; Hu, Y.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Lv, J.; Li, J.; Zhang, J.; Zheng, L. Chromium detoxification in arbuscular mycorrhizal symbiosis mediated by sulfur uptake and metabolism. Environ. Exp. Bot. 2018, 147, 43–52. [Google Scholar] [CrossRef]
- Gai, J.; Fan, J.; Zhang, S.; Mi, N.; Christie, P.; Li, X.; Feng, G. Direct effects of soil cadmium on the growth and activity of arbuscular mycorrhizal fungi. Rhizosphere 2018, 7, 43–48. [Google Scholar] [CrossRef]
- Martínez-García, L.B.; De Deyn, G.B.; Pugnaire, F.I.; Kothamasi, D.; van der Heijden, M.G. Symbiotic soil fungi enhance ecosystem resilience to climate change. Glob. Chang. Biol. 2017, 23, 5228–5236. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 442. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Lavado, R.S.; Giacometti, R. Interaction of plants and arbuscular mycorrhizal fungi in responses to arsenic stress: A collaborative tale useful to manage contaminated soils. In Mechanisms of Arsenic Toxicity and Tolerance in Plants; Springer: Singapore, 2018; pp. 239–255. [Google Scholar]
- González-Guerrero, M.; Benabdellah, K.; Valderas, A.; Azcón-Aguilar, C.; Ferrol, N. GintABC1 encodes a putative ABC transporter of the MRP subfamily induced by Cu, Cd, and oxidative stress in Glomus intraradices. Mycorrhiza 2010, 20, 137–146. [Google Scholar] [CrossRef]
- Zhu, R.; Zheng, Z.; Li, T.; He, S.; Zhang, X.; Wang, Y.; Liu, T. Effect of tea plantation age on the distribution of glomalin-related soil protein in soil water-stable aggregates in southwestern China. Environ. Sci. Pollut. Res. 2018, 26, 1973–1982. [Google Scholar] [CrossRef]
- Zhang, Z.; Mallik, A.; Zhang, J.; Huang, Y.; Zhou, L. Effects of arbuscular mycorrhizal fungi on inoculated seedling growth and rhizosphere soil aggregates. Soil Tillage Res. 2019, 194, 104340. [Google Scholar] [CrossRef]
- Ji, L.; Tan, W.; Chen, X. Arbuscular mycorrhizal mycelial networks and glomalin-related soil protein increase soil aggregation in Calcaric Regosol under well-watered and drought stress conditions. Soil Tillage Res. 2019, 185, 1–8. [Google Scholar] [CrossRef]
- Ker, K.; Charest, C. Nickel remediation by AM-colonized sunflower. Mycorrhiza 2010, 20, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Y.; Scheller, H.V.; Ghosh, A.; Ban, Y.; Chen, H.; Tang, M. Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils. Soil Biol. Biochem. 2015, 86, 146–158. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Huang, L.; Chen, B. Arbuscular mycorrhiza and plant chromium tolerance. Soil Ecol. Lett. 2019, 1, 94–104. [Google Scholar] [CrossRef]
- Miransari, M. Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol. Adv. 2011, 29, 645–653. [Google Scholar] [CrossRef]
- Shalaby, A.M. Responses of arbuscular mycorrhizal fungal spores isolated from heavy metal-polluted and unpolluted soil to Zn, Cd, Pb and their interactions in vitro. Pak. J. Biol. Sci. 2003, 6, 1416–1422. [Google Scholar] [CrossRef]
- Kullu, B.; Patra, D.K.; Acharya, S.; Pradhan, C.; Patra, H.K. AM fungi mediated bioaccumulation of hexavalent chromium in Brachiaria mutica-a mycorrhizal phytoremediation approach. Chemosphere 2020, 258, 127337. [Google Scholar] [CrossRef]
- Janoušková, M.; Pavlíková, D. Cadmium immobilization in the rhizosphere of arbuscular mycorrhizal plants by the fungal extraradical mycelium. Plant Soil 2010, 332, 511–520. [Google Scholar] [CrossRef]
- Wang, Q.; Mei, D.; Chen, J.; Lin, Y.; Liu, J.; Lu, H.; Yan, C. Sequestration of heavy metal by glomalin-related soil protein: Implication for water quality improvement in mangrove wetlands. Water Res. 2019, 148, 142–152. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, L.; Shi, Z.Y.; Li, Y.J.; Song, Z.M. Effects of AM inoculation and organic amendment, alone or in combination, on growth, P nutrition, and heavy-metal uptake of tobacco in Pb-Cd-contaminated soil. J. Plant Growth Regul. 2012, 31, 549–559. [Google Scholar] [CrossRef]
