Elucidating the Effect of Nutritional Imbalances of N and K on the Infection of Verticillium dahliae in Olive
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolate and Inoculum Preparation
2.2. Treatments
2.3. Viability of Verticillium dahliae Microsclerotia
2.3.1. Preparation of Artificially Pathogen-Colonized Substrate
2.3.2. Application of Treatments
2.3.3. Assessment
2.4. Verticillium Wilt Development
2.4.1. Plant Material
2.4.2. Application of Treatments and Plant Inoculation
2.4.3. Assessment
2.5. Effect of Root Exudates from Non-Inoculated Plants Grown under Different Treatments
2.5.1. Collection of Root Exudates
2.5.2. Viability of Verticillium dahliae Microsclerotia
2.5.3. Viability of Verticillium dahliae Conidia
2.6. Data Analyses
3. Results
3.1. Viability of Verticillium dahliae Microsclerotia
3.2. Verticillium Wilt Development
3.3. Effects of Root Exudates Collected from Non-Inoculated Plants Grown under Different Treatments
3.3.1. Viability of Verticillium dahliae Microsclerotia
3.3.2. Viability of Verticillium dahliae Conidia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pegg, G.F.; Brady, B.L. Verticillium wilts; Cromwell Press: Trowbridge, UK, 2002. [Google Scholar]
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: A case study to implement an integrated strategy to control a soil-borne pathogen. Plant. Soil 2011, 344, 1–50. [Google Scholar] [CrossRef]
- Jiménez-Díaz, R.M.; Cirulli, M.; Bubici, G.; Jiménez-Gasco, M.; Antoniou, P.P.; Tjamos, E.C. Verticillium wilt, a major threat to olive production: Current status and future prospects for its management. Plant. Dis. 2012, 96, 304–329. [Google Scholar] [CrossRef]
- Montes-Osuna, N.; Mercado-Blanco, J. Verticillium wilt of olive and its control: What did we learn during the last decade? Plants 2020, 9, 735. [Google Scholar] [CrossRef]
- López-Moral, A.; Agustí-Brisach, C.; Trapero, A. Plant Biostimulants: New Insights into the Biological Control of Verticillium Wilt of Olive. Front. Plant. Sci. 2021, 12, 662178. [Google Scholar] [CrossRef]
- López-Escudero, F.J.; Castillo, L.F.R.; Blanco-López, M.A.; Trapero, A. La verticilosis, un grave problema de la olivicultura actual. Agric. Rev. Agropecu. Y Ganad. 2011, 937, 106–110. [Google Scholar]
