Crosstalk between Yeast Cell Plasma Membrane Ergosterol Content and Cell Wall Stiffness under Acetic Acid Stress Involving Pdr18
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
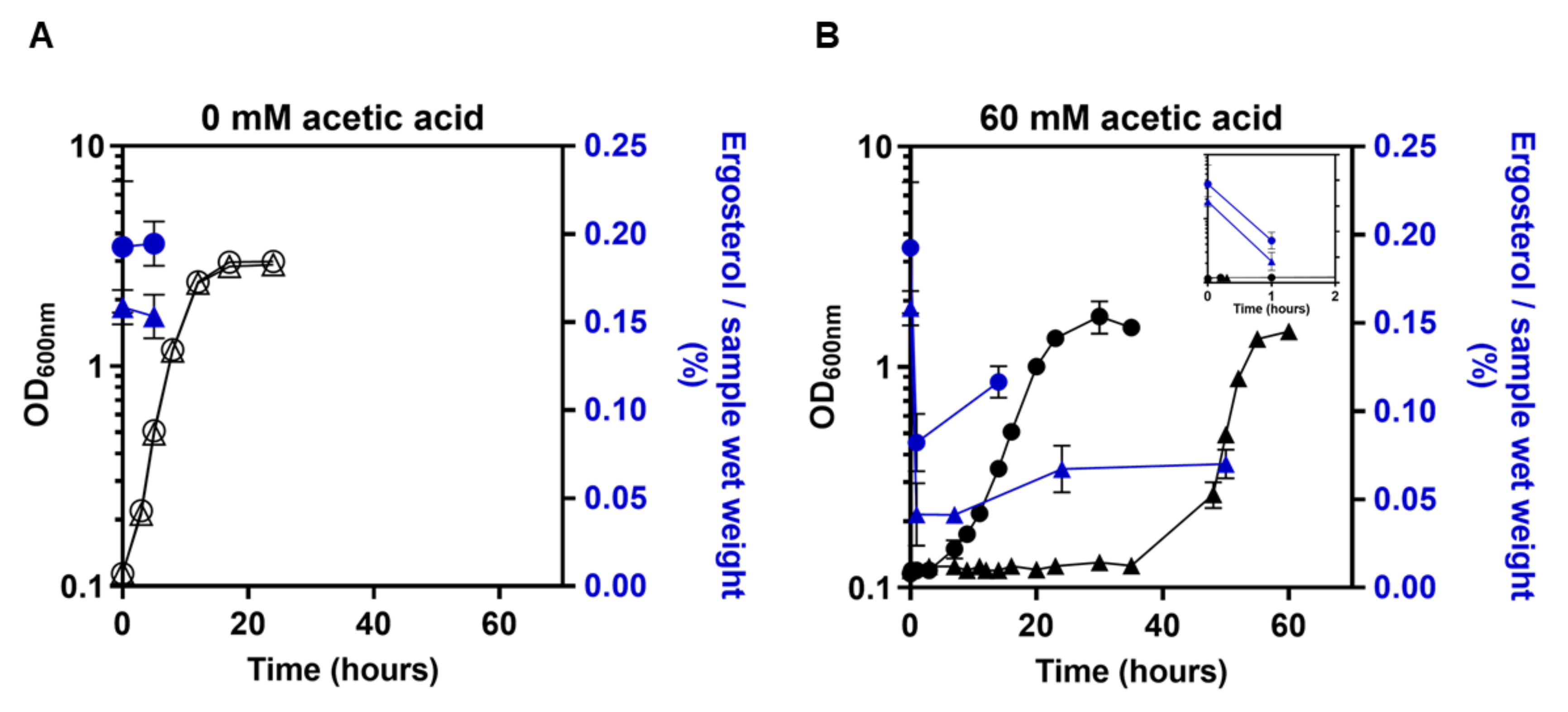
2.2. Ergosterol Quantification
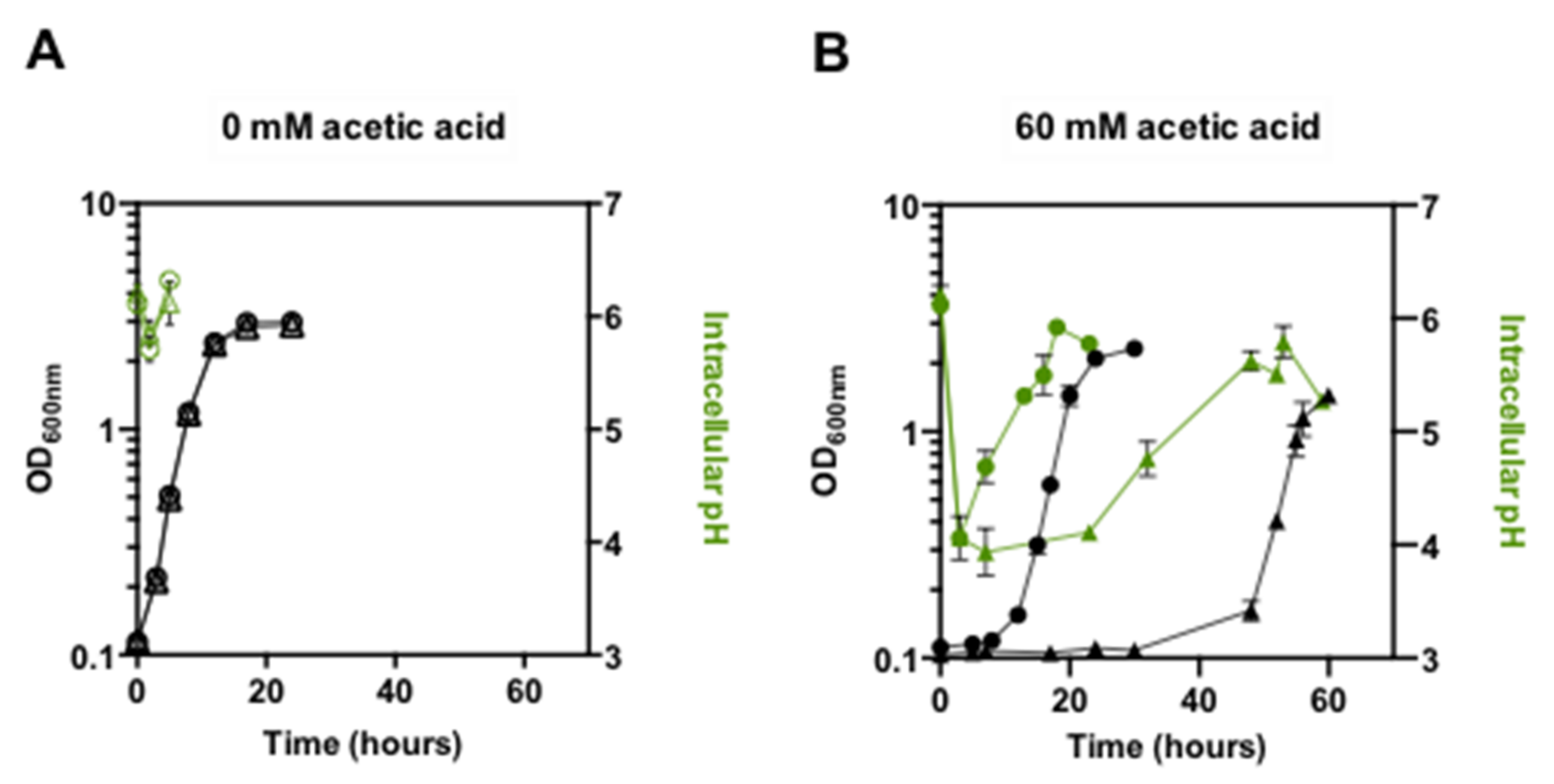
2.3. Determination of Intracellular pH (pHi) by Flow Cytometry
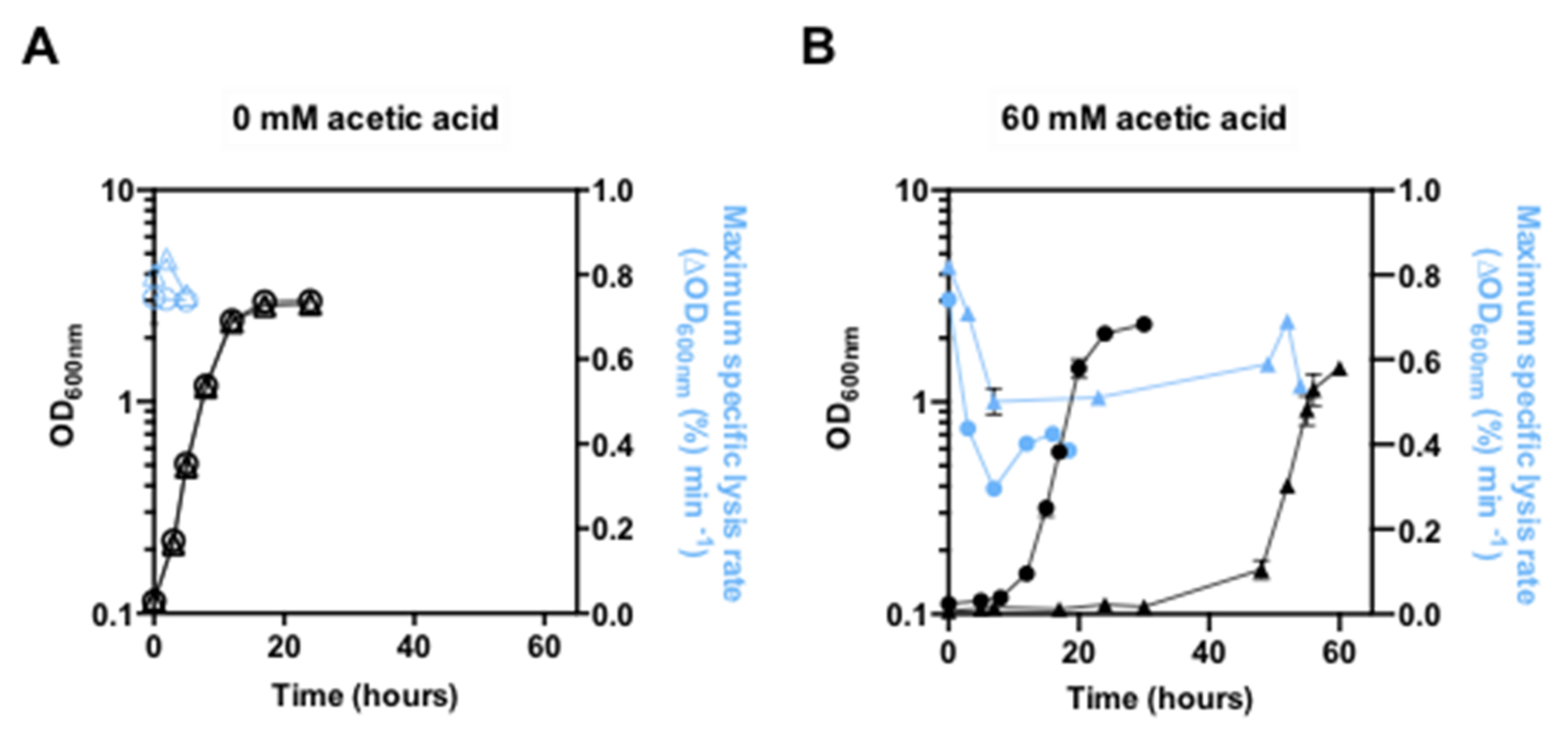
2.4. Yeast Cell Wall Susceptibility to Lyticase
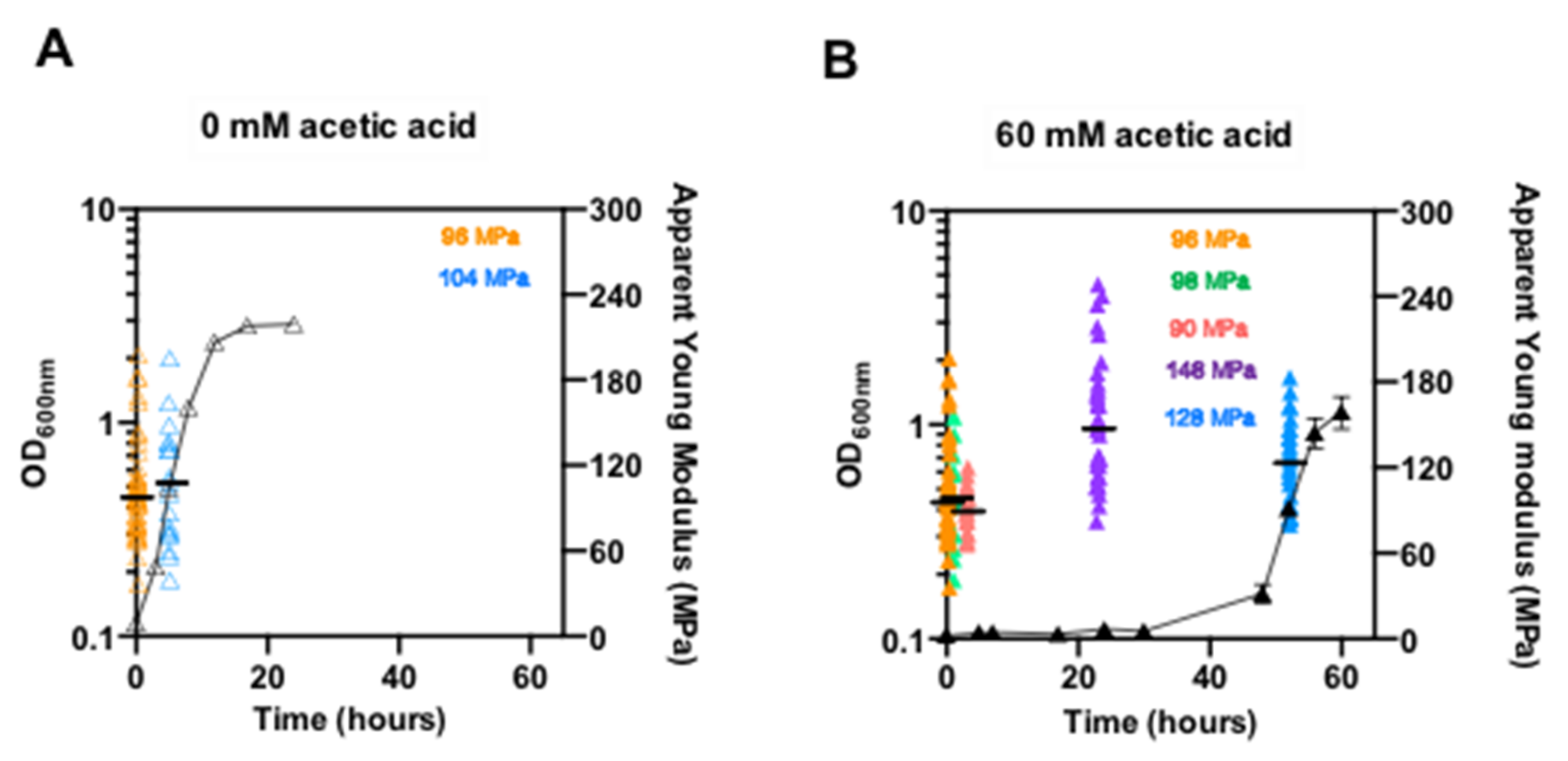
2.5. Measurement of the Young’s Modulus of the Cell Surface by Atomic Force Microscopy (AFM)
2.6. Transcriptional Analysis of Cell Wall Biosynthetic and Regulatory Genes
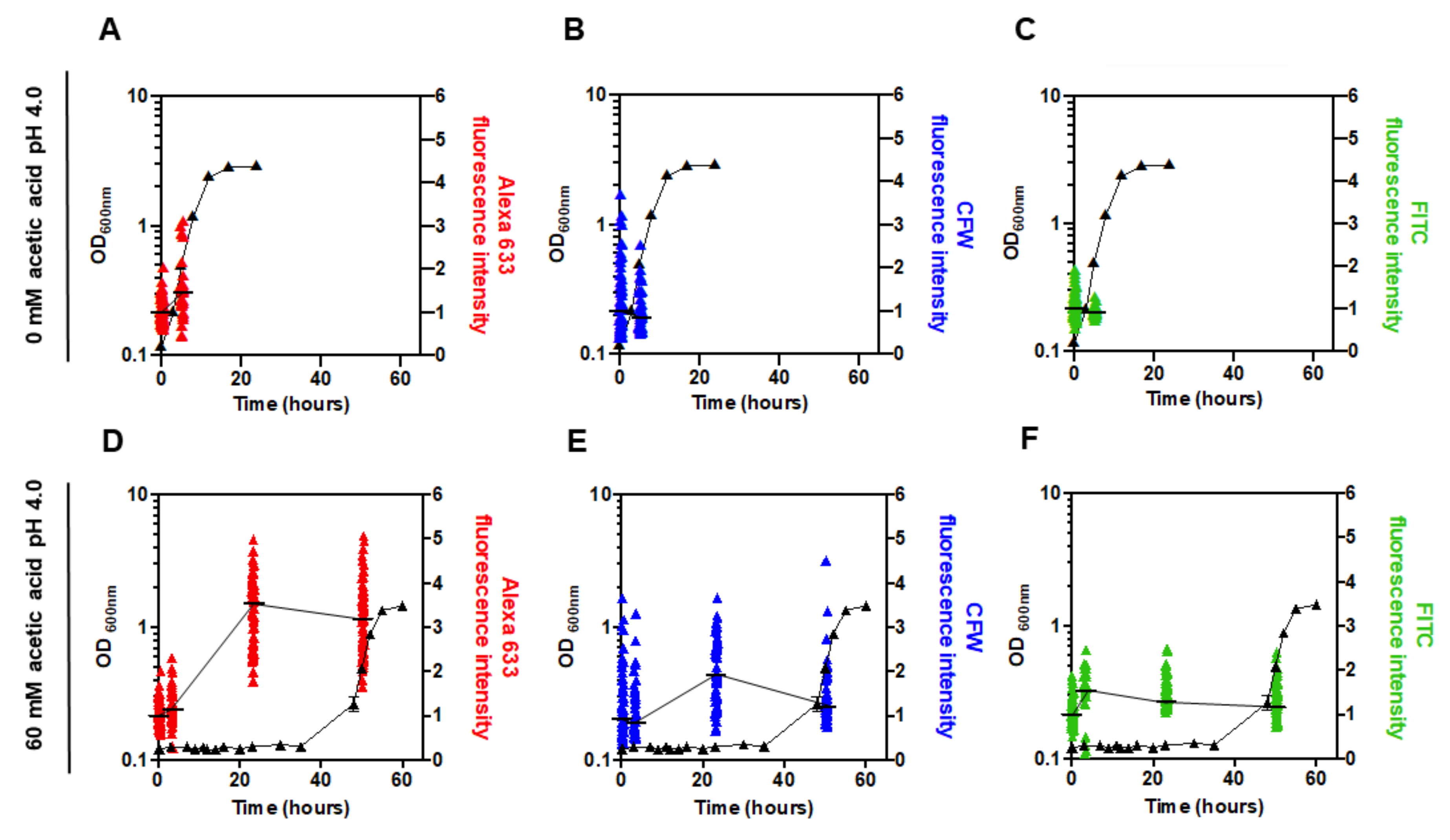
2.7. Assessment of Cell Wall Polysaccharide Content by Fluorescence Microscopy
3. Results
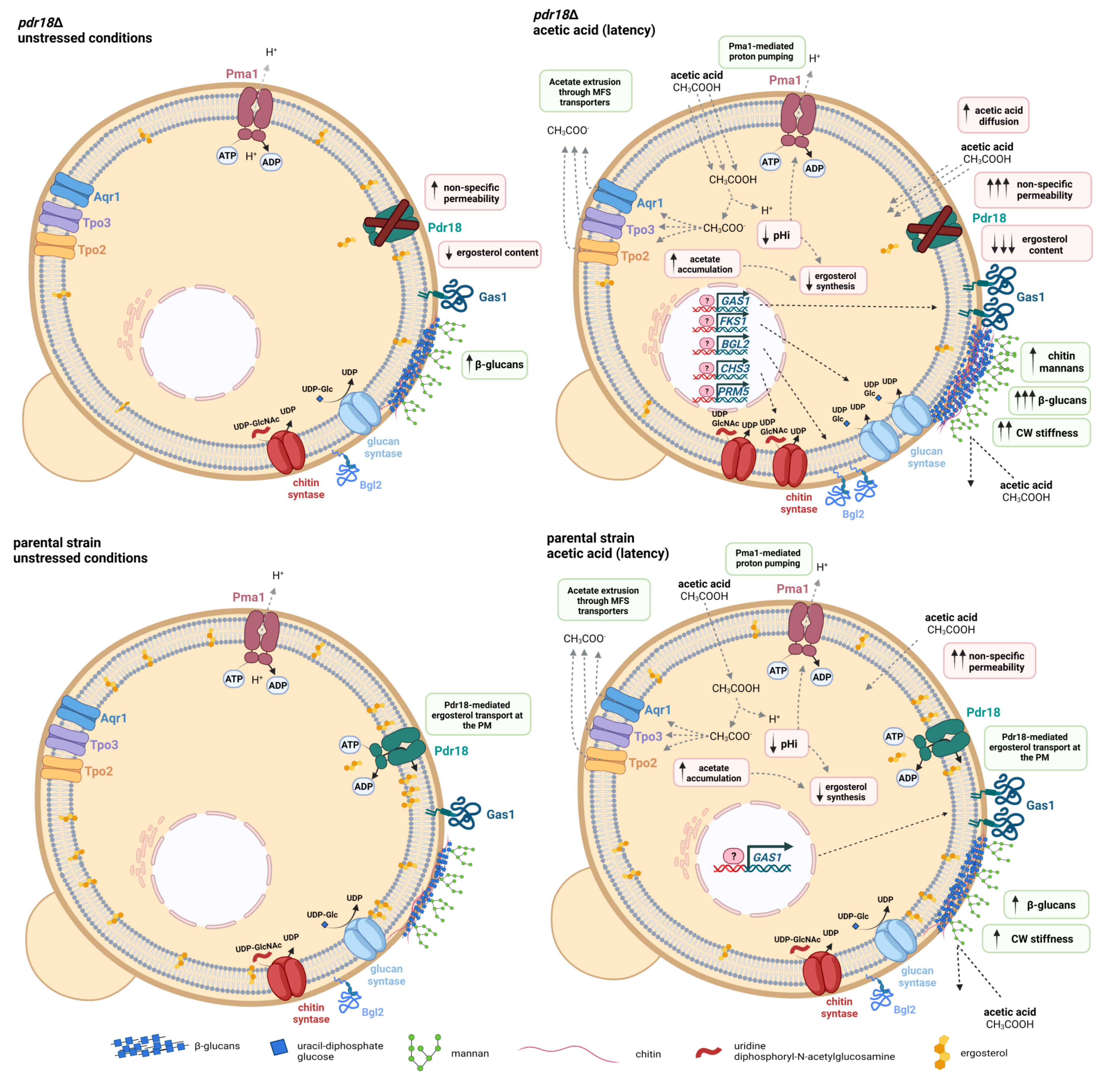
3.1. Expression of PDR18 Is Required to Counteract the Decrease of Ergosterol Content and to Reduce the Time for Intracellular pH (pHi) Recovery following Acidification Induced by Acetic Acid Stress
3.2. Expression of PDR18 Is Required for Maximum Resistance of Yeast Cells to Lyticase Activity Induced under Acetic Acid Stress
3.3. The Stiffness of pdr18Δ Cell Wall Increases during Acetic-Acid-Induced Latency
3.4. Transcriptional Profiles of Cell Wall Biosynthetic Genes in pdr18Δ Cells’ Response to Acetic Acid Stress
3.5. The Content of the Major Cell Wall Polysaccharides Increases in pdr18Δ Cells during Acetic Acid-Induced Latency
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palma, M.; Sá-Correia, I. Physiological Genomics of the Highly Weak-Acid-Tolerant Food Spoilage Yeasts of Zygosaccharomyces bailii sensu lato. In Progress in Molecular and Subcellular Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 58, pp. 85–109. ISBN 9783030130350. [Google Scholar]
- Palma, M.; Guerreiro, J.F.; Sá-Correia, I. Adaptive Response and Tolerance to Acetic Acid in Saccharomyces cerevisiae and Zygosaccharomyces bailii: A Physiological Genomics Perspective. Front. Microbiol. 2018, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.T.; Romaní, A.; Costa, C.E.; Sá-Correia, I.; Domingues, L. Molecular and physiological basis of Saccharomyces cerevisiae tolerance to adverse lignocellulose-based process conditions. Appl. Microbiol. Biotechnol. 2019, 103, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. Dissociation constants of organic acids and bases. In CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2003; pp. 8–46. [Google Scholar]
- Casal, M.; Cardoso, H.; Leão, C. Mechanisms regulating the transport of acetic acid in Saccharomyces cerevisiae. Microbiology 1996, 142, 1385–1390. [Google Scholar] [CrossRef]