- Chen, B.; Nayuki, K.; Kuga, Y.; Zhang, X.; Wu, S.; Ohtomo, R. Uptake and intraradical immobilization of cadmium by arbuscular mycorrhizal fungi as revealed by a stable isotope tracer and synchrotron radiation μX-ray fluorescence analysis. Microbes Environ. 2018, 33, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xiao, X.; Zhu, Y.-G.; Smith, F.A.; Xie, Z.M.; Smith, S.E. The arbuscular mycorrhizal fungus Glomus mosseae gives contradictory effects on phosphorus and arsenic acquisition by Medicago sativa Linn. Sci. Total Environ. 2007, 379, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Lv, J.; Li, G.; Zhang, Z.; Zhang, J. Chromium immobilization by extra-and intraradical fungal structures of arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42. [Google Scholar] [CrossRef]
- Danh, L.T.; Truong, P.; Mammucari, R.; Foster, N. A critical review of the arsenic uptake mechanisms and phytoremediation potential of Pteris vittata. Int. J. Phytoremediat. 2014, 16, 429–453. [Google Scholar] [CrossRef]
- Otones, V.; Álvarez-Ayuso, E.; García-Sánchez, A.; Santa Regina, I.; Murciego, A. Arsenic distribution in soils and plants of an arsenic impacted former mining area. Environ. Pollut. 2011, 159, 2637–2647. [Google Scholar] [CrossRef]
- Gupta, S.; Thokchom, S.D.; Kapoor, R. Arbuscular mycorrhiza improves photosynthesis and restores alteration in sugar metabolism in Triticum aestivum L. grown in arsenic contaminated soil. Front. Plant Sci. 2021, 12, 334. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Xie, X.; Wu, Y.; Liang, F.; Tang, M. Arbuscular mycorrhizal fungi promote lead immobilization by increasing the polysaccharide content within pectin and inducing cell wall peroxidase activity. Chemosphere 2021, 267, 128924. [Google Scholar] [CrossRef]
- Pigna, M.; Cozzolino, V.; Violante, A.; Meharg, A.A. Influence of phosphate on the arsenic uptake by wheat (Triticum durum L.) irrigated with arsenic solutions at three different concentrations. Water Air Soil Pollut. 2009, 197, 371–380. [Google Scholar] [CrossRef]
- Chandrakar, V.; Naithani, S.C.; Keshavkant, S. Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: A review. Biologia 2016, 71, 367–377. [Google Scholar] [CrossRef]
- Stoeva, N.; Berova, M.; Zlatev, Z. Physiological response of maize to arsenic contamination. Biol. Plant. 2003, 46, 449–452. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Majumder, B.; Das, S.; Biswas, S.; Mazumdar, A.; Biswas, A.K. Differential responses of photosynthetic parameters and its influence on carbohydrate metabolism in some contrasting rice (Oryza sativa L.) genotypes under arsenate stress. Ecotoxicology 2020, 29, 912–931. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Singla, P. Arsenic toxicity in crop plants: Physiological effects and tolerance mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon's role in abiotic and biotic plant stresses. Ann. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef]
- Finnegan, P.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Codling, E.; Chaney, R.; Green, C. Accumulation of lead and arsenic by potato grown on lead–arsenate-contaminated orchard soils. Commun. Soil Sci. Plant Anal. 2016, 47, 799–807. [Google Scholar] [CrossRef]
- Bustingorri, C.; Balestrasse, K.B.; Lavado, R.S. Effects of high arsenic and fluoride soil concentrations on soybean plants. Phyton 2015, 84, 407–416. [Google Scholar]
- Das, H.; Mitra, A.K.; Sengupta, P.; Hossain, A.; Islam, F.; Rabbani, G. Arsenic concentrations in rice, vegetables, and fish in Bangladesh: A preliminary study. Environ. Int. 2004, 30, 383–387. [Google Scholar] [CrossRef]