- Trapero, A.; López-Escudero, F.J.; Blanco-López, M.A. Enfermedades. In El Cultivo del Olivo, 7th ed.; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi Prensa: Madrid, Spain, 2017; pp. 735–793. [Google Scholar]
- Pérez-Rodríguez, M.; Roca, L.F.; López-Escudero, F.J. Book of Abstracts: Influencia de la fertirrigación en la Verticilosis del Olivo; Congreso Nacional de la Sociedad Española de Fitopatología (SEF): Palencia, Spain, 2016; p. 251. [Google Scholar]
- Huber, D.M.; Thompson, I.A. Nitrogen and plant disease. In Mineral Nutrition and Plant Disease; Datnoff, L.E., Wade, H.E., Huber, D.M., Eds.; The American Phytopathological Society: St. Paul, NY, USA, 2007; pp. 31–44. [Google Scholar]
- Roca, L.F.; Romero, J.; Bohórquez, J.M.; Alcántara, E.; Fernández-Escobar, R.; Trapero, A. Nitrogen status affects growth, chlorophyll content and infection by Fusicladium oleagineum in olive. Crop. Prot. 2018, 109, 80–85. [Google Scholar] [CrossRef]
- Prabhu, A.S.; Fageria, N.K.; Huber, D.M.; Rodrigues, F.A. Potasium and plant disease. In Mineral Nutrition and Plant Disease; Datnoff, L.E., Wade, H.E., Huber, D.M., Eds.; The American Phytopathological Society: St. Paul, NY, USA, 2007; pp. 57–78. [Google Scholar]
- Pettigrew, W.T.P. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, C.; Debez, A.; Adbely, C. Potassium deficiency in plants: Effects and signaling cascades. Acta Physiol. Plant. 2014, 36, 1055–1070. [Google Scholar] [CrossRef]
- Fontana, J.E.; Wang, G.; Sun, R.; Xue, H.; Li, Q.; Liu, J.; Davis, K.E.; Thornburg, T.E.; Zhang, B.; Zhang, Z.; et al. Impact of potassium deficiency on cotton growth, development and potential microRNA-mediated mechanism. Plant Physiol. Biochem. 2020, 153, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, M.; Alcántara, E.; Amaro, M.; Serrano, N.; Lorite, I.J.; Arquero, O.; Orgaz, F.; López-Escudero, F.J. The influence of irrigation frequency on the onset and development of Verticillium wilt. Plant. Dis. 2015, 99, 488–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-Rodríguez, M.; Serrano, N.; Arquero, O.; Orgaz, F.; Moral, J.; López-Escudero, F.J. The