- Kawahata, M.; Masaki, K.; Fujii, T.; Iefuji, H. Yeast genes involved in response to lactic acid and acetic acid: Acidic conditions caused by the organic acids in Saccharomyces cerevisiae cultures induce expression of intracellular metal metabolism genes regulated by Aft1p. FEMS Yeast Res. 2006, 6, 924–936. [Google Scholar] [CrossRef]
- Almeida, B.; Ohlmeier, S.; Almeida, A.J.; Madeo, F.; Leão, C.; Rodrigues, F.; Ludovico, P. Yeast protein expression profile during acetic acid-induced apoptosis indicates causal involvement of the TOR pathway. Proteomics 2009, 9, 720–732. [Google Scholar] [CrossRef]
- Mira, N.P.; Palma, M.; Guerreiro, J.F.; Sá-Correia, I. Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb. Cell Fact. 2010, 9, 79. [Google Scholar] [CrossRef]
- Abbott, D.A.; Knijnenburg, T.A.; De Poorter, L.M.I.; Reinders, M.J.T.; Pronk, J.T.; Van Maris, A.J.A. Generic and specific transcriptional responses to different weak organic acids in anaerobic chemostat cultures of Saccharomyces cerevisiae. FEMS Yeast Res. 2007, 7, 819–833. [Google Scholar] [CrossRef][Green Version]
- Li, B.Z.; Yuan, Y.J. Transcriptome shifts in response to furfural and acetic acid in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 86, 1915–1924. [Google Scholar] [CrossRef]
- Mira, N.P.; Becker, J.D.; Sá-Correia, I. Genomic Expression Program Involving the Haa1p-Regulon in Saccharomyces cerevisiae Response to Acetic Acid. Omi. A J. Integr. Biol. 2010, 14, 587–601. [Google Scholar] [CrossRef]
- Bajwa, P.K.; Ho, C.Y.; Chan, C.K.; Martin, V.J.J.; Trevors, J.T.; Lee, H. Transcriptional profiling of Saccharomyces cerevisiae T2 cells upon exposure to hardwood spent sulphite liquor: Comparison to acetic acid, furfural and hydroxymethylfurfural. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2013, 103, 1281–1295. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, J.; Fan, L.; Chen, Q. RNA-Seq-based transcriptomic and metabolomic analysis reveal stress responses and programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 42659. [Google Scholar] [CrossRef]
- Antunes, M.; Palma, M.; Sá-Correia, I. Transcriptional profiling of Zygosaccharomyces bailii early response to acetic acid or copper stress mediated by ZbHaa1. Sci. Rep. 2018, 8, 14122. [Google Scholar] [CrossRef]
- Longo, V.; Ždralević, M.; Guaragnella, N.; Giannattasio, S.; Zolla, L.; Timperio, A.M. Proteome and metabolome profiling of wild-type and YCA1-knock-out yeast cells during acetic acid-induced programmed cell death. J. Proteomics 2015, 128, 173–188. [Google Scholar] [CrossRef]