- Panaullah, G.M.; Alam, T.; Hossain, M.B.; Loeppert, R.H.; Lauren, J.G.; Meisner, C.A.; Ahmed, Z.U.; Duxbury, J.M. Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 2009, 317, 31–39. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Loira, B.; Cebrian, M.E.; Franco-Marina, F.; Lopez-Carrillo, L. Arsenic metabolism and cancer risk: A meta-analysis. Environ. Res. 2017, 156, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic behaviour in soil-plant system: Biogeochemical reactions and chemical speciation influences. In Enhancing Cleanup of Environmental Pollutants; Springer: Cham, Switzerland, 2017; pp. 97–140. [Google Scholar]
- Lee, J.-T.; Yu, W.-. C Evaluation of legume growth in arsenic-polluted acidic soils with various pH values. J. Water Sustain. 2012, 2, 1. [Google Scholar]
- Ullrich-Eberius, C.; Sanz, A.; Novacky, A. Evaluation of arsenate-and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba G1. J. Exp. Bot. 1989, 40, 119–128. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Diehn, T.A.; Bienert, G.P. Metalloido-porins: Essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci. 2015, 238, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ma, J.F.; Meharg, A.; McGrath, S. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, P.M.; Hakvoort, H.W.; Bliek, M.; Souer, E.; Schat, H. Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant J. 2006, 45, 917–929. [Google Scholar] [CrossRef]
- Boorboori, M.R.; Gao, Y.; Wang, H.; Fang, C. Usage of Si, P, Se, and Ca decrease arsenic concentration/toxicity in rice, a review. Appl. Sci. 2021, 11, 8090. [Google Scholar] [CrossRef]
- Wu, F.; Ye, Z.; Wong, M.H. Intraspecific differences of arbuscular mycorrhizal fungi in their impacts on arsenic accumulation by Pteris vittata L. Chemosphere 2009, 76, 1258–1264. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Han, Y.-H.; Chen, Y.; Zhu, L.-J.; Ma, L.Q. Arsenic transformation and plant growth promotion characteristics of As-resistant endophytic bacteria from As-hyperaccumulator Pteris vittata. Chemosphere 2016, 144, 1233–1240. [Google Scholar] [CrossRef]
- Spagnoletti, F.; Lavado, R.S. The arbuscular mycorrhiza Rhizophagus intraradices reduces the negative effects of arsenic on soybean plants. Agronomy 2015, 5, 188–199. [Google Scholar] [CrossRef]
- Schneider, J.; Stürmer, S.L.; Guilherme, L.R.G.; de Souza Moreira, F.M.; de Sousa Soares, C.R.F. Arbuscular mycorrhizal fungi in arsenic-contaminated areas in Brazil. J. Hazard. Mater. 2013, 262, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Chavez, C.; D'haen, J.; Vangronsveld, J.; Dodd, J. Copper sorption and accumulation by the extraradical mycelium of different Glomus spp. (arbuscular mycorrhizal fungi) isolated from the same polluted soil. Plant Soil 2002, 240, 287–297. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, F.; Hua, J.; Ma, Z. Prediction of the distribution of arbuscular mycorrhizal fungi in the metal (loid)-contaminated soils by the arsenic concentration in the fronds of Pteris vittata L. J. Soils Sediments 2018, 18, 2544–2551. [Google Scholar] [CrossRef]
- Su, S.; Zeng, X.; Bai, L.; Williams, P.N.; Wang, Y.; Zhang, L.; Wu, C. Inoculating chlamydospores of Trichoderma asperellum SM-12F1 changes arsenic availability and enzyme activity in soils and improves water spinach growth. Chemosphere 2017, 175, 497–504. [Google Scholar] [CrossRef]
- Li, J.; Chen, B.; Zhang, X.; Hao, Z.; Zhang, X.; Zhu, Y. Arsenic transformation and volatilization by arbuscular mycorrhizal symbiosis under axenic conditions. J. Hazard. Mater. 2021, 413, 125390. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Balestrasse, K.; Lavado, R.S.; Giacometti, R. Arbuscular mycorrhiza detoxifying response against arsenic and pathogenic fungus in soybean. Ecotoxicol. Environ. Saf. 2016, 133, 47–56. [Google Scholar] [CrossRef]