effect of short irrigation frequencies on the development of Verticillium wilt in the susceptible olive cultivar ‘Picual’ under field conditions. Plant. Dis. 2016, 100, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Blanco, J.; Rodríguez-Jurado, D.; Parrilla-Araujo, S.; Jiménez-Díaz, R.M. Simultaneous detection of the defoliating and nondefoliating Verticilium dahliae pathotypes in infected olive plants by duplex, nested polymerase chain reaction. Plant. Dis. 2007, 87, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Mulero-Aparicio, A.; Agustí-Brisach, C.; Varo, A.; López-Escudero, F.J.; Trapero, A. A non-pathogenic strain of Fusarium oxysporum as a potential biocontrol agent against Verticillium wilt of olive. Biol. Control. 2019, 139, 104045. [Google Scholar] [CrossRef]
- Huisman, O.C.; Ashworth, L.J. Quantitative assessment of Verticillium albo-atrum in field soils: Procedural and substrate improvements. Phytopathology 1974, 64, 1043–1044. [Google Scholar] [CrossRef]
- Butterfield, E.J.; DeVay, J.E. Reassessment of soil assays for Verticillium dahliae. Phytopathology 1977, 67, 1073–1078. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.C.; Trapero, A. Selection and evaluation of micro-organisms for biocontrol of Verticillium dahliae in olive. J. Appl. Microbiol. 2016, 121, 767–777. [Google Scholar] [CrossRef] [PubMed]
- López-Escudero, F.J.; Del Río, C.; Caballero, J.M.; Blanco-López, M.A. Evaluation of olive cultivars for resistance to Verticillium dahliae. Eur. J. Plant. Pathol. 2004, 110, 79–85. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.C.; Agustí-Brisach, C.; García-Ortiz-Civantos, C.; Fernández-Hernández, A.; Mulero-Aparicio, A.; Trapero, A. Evaluation of organic amendments from agro-industry waste for the control of Verticillium wilt of olive. Plant. Pathol. 2018, 67, 860–870. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; Willey: New York, NY, USA, 1990. [Google Scholar]
- Aulakh, M.S.; Wassman, R.; Bueno, C.; Kreuzwieser, J.; Rennenberg, H. Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant. Biol. 2001, 3, 139–148. [Google Scholar] [CrossRef]