- Godinho, C.P.; Prata, C.S.; Pinto, S.N.; Cardoso, C.; Bandarra, N.M.; Fernandes, F.; Sá-Correia, I. Pdr18 is involved in yeast response to acetic acid stress counteracting the decrease of plasma membrane ergosterol content and order. Sci. Rep. 2018, 8, 7860. [Google Scholar] [CrossRef]
- Lindberg, L.; Santos, A.X.S.; Riezman, H.; Olsson, L.; Bettiga, M. Lipidomic Profiling of Saccharomyces cerevisiae and Zygosaccharomyces bailii Reveals Critical Changes in Lipid Composition in Response to Acetic Acid Stress. PLoS ONE 2013, 8, e73936. [Google Scholar] [CrossRef]
- Lindahl, L.; Genheden, S.; Eriksson, L.A.; Olsson, L.; Bettiga, M. Sphingolipids contribute to acetic acid resistance in Zygosaccharomyces bailii. Biotechnol. Bioeng. 2016, 113, 744–753. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Vitorino, M.V.; Godinho, C.P.; Bourbon-Melo, N.; Robalo, T.T.; Fernandes, F.; Rodrigues, M.S.; Sá-Correia, I. Yeast adaptive response to acetic acid stress involves structural alterations and increased stiffness of the cell wall. Sci. Rep. 2021, 11, 12652. [Google Scholar] [CrossRef]
- Francois, J.M. Cell Surface Interference with Plasma Membrane and Transport Processes in Yeasts. Adv. Exp. Med. Biol. 2016, 892, 11–31. [Google Scholar]
- Schuberth, C.; Wedlich-Söldner, R. Building a patchwork—The yeast plasma membrane as model to study lateral domain formation. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 767–774. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef]
- Dague, E.; Bitar, R.; Ranchon, H.; Durand, F.; Yken, H.M.; François, J.M. An atomic force microscopy analysis of yeast mutants defective in cell wall architecture. Yeast 2010, 27, 673–684. [Google Scholar] [CrossRef]
- Schiavone, M.; Formosa-Dague, C.; Elsztein, C.; Teste, M.-A.; Martin-Yken, H.; De Morais, M.; Dague, E.; François, J. An Atomic Force Microscopy study of yeast response to ethanol stress: Evidence for a role of the plasma membrane in the nanomechanical properties of the cell walls. Appl. Environ. Microbiol. 2016, 82, 4789–4801. [Google Scholar] [CrossRef]
- Pillet, F.; Lemonier, S.; Schiavone, M.; Formosa, C.; Martin-Yken, H.; Francois, J.M.; Dague, E. Uncovering by Atomic Force Microscopy of an original circular structure at the yeast cell surface in response to heat shock. BMC Biol. 2014, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef] [PubMed]
- Eisenkolb, M.; Zenzmaier, C.; Leitner, E.; Schneiter, R. A specific structural requirement for ergosterol in long-chain fatty acid synthesis mutants important for maintaining raft domains in yeast. Mol. Biol. Cell 2002, 13, 4414–4428. [Google Scholar] [CrossRef] [PubMed]