- Maldonado-Mendoza, I.E.; Harrison, M.J. RiArsB and RiMT-11: Two novel genes induced by arsenate in arbuscular mycorrhiza. Fungal Biol. 2018, 122, 121–130. [Google Scholar] [CrossRef]
- Lomax, C.; Liu, W.J.; Wu, L.; Xue, K.; Xiong, J.; Zhou, J.; McGrath, S.P.; Meharg, A.A.; Miller, A.J.; Zhao, F.J. Methylated arsenic species in plants originate from soil microorganisms. New Phytol. 2012, 193, 665–672. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, G.; Teng, Y.; Wang, J.; Rhee, J.S.; Wang, S.; Li, F. Screening and assessment of solidification/stabilization amendments suitable for soils of lead-acid battery contaminated site. J. Hazard. Mater. 2015, 288, 140–146. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Jiang, X.; Chen, B.; Zhang, X. Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol. Environ. Saf. 2018, 157, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Vodnik, D.; Grčman, H.; Maček, I.; Van Elteren, J.; Kovačevič, M. The contribution of glomalin-related soil protein to Pb and Zn sequestration in polluted soil. Sci. Total Environ. 2008, 392, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, F.; Carmona, M.; Gómez, N.E.T.; Chiocchio, V.; Lavado, R.S. Arbuscular mycorrhiza reduces the negative effects of M. phaseolina on soybean plants in arsenic-contaminated soils. Appl. Soil Ecol. 2017, 121, 41–47. [Google Scholar] [CrossRef]
- Christophersen, H.M.; Smith, F.A.; Smith, S.E. Unraveling the influence of arbuscular mycorrhizal colonization on arsenic tolerance in Medicago: Glomus mosseae is more effective than G. intraradices, associated with lower expression of root epidermal Pi transporter genes. Front. Physiol. 2012, 3, 91. [Google Scholar] [CrossRef]
- Zafarzadeh, A.; Rahimzadeh, H.; Mahvi, A.H. Health risk assessment of heavy metals in vegetables in an endemic esophageal cancer region in Iran. Health Scope 2018, 7, 7. [Google Scholar] [CrossRef]
- McBride, M.B. Chemisorption and precipitation of inorganic ions. In Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994; pp. 121–168. [Google Scholar]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y.; Hua, X. Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environ. Int. 2019, 128, 430–437. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Kasemodel, M.; Sakamoto, I.; Varesche, M.; Rodrigues, V. Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Sci. Total Environ. 2019, 675, 367–379. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Zeng, G.; Qu, D.; Gu, J.; Zhou, M.; Chai, L. Cadmium-induced oxidative stress and response of the ascorbate–glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere 2007, 69, 99–107. [Google Scholar] [CrossRef]
- Beryllium, I. Cadmium, mercury, and exposures in the glass manufacturing industry. Working Group views and expert opinions, Lyon, 9–16 February 1993. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 58, 1–415. [Google Scholar]
- Fang, W.; Wei, Y.; Liu, J. Comparative characterization of sewage sludge compost and soil: Heavy metal leaching characteristics. J. Hazard. Mater. 2016, 310, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dusek, J.; Vogel, T.; Lichner, L.; Cipakova, A. Short-term transport of cadmium during a heavy-rain event simulated by a dual-continuum approach. J. Plant Nutr. Soil Sci. 2010, 173, 536–547. [Google Scholar] [CrossRef]
- Mi, Y.; Zhan, F.; Li, B.; Qin, L.; Wang, J.; Zu, Y.; Li, Y. Distribution characteristics of cadmium and lead in particle size fractions of farmland soils in a lead–zinc mine area in Southwest China. Environ. Syst. Res. 2018, 7, 14. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Aggarwal, N. Effect of mycorrhizal inoculations on heavy metal uptake and stress alleviation of Cajanus cajan (L.) Millsp. genotypes grown in cadmium and lead contaminated soils. Plant Growth Regul. 2012, 66, 9–26. [Google Scholar] [CrossRef]