- López-Moral, A.; Agustí-Brisach, C.; Llorens, E.; Scalschi, L.; Sánchez-Rodríguez, A.R.; García-Agustín, P.; Trapero, A. Book of Abstracts: Bioestimulantes e Inductores de Resistencia como Alternativas para el Control Biológico de la Verticilosis del Olivo; XVI Congreso nacional de Ciencias Hortícolas (SECH): Córdoba, Spain, 2021; p. 68. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Bioestadística, 2nd ed.; McGraw-Hill: Bogotá, Colombia, 1985. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Education: Singapore, 2010. [Google Scholar]
- Anonimous. Analytical Software, Statistix10; User’s Manual; Anonimous: Tallahassee, FL, USA, 2013. [Google Scholar]
- Guardiola, J.L.; García-Luis, A. Fisiología Vegetal I: Nutrición y Transporte; Editorial Síntesis: Madrid, Spain, 1990; pp. 219–220. [Google Scholar]
- Llorens, E.; García-Agustín, P.; Lapeña, L. Advances in induced resistance by natural compounds: Towards new options for woody crop protection. Sci. Agric. 2017, 74, 90–100. [Google Scholar] [CrossRef]
| Treatment | Compound [mM] | Element [mM] |
|---|---|---|
| Control N-K | Ca(NO3)2 [1.5] KNO3 [2.0] | N [5.0] K [2.0] |
| N and Na excess (↑N+↑Na) | Ca(NO3)2 [1.5] KNO3 [2.0] NaNO3 [45.0] | N [50.0] K [2.0] Na [45.0] |
| NaCl excess (↑NaCl) | Ca(NO3)2 [1.5] KNO3[2.0] NaCl [45.0] | N [5.0] K [2.0] Na [45.0] |
| K deficiency (↓K) | Ca(NO3)2 [1.5] NaNO3 [2.0] | N [5.0] Na [2.0] |
| N and Na excess and K deficiency (↑N+↑Na+↓K) | Ca(NO3)2 [1.5] NaNO3 [47.0] | N [50.0] Na [47.0] |
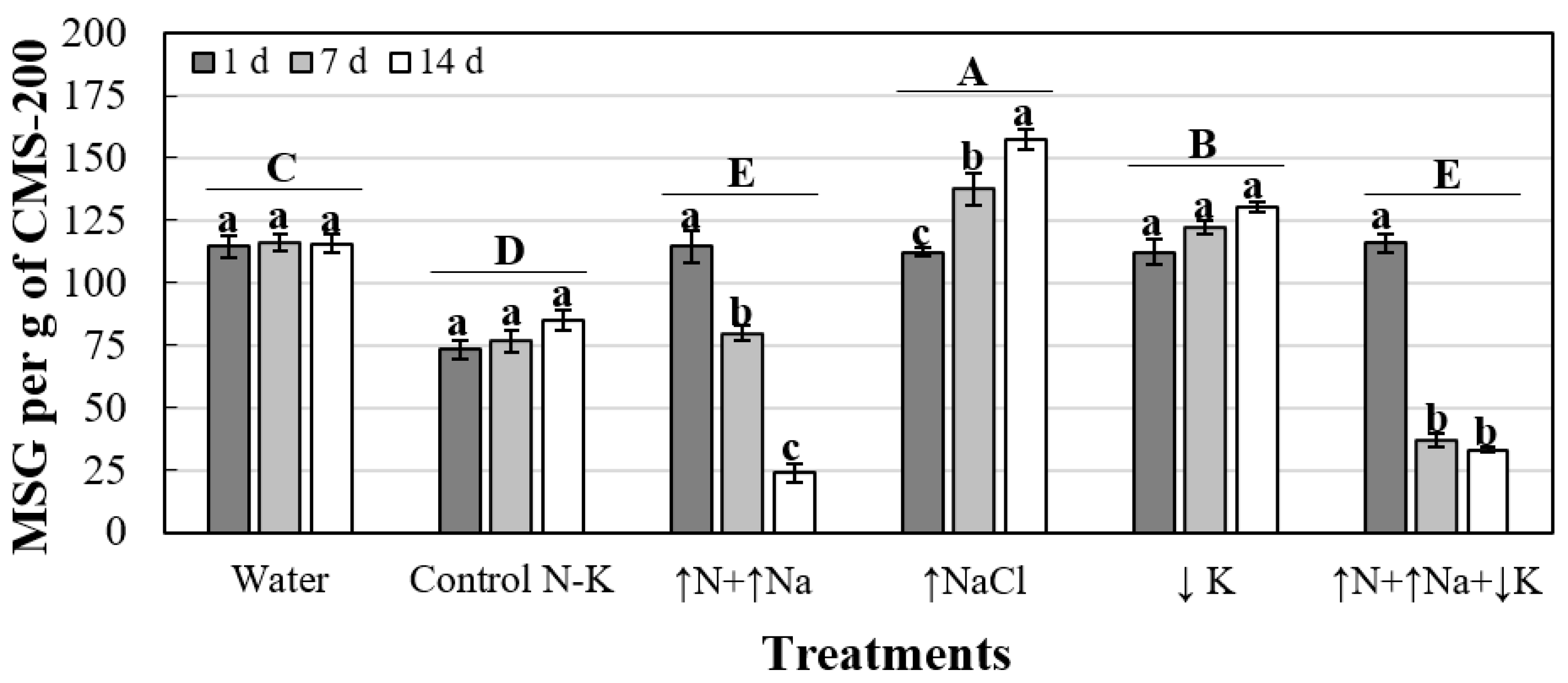
| Treatment | MSG (MS Per g of CMS-200) a | ||
|---|---|---|---|
| 1 Day | 7 Days | 14 Days | |
| Water | 114.8 ± 4.4 a | 116.3 ± 3.6 b | 115.7 ± 3.6 c |
| Control N-K | 73.2 ± 3.9 b | 76.5 ± 4.5 c | 85.2 ± 3.9 d |
| ↑N+↑Na | 114.5 ± 6.2 a | 79.9 ± 3.1 c | 23.7 ± 3.5 e |
| ↑NaCl | 112.4 ± 1.6 a | 137.7 ± 6.4 a | 157.6 ± 4.2 a |
| ↓K | 112.4 ± 5.1 a | 122.2 ± 2.5 b | 130.6 ± 2.0 b |
| ↑N+↑Na+↓K | 116.1 ± 3.8 a | 36.8 ± 2.7 d | 33.1 ± 1.1 e |
| P-value (α = 0.05) | 0.0004 | ≤0.0001 | ≤0.0001 |
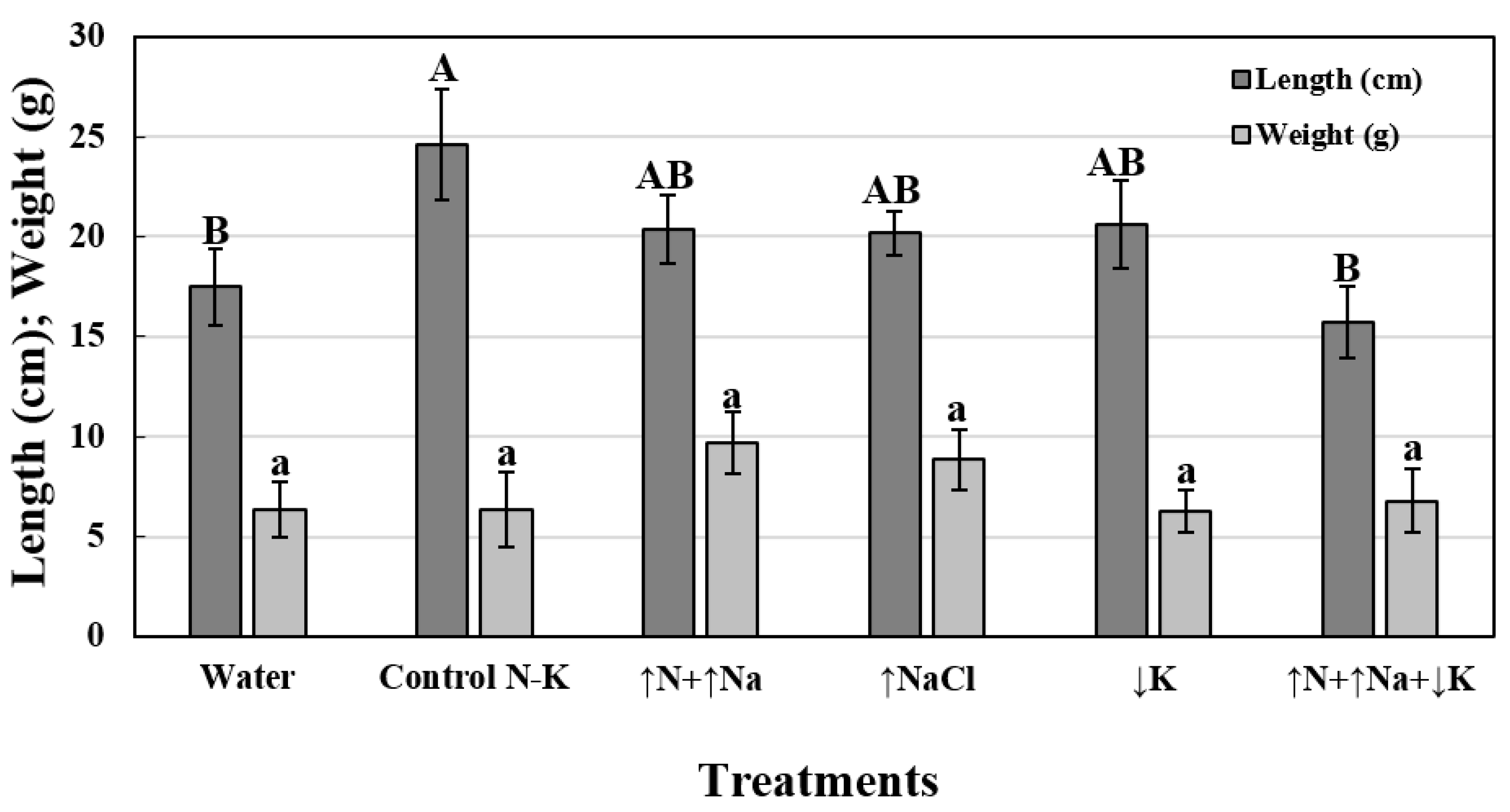
| Treatment | Incidence (%) b | Mortality (%) b | RDS (%) c,e | RAUDPC (%) d,e |
|---|---|---|---|---|