- Parks, L.W.; Casey, W.M. Physiological Implications of Sterol Biosynthesis in Yeast. Annu. Rev. Microbiol. 1995, 49, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Dickey, A.N.; Yim, W.-S.; Faller, R. Using ergosterol to mitigate the deleterious effects of ethanol on bilayer structure. J. Phys. Chem. B 2009, 113, 2388–2397. [Google Scholar] [CrossRef]
- Muñiz, M.; Zurzolo, C. Sorting of GPI-anchored proteins from yeast to mammals—Common pathways at different sites? J. Cell Sci. 2014, 127, 2793–2801. [Google Scholar] [CrossRef]
- Saha, S.; Anilkumar, A.A.; Mayor, S. GPI-anchored protein organization and dynamics at the cell surface. J. Lipid Res. 2016, 57, 159–175. [Google Scholar] [CrossRef]
- Zurzolo, C.; Simons, K. Glycosylphosphatidylinositol-anchored proteins: Membrane organization and transport. Biochim. Biophys. Acta Biomembr. 2016, 1858, 632–639. [Google Scholar] [CrossRef]
- Sesana, S.; Re, F.; Bulbarelli, A.; Salerno, D.; Cazzaniga, E.; Masserini, M. Membrane features and activity of GPI-anchored enzymes: Alkaline phosphatase reconstituted in model membranes. Biochemistry 2008, 47, 5433–5440. [Google Scholar] [CrossRef]
- Sharma, P.; Varma, R.; Sarasij, R.C.; Ira; Gousset, K.; Krishnamoorthy, G.; Rao, M.; Mayor, S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 2004, 116, 577–589. [Google Scholar] [CrossRef]
- Kock, C.; Arlt, H.; Ungermann, C.; Heinisch, J.J. Yeast cell wall integrity sensors form specific plasma membrane microdomains important for signalling. Cell. Microbiol. 2016, 18, 1251–1267. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef]
- Morimoto, Y.; Tani, M. Synthesis of mannosylinositol phosphorylceramides is involved in maintenance of cell integrity of yeast Saccharomyces cerevisiae. Mol. Microbiol. 2015, 95, 706–722. [Google Scholar] [CrossRef]
- Caro, L.H.P.; Tettelin, H.; Vossen, J.H.; Ram, A.F.J.; Van Den Ende, H.; Klis, F.M. In Silicio identification of glycosyl-phosphatidylinositol-anchored plansma-membrane and cell wall proteins of Saccharomyces cerevisiae. Yeast 1997, 15, 1477–1489. [Google Scholar] [CrossRef]
- Sá-Correia, I.; Godinho, C.P. Exploring the biological function of efflux pumps for the development of superior industrial yeasts. Curr. Opin. Biotechnol. 2022, 74, 32–41. [Google Scholar] [CrossRef]
- Dos Santos, S.C.; Teixeira, M.C.; Dias, P.J.; Sá-Correia, I. MFS transporters required for multidrug/multixenobiotic (MD/MX) resistance in the model yeast: Understanding their physiological function through post-genomic approaches. Front. Physiol. 2014, 5, 180. [Google Scholar] [CrossRef]
- Buechel, E.R.; Pinkett, H.W. Transcription factors and ABC transporters: From pleiotropic drug resistance to cellular signaling in yeast. FEBS Lett. 2020, 594, 3943–3964. [Google Scholar] [CrossRef]