- Wahid, A.; Arshad, M.; Farooq, M. Cadmium phytotoxicity: Responses, mechanisms and mitigation strategies: A review. In Organic Farming, Pest Control and Remediation of Soil Pollutants; Springer: Dordrecht, The Netherlands, 2009; pp. 371–403. [Google Scholar]
- Vivas, A.; Vörös, A.; Biró, B.; Barea, J.; Ruiz-Lozano, J.; Azcón, R. Beneficial effects of indigenous Cd-tolerant and Cd-sensitive Glomus mosseae associated with a Cd-adapted strain of Brevibacillus sp. in improving plant tolerance to Cd contamination. Appl. Soil Ecol. 2003, 24, 177–186. [Google Scholar] [CrossRef]
- Li, R.-Y.; Ago, Y.; Liu, W.-J.; Mitani, N.; Feldmann, J.; McGrath, S.P.; Ma, J.F.; Zhao, F.-J. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009, 150, 2071–2080. [Google Scholar] [CrossRef]
- Sun, H.; Xie, Y.; Zheng, Y.; Lin, Y.; Yang, F. The enhancement by arbuscular mycorrhizal fungi of the Cd remediation ability and bioenergy quality-related factors of five switchgrass cultivars in Cd-contaminated soil. PeerJ 2018, 6, e4425. [Google Scholar] [CrossRef]
- Jiang, Q.-Y.; Zhuo, F.; Long, S.-H.; Zhao, H.-D.; Yang, D.-J.; Ye, Z.-H.; Li, S.-S.; Jing, Y.-X. Can arbuscular mycorrhizal fungi reduce Cd uptake and alleviate Cd toxicity of Lonicera japonica grown in Cd-added soils? Sci. Rep. 2016, 6, 21805. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Yue, X.; He, Y.; Xia, Y.; Wang, Y. Arbuscular mycorrhizal fungi enhance antioxidant defense in the leaves and the retention of heavy metals in the roots of maize. Environ. Sci. Pollut. Res. 2018, 25, 24338–24347. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int. J. Phytoremediat. 2019, 21, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Li, B.; Jiang, M.; Li, T.; He, Y.; Li, Y.; Wang, Y. Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int. J. Phytoremediat. 2019, 21, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Chavez, M.; Carrillo-Gonzalez, R.; Wright, S.; Nichols, K. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardeza, M.L.; Ferri, A.; Cornejo, P.; Gomez, E. Distribution of chromium species in a Cr-polluted soil: Presence of Cr (III) in glomalin related protein fraction. Sci. Total Environ. 2014, 493, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Gunathilakae, N.; Yapa, N.; Hettiarachchi, R. Effect of arbuscular mycorrhizal fungi on the cadmium phytoremediation potential of Eichhornia crassipes (Mart.) Solms. Groundw. Sustain. Dev. 2018, 7, 477–482. [Google Scholar] [CrossRef]
- Audet, P.; Charest, C. Allocation plasticity and plant–metal partitioning: Meta-analytical perspectives in phytoremediation. Environ. Pollut. 2008, 156, 290–296. [Google Scholar] [CrossRef]
- Gabarrón, M.; Faz, A.; Acosta, J. Effect of different industrial activities on heavy metal concentrations and chemical distribution in topsoil and road dust. Environ. Earth Sci. 2017, 76, 129. [Google Scholar] [CrossRef]
- Moosavi, S.; Seghatoleslami, M. Phytoremediation: A review. Adv. Agric. Biol. 2013, 1, 5–11. Available online: www.pscipub.com/AAB (accessed on 8 November 2021).
- Cheng, H.; Hu, Y. Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: A review. Environ. Pollut. 2010, 158, 1134–1146. [Google Scholar] [CrossRef]
- Gupta, V.; Jatav, P.K.; Verma, R.; Kothari, S.L.; Kachhwaha, S. Nickel accumulation and its effect on growth, physiological and biochemical parameters in millets and oats. Environ. Sci. Pollut. Res. 2017, 24, 23915–23925. [Google Scholar] [CrossRef]