| Control N-K | 58.3 ab | 16.7 ab | 69.3 ± 5.3 b | 108.7 ± 14.7 b |
| ↑N+↑Na | 58.3 ab | 25.0 a | 80.0 ± 12.2 ab | 63.4 ± 7.2 c |
| ↑NaCl | 66.7 ab | 8.3 b | 122.7 ± 27.4 a | 119.6 ± 9.0 ab |
| ↓K | 75.0 a | 25.0 a | 100.0 ± 4.6 ab | 147.0 ± 11.9 a |
| ↑N+↑Na+↓K | 50.0 b | 25.0 a | 85.3 ± 14.1 ab | 89.9 ± 5.9 bc |
| Water (+) f | 66.7 ab | 16.7 ab | 100.0 ± 10.6 ab | 100.0 ± 22.4 b |
| Water (−) g | 0.0 c | 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 d |
| P-value (α = 0.05) | ≤0.0001 | ≤0.0001 | 0.0014 | ≤0.0001 |
| Treatments | CG (%) b,d | MSG (MS Per g of Soil c,d) |
|---|---|---|
| Water | 83.0 ± 1.0 c | 40.5 ± 0.5 a |
| Control N-K | 88.0 ± 0.6 b | 32.5 ± 1.6 c |
| ↑N+↑Na | 85.8 ± 2.3 bc | 27.9 ± 0.3 d |
| ↑NaCl | 93.5 ± 2.4 a | 33.2 ± 1.6 bc |
| ↓K | 82.4 ± 0.6 c | 28.4 ± 1.1 d |
| ↑N+↑Na+↓K | 88.0 ± 0.8 b | 25.9 ± 0.9 d |
| CaSO4 (−) e | 81.2 ± 1.9 c | 36.3 ± 1.0 b |
| P-value (α = 0.05) | 0.0021 | 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Moral, A.; Agustí-Brisach, C.; Ruiz-Blancas, C.; Antón-Domínguez, B.I.; Alcántara, E.; Trapero, A. Elucidating the Effect of Nutritional Imbalances of N and K on the Infection of Verticillium dahliae in Olive. J. Fungi 2022, 8, 139. https://doi.org/10.3390/jof8020139
López-Moral A, Agustí-Brisach C, Ruiz-Blancas C, Antón-Domínguez BI, Alcántara E, Trapero A. Elucidating the Effect of Nutritional Imbalances of N and K on the Infection of Verticillium dahliae in Olive. Journal of Fungi. 2022; 8(2):139. https://doi.org/10.3390/jof8020139
Chicago/Turabian StyleLópez-Moral, Ana, Carlos Agustí-Brisach, Cristina Ruiz-Blancas, Begoña I. Antón-Domínguez, Esteban Alcántara, and Antonio Trapero. 2022. "Elucidating the Effect of Nutritional Imbalances of N and K on the Infection of Verticillium dahliae in Olive" Journal of Fungi 8, no. 2: 139. https://doi.org/10.3390/jof8020139
APA StyleLópez-Moral, A., Agustí-Brisach, C., Ruiz-Blancas, C., Antón-Domínguez, B. I., Alcántara, E., & Trapero, A. (2022). Elucidating the Effect of Nutritional Imbalances of N and K on the Infection of Verticillium dahliae in Olive. Journal of Fungi, 8(2), 139. https://doi.org/10.3390/jof8020139