- Ullah, A.; Orij, R.; Brul, S.; Smits, G.J. Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2012, 78, 8377–8387. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, A.; Łukaszewicz, M.; Bartosiewicz, D.; Sigler, K. Cell ATP level of Saccharomyces cerevisiae sensitively responds to culture growth and drug-inflicted variations in membrane integrity and PDR pump activity. Biochem. Biophys. Res. Commun. 2010, 395, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Godinho, C.P.; Cabrito, T.R.; Mira, N.P.; Sá-Correia, I. Increased expression of the yeast multidrug resistance ABC transporter Pdr18 leads to increased ethanol tolerance and ethanol production in high gravity alcoholic fermentation. Microb. Cell Fact. 2012, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Cabrito, T.R.; Teixeira, M.C.; Singh, A.; Prasad, R.; Sá-Correia, I. The yeast ABC transporter Pdr18 (ORF YNR070w) controls plasma membrane sterol composition, playing a role in multidrug resistance. Biochem. J. 2011, 440, 195–202. [Google Scholar] [CrossRef]
- Godinho, C.P.; Costa, R.; Sá-Correia, I. The ABC transporter Pdr18 is required for yeast thermotolerance due to its role in ergosterol transport and plasma membrane properties. Environ. Microbiol. 2021, 23, 69–80. [Google Scholar] [CrossRef]
- Godinho, C.P.; Dias, P.J.; Ponçot, E.; Sá-Correia, I. The Paralogous Genes PDR18 and SNQ2, Encoding Multidrug Resistance ABC Transporters, Derive From a Recent Duplication Event, PDR18 Being Specific to the Saccharomyces Genus. Front. Genet. 2018, 9, 476. [Google Scholar] [CrossRef]
- Demuyser, L.; Van Dyck, K.; Timmermans, B.; Van Dijck, P. Inhibition of Vesicular Transport Influences Fungal Susceptibility to Fluconazole. Antimicrob. Agents Chemother. 2019, 63, e01998-18. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Lee-Yang, W.; Ciblak, M.A.; Frade, J.P.; Brandt, M.E.; Hajjeh, R.A.; Harrison, L.H.; Sofair, A.N.; Warnock, D.W. Comparison of Visual and Spectrophotometric Methods of Broth Microdilution MIC End Point Determination and Evaluation of a Sterol Quantitation Method for in Vitro Susceptibility Testing of Fluconazole and Itraconazole Against Trailing and Nontrailing Cand. Antimicrob. Agents Chemother. 2002, 46, 2477–2481. [Google Scholar] [CrossRef]
- Simões, T.; Mira, N.P.; Fernandes, A.R.; Sá-Correia, I. The SPI1 gene, encoding a glycosylphosphatidylinositol-anchored cell wall protein, plays a prominent role in the development of yeast resistance to lipophilic weak-acid food preservatives. Appl. Environ. Microbiol. 2006, 72, 7168–7175. [Google Scholar] [CrossRef]