- Balakhnina, T.I.; Borkowska, A.; Nosalewicz, M.; Nosalewicz, A.; Wlodarczyk, T.M.; Kosobryukhov, A.A.; Fomina, I.R. Effect of temperature on oxidative stress induced by lead in the leaves of Plantago major L. Int. Agrophys. 2016, 30, 285–292. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Ogundiran, M.B.; Togun, A.O. Soil amendment with compost and crop growth stages influenced heavy metal uptake and distribution in maize crop grown on lead-acid battery waste contaminated soil. J. Environ. Chem. Eng. 2018, 6, 4809–4819. [Google Scholar] [CrossRef]
- Cao, S.; Duan, X.; Zhao, X.; Wang, B.; Ma, J.; Fan, D.; Sun, C.; He, B.; Wei, F.; Jiang, G. Health risk assessment of various metal (loid) s via multiple exposure pathways on children living near a typical lead-acid battery plant, China. Environ. Pollut. 2015, 200, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Saputra, H.M.; Mandia, S.; Retnoaji, B.; Wijayanti, N. Antioxidant properties of liverwort (Marchantia polymorpha L.) to lead-induced oxidative stress on HEK293 cells. J. Biol. Sci. 2016, 16, 77–85. [Google Scholar] [CrossRef]
- Salazar, M.J.; Menoyo, E.; Faggioli, V.; Geml, J.; Cabello, M.; Rodriguez, J.H.; Marro, N.; Pardo, A.; Pignata, M.L.; Becerra, A.G. Pb accumulation in spores of arbuscular mycorrhizal fungi. Sci. Total Environ. 2018, 643, 238–246. [Google Scholar] [CrossRef]
- González-Chávez, M.d.C.A.; Carrillo-González, R.; Cuellar-Sánchez, A.; Delgado-Alvarado, A.; Suárez-Espinosa, J.; Ríos-Leal, E.; Solís-Domínguez, F.A.; Maldonado-Mendoza, I.E. Phytoremediation assisted by mycorrhizal fungi of a Mexican defunct lead-acid battery recycling site. Sci. Total Environ. 2019, 650, 3134–3144. [Google Scholar] [CrossRef]
- Chen, L.; Hu, X.; Yang, W.; Xu, Z.; Zhang, D.; Gao, S. The effects of arbuscular mycorrhizal fungi on sex-specific responses to Pb pollution in Populus cathayana. Ecotoxicol. Environ. Saf. 2015, 113, 460–468. [Google Scholar] [CrossRef]
- Baghaie, A.H.; Aghilizefreei, A. Neighbor presence of plant growth-promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) can increase sorghum phytoremediation efficiency in a soil treated with Pb polluted cow manure. J. Hum. Environ. Health Promot. 2019, 5, 153–159. [Google Scholar] [CrossRef][Green Version]
- Khan, M.A.; Ramzani, P.M.A.; Zubair, M.; Rasool, B.; Khan, M.K.; Ahmed, A.; Khan, S.A.; Turan, V.; Iqbal, M. Associative effects of lignin-derived biochar and arbuscular mycorrhizal fungi applied to soil polluted from Pb-acid batteries effluents on barley grain safety. Sci. Total Environ. 2020, 710, 136294. [Google Scholar] [CrossRef]
- Viti, C.; Marchi, E.; Decorosi, F.; Giovannetti, L. Molecular mechanisms of Cr (VI) resistance in bacteria and fungi. FEMS Microbiol. Rev. 2014, 38, 633–659. [Google Scholar] [CrossRef] [PubMed]
- de los Angeles Beltrán-Nambo, M.; Rojas-Jacuinde, N.; Martinez-Trujillo, M.; Jaramillo-López, P.F.; Romero, M.G.; Carreón-Abud, Y. Differential strategies of two species of arbuscular mycorrhizal fungi in the protection of maize plants grown in chromium-contaminated soils. BioMetals 2021, 34, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Dhal, B.; Thatoi, H.; Das, N.; Pandey, B. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Nigam, H.; Das, M.; Chauhan, S.; Pandey, P.; Swati, P.; Yadav, M.; Tiwari, A. Effect of chromium generated by solid waste of tannery and microbial degradation of chromium to reduce its toxicity: A review. Adv. Appl. Sci. Res. 2015, 6, 129–136. [Google Scholar]
- Dayan, A.; Paine, A. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef]
- Kumar, P. Soil applied glycine betaine with Arbuscular mycorrhizal fungi reduces chromium uptake and ameliorates chromium toxicity by suppressing the oxidative stress in three genetically different Sorghum (Sorghum bicolor L.) cultivars. BMC Plant Biol. 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Kováčik, J.; Babula, P.; Hedbavny, J.; Kryštofová, O.; Provaznik, I. Physiology and methodology of chromium toxicity using alga Scenedesmus quadricauda as model object. Chemosphere 2015, 120, 23–30. [Google Scholar] [CrossRef]