- Sader, J.E.; Borgani, R.; Gibson, C.T.; Haviland, D.B.; Higgins, M.J.; Kilpatrick, J.I.; Lu, J.; Mulvaney, P.; Shearer, C.J.; Slattery, A.D.; et al. A virtual instrument to standardise the calibration of atomic force microscope cantilevers. Rev. Sci. Instrum. 2016, 87, 93711. [Google Scholar] [CrossRef]
- Collart, M.A.; Oliviero, S. Preparation of Yeast RNA. In Current Protocols in Molecular Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1993. [Google Scholar] [CrossRef]
- Pradhan, A.; Avelar, G.M.; Bain, J.M.; Childers, D.S.; Larcombe, D.E.; Netea, M.G.; Shekhova, E.; Munro, C.A.; Brown, G.D.; Erwig, L.P.; et al. Hypoxia Promotes Immune Evasion by Triggering β-Glucan Masking on the Candida albicans Cell Surface via Mitochondrial and cAMP-Protein Kinase A Signaling. MBio 2018, 9, e01318-18. [Google Scholar] [CrossRef] [PubMed]
- Narendranath, N.V.; Thomas, K.C.; Michael, W.; Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Acetic Acid and Lactic Acid Inhibition of Growth of Saccharomyces cerevisiae by Different Mechanisms. J. Am. Soc. Brew. Chem. 2001, 59, 187–194. [Google Scholar]
- Ramos, S.; Balbín, M.; Raposo, M.; Valle, E.; Pardo, L.A. The mechanism of intracellular acidification induced by glucose in Saccharomyces cerevisiae. J. Gen. Microbiol. 1989, 135, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, S.; Fernández-Niño, M.; González-Ramos, D.; van Maris, A.J.A.; Nevoigt, E. The fraction of cells that resume growth after acetic acid addition is a strain-dependent parameter of acetic acid tolerance in Saccharomyces cerevisiae. FEMS Yeast Res. 2014, 14, 642–653. [Google Scholar] [CrossRef]
- Gohlke, S.; Muthukrishnan, S.; Merzendorfer, H. In Vitro and In Vivo studies on the structural organization of Chs3 from Saccharomyces cerevisiae. Int. J. Mol. Sci. 2017, 18, 702. [Google Scholar] [CrossRef]
- Douglas, C.M. Fungal β(1,3)-D-glucan synthesis. Med. Mycol. Suppl. 2001, 39, 55–66. [Google Scholar] [CrossRef]
- Jung, U.S.; Sobering, A.K.; Romeo, M.J.; Levin, D.E. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 2002, 46, 781–789. [Google Scholar] [CrossRef]
- Ram, A.F.J.; Kapteyn, J.C.; Montijn, R.C.; Caro, L.H.P.; Douwes, J.E.; Baginsky, W.; Mazur, P.; Van Den Ende, H.; Klis, F.M. Loss of the plasma membrane-bound protein Gas1p in Saccharomyces cerevisiae results in the release of β1,3-glucan into the medium and induces a compensation mechanism to ensure cell wall integrity. J. Bacteriol. 1998, 180, 1418–1424. [Google Scholar] [CrossRef]
- Aimanianda, V.; Simenel, C.; Garnaud, C.; Clavaud, C.; Tada, R.; Barbin, L.; Mouyna, I.; Heddergott, C.; Popolo, L.; Ohya, Y.; et al. The dual activity responsible for the elongation and branching of β-(1,3)- glucan in the fungal cell wall. MBio 2017, 8, e00619-17. [Google Scholar] [CrossRef]
- Blanco, N.; Sanz, A.B.; Rodríguez-Peña, J.M.; Nombela, C.; Farkaš, V.; Hurtado-Guerrero, R.; Arroyo, J. Structural and functional analysis of yeast Crh1 and Crh2 transglycosylases. FEBS J. 2015, 282, 715–731. [Google Scholar] [CrossRef]
- Maeda, H.; Ishida, N. Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J. Biochem. 1967, 62, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Chandrasekaran, G.; Brul, S.; Smits, G.J. Yeast adaptation to weak acids prevents futile energy expenditure. Front. Microbiol. 2013, 4, 142. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Tani, M. Mannosylinositol phosphorylceramides and ergosterol coodinately maintain cell wall integrity in the yeast Saccharomyces cerevisiae. FEBS J. 2018, 285, 2405–2427. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Botet, J.; Rodríguez-Peña, J.M.; Bermejo, C.; Ribas, J.C.; Revuelta, J.L.; Nombela, C.; Arroyo, J. Genomic profiling of fungal cell wall-interfering compounds: Identification of a common gene signature. BMC Genomics 2015, 16, 683. [Google Scholar] [CrossRef]
- Zlotnik, H.; Pilar Fernandez, M.; Bowers, B.; Cabib, E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J. Bacteriol. 1984, 159, 1018–1026. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, Y.; Zhang, X.; Xu, Y.; Dai, H.; Xiao, W. Deletion of yeast CWP genes enhances cell permeability to genotoxic agents. Toxicol. Sci. 2008, 103, 68–76. [Google Scholar] [CrossRef]
- De Nobel, J.G.; Klis, F.M.; Priem, J.; Munnik, T.; Van Den Ende, H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast 1990, 6, 491–499. [Google Scholar] [CrossRef]
- Jigami, Y.; Odani, T. Mannosylphosphate transfer to yeast mannan. Biochim. Biophys. Acta Gen. Subj. 1999, 1426, 335–345. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef]
- Bagnat, M.; Keränen, S.; Shevchenko, A.; Shevchenko, A.; Simons, K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 2000, 97, 3254–3259. [Google Scholar] [CrossRef]
- Coradini, A.L.V.; da Silveira Bezerra de Mello, F.; Furlan, M.; Maneira, C.; Carazzolle, M.F.; Pereira, G.A.G.; Teixeira, G.S. QTL mapping of a Brazilian bioethanol strain links the cell wall protein-encoding gene GAS1 to low pH tolerance in S. cerevisiae. Biotechnol. Biofuels 2021, 14, 239. [Google Scholar] [CrossRef]
- Pinheiro, S.; Pandey, S.; Pelet, S. Cellular heterogeneity: Yeast-side story. Fungal Biol. Rev. 2022, 39, 34–45. [Google Scholar] [CrossRef]
- Holland, S.L.; Reader, T.; Dyer, P.S.; Avery, S. V Phenotypic heterogeneity is a selected trait in natural yeast populations subject to environmental stress. Environ. Microbiol. 2014, 16, 1729–1740. [Google Scholar] [CrossRef]