- Gill, R.A.; Zang, L.; Ali, B.; Farooq, M.A.; Cui, P.; Yang, S.; Ali, S.; Zhou, W. Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 2015, 120, 154–164. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Ullah, S.; Ali, R.; Mahmood, S.; Atif Riaz, M.; Akhtar, K. Differential growth and metal accumulation response of Brachiaria mutica and Leptochloa fusca on cadmium and lead contaminated soil. Soil Sediment Contam. Int. J. 2020, 29, 844–859. [Google Scholar] [CrossRef]
- Komal, T.; Mustafa, M.; Ali, Z.; Kazi, A.G. Heavy metal uptake and transport in plants. In Heavy Metal Contamination of Soils; Springer: Cham, Switzerland, 2015; pp. 181–194. [Google Scholar]
- Singh, M.; Srivastava, P.; Verma, P.; Kharwar, R.; Singh, N.; Tripathi, R. Soil fungi for mycoremediation of arsenic pollution in agriculture soils. J. Appl. Microbiol. 2015, 119, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardeza, M.L.; Calonne-Salmon, M.; Gómez, E.; Declerck, S. Short-term chromium (VI) exposure increases phosphorus uptake by the extraradical mycelium of the arbuscular mycorrhizal fungus Rhizophagus irregularis MUCL 41833. Chemosphere 2017, 187, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Su, D.; Lv, J.; Li, G.; Zhang, Z. Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM–EDS, TEM–EDS, and XAFS. Environ. Sci. Technol. 2015, 49, 14036–14047. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.L.; Avery, S.V. Chromate toxicity and the role of sulfur. Metallomics 2011, 3, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Sobrino-Plata, J.; Meyssen, D.; Cuypers, A.; Escobar, C.; Hernández, L.E. Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil 2014, 377, 369–381. [Google Scholar] [CrossRef]

| Metal(loid)s | Content Measured in Different Plants (µg/g DW) | WHO * (mg/kg) | Canada (in Row Herbal Materials) (mg/kg) | China (Herbal Material) (mg/kg) | India | |
|---|---|---|---|---|---|---|
| Food (mg/kg) | Water (mg/L) | |||||
| Arsenic | 0.02–7 | Nil | 5 | 2 | 1.1 | 0.05 |
| Cadmium | 0.1–2.4 | 0.3 | 0.3 | 1 | 1.5 | 0.01 |
| Lead | 1–13 | 10 | 10 | 10 | 2.5 | 0.1 |
| Chromium | 0.2–1 | Nil | 2 | Nil | 20 | 0.05 |
| Arsenic Compounds | Acronyms | Chemical Formula |
|---|---|---|
| Arsenate | As (V) | As(O−)3 |
| Arsenite | As (III) | O=As(O−)3 |
| Methylarsonate | MMA | CH3AsO(O−)2 |
| Dimethylarsinate | DMA | (CH3)2AsO(O−) |
| Trimethylarsin oxide | TMAO | (CH3)3AsO |
| Tetramethylarsonium ion | TETRA | (CH3)4As+ |
| Arsenobetain | AB | (CH3)3As+CH2COO− |
| Trimethylarsoniopropionate | TMAP | (CH3)3As+CH2CH2COO− |
| Arsenocholine | AC | (CH3)3As+CH2CH2O− |
| Dimethylarsinoylacetate | DMAA | (CH3)2(O)As+CH2COO− |
| Dimethylarsinoylpropionate | DMAP | (CH3)2(O)As+CH2CH2COO− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boorboori, M.R.; Zhang, H.-Y. Arbuscular Mycorrhizal Fungi Are an Influential Factor in Improving the Phytoremediation of Arsenic, Cadmium, Lead, and Chromium. J. Fungi 2022, 8, 176. https://doi.org/10.3390/jof8020176
Boorboori MR, Zhang H-Y. Arbuscular Mycorrhizal Fungi Are an Influential Factor in Improving the Phytoremediation of Arsenic, Cadmium, Lead, and Chromium. Journal of Fungi. 2022; 8(2):176. https://doi.org/10.3390/jof8020176
Chicago/Turabian StyleBoorboori, Mohammad Reza, and Hai-Yang Zhang. 2022. "Arbuscular Mycorrhizal Fungi Are an Influential Factor in Improving the Phytoremediation of Arsenic, Cadmium, Lead, and Chromium" Journal of Fungi 8, no. 2: 176. https://doi.org/10.3390/jof8020176
APA StyleBoorboori, M. R., & Zhang, H.-Y. (2022). Arbuscular Mycorrhizal Fungi Are an Influential Factor in Improving the Phytoremediation of Arsenic, Cadmium, Lead, and Chromium. Journal of Fungi, 8(2), 176. https://doi.org/10.3390/jof8020176