- Schmidt, C.L.; Grey, M.; Schmidt, M.; Brendel, M.; Henriques, J.A. Allelism of Saccharomyces cerevisiae genes PSO6, involved in survival after 3-CPs+UVA induced damage, and ERG3, encoding the enzyme sterol C-5 desaturase. Yeast 1999, 15, 1503–1510. [Google Scholar] [CrossRef]
- Pfaller, M.; Riley, J. Effects of fluconazole on the sterol and carbohydrate composition of four species of Candida. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 152–156. [Google Scholar] [CrossRef]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
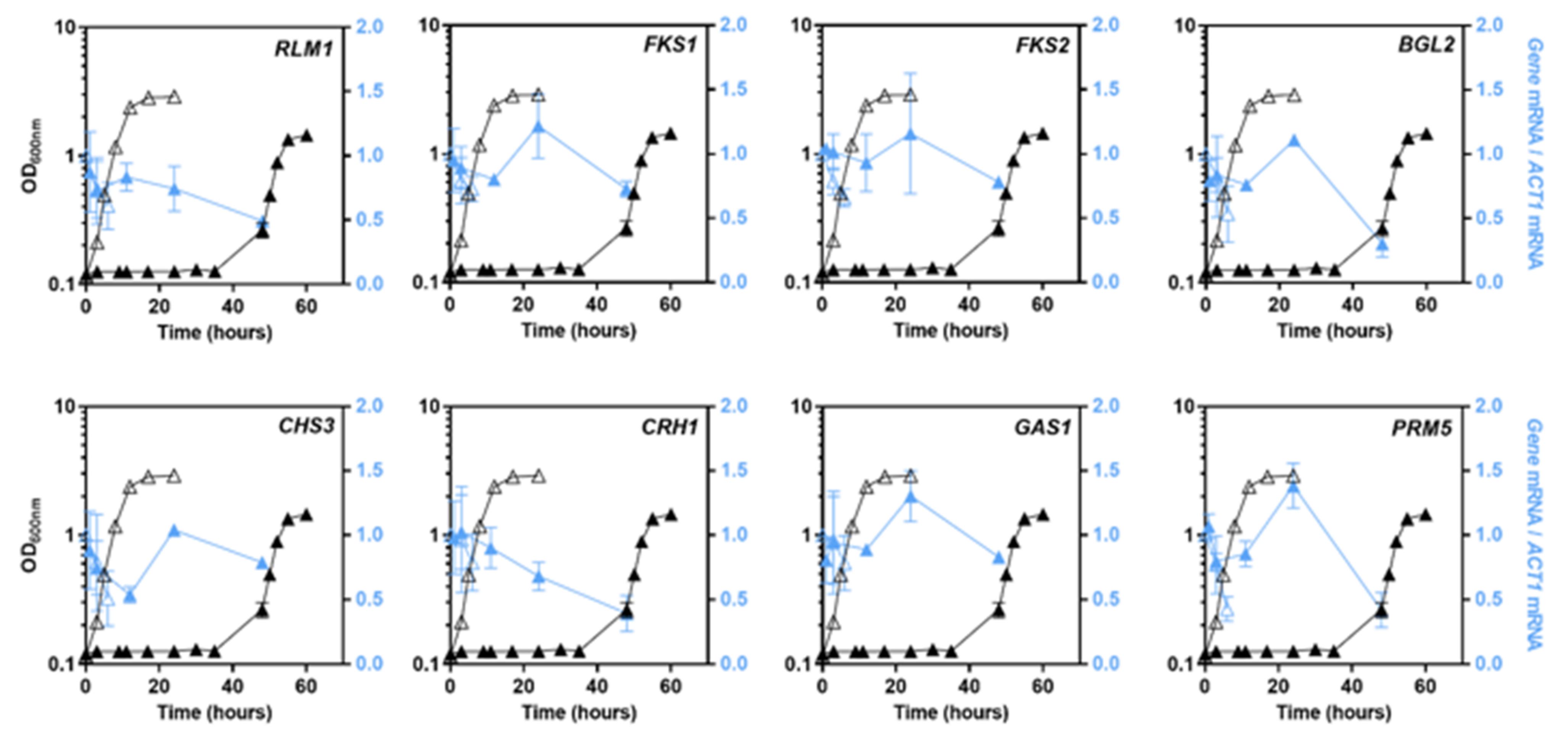
| Gene | Function of the Encoded Protein | Bibliographic References |
|---|---|---|
| CHS3 | Major chitin synthase required for the synthesis of the majority of cell wall chitin | [58] |
| FKS1 | β-1-3-glucan synthase | [59] |
| FKS2 | Fks1 paralog, involved in β-1-3-glucan synthesis | |
| RLM1 | Transcription factor responsible for the transcriptional activation of the majority of genes induced in response to cell wall stress through the CWI pathway | [60] |
| GAS1 | β-1,3-glucanosyltransferase involved in cell wall remodeling-elongation of (1→3)-β-D-glucan chains and branching | [61,62] |
| CRH1 | Chitin transglycosylase involved in the transfer of chitin to β-1-6 and β-1-3 glucans in the cell wall | [63] |
| BGL2 | Endo-beta-1,3-glucanase involved cell wall remodeling necessary for branching of the β-1-3 glucans in the cell wall | [62] |
| PRM5 | Pheromone-regulated protein and an Rlm1 target; a hallmark of CWI pathway activation | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, R.A.; Godinho, C.P.; Vitorino, M.V.; Robalo, T.T.; Fernandes, F.; Rodrigues, M.S.; Sá-Correia, I. Crosstalk between Yeast Cell Plasma Membrane Ergosterol Content and Cell Wall Stiffness under Acetic Acid Stress Involving Pdr18. J. Fungi 2022, 8, 103. https://doi.org/10.3390/jof8020103
Ribeiro RA, Godinho CP, Vitorino MV, Robalo TT, Fernandes F, Rodrigues MS, Sá-Correia I. Crosstalk between Yeast Cell Plasma Membrane Ergosterol Content and Cell Wall Stiffness under Acetic Acid Stress Involving Pdr18. Journal of Fungi. 2022; 8(2):103. https://doi.org/10.3390/jof8020103
Chicago/Turabian StyleRibeiro, Ricardo A., Cláudia P. Godinho, Miguel V. Vitorino, Tiago T. Robalo, Fábio Fernandes, Mário S. Rodrigues, and Isabel Sá-Correia. 2022. "Crosstalk between Yeast Cell Plasma Membrane Ergosterol Content and Cell Wall Stiffness under Acetic Acid Stress Involving Pdr18" Journal of Fungi 8, no. 2: 103. https://doi.org/10.3390/jof8020103
APA StyleRibeiro, R. A., Godinho, C. P., Vitorino, M. V., Robalo, T. T., Fernandes, F., Rodrigues, M. S., & Sá-Correia, I. (2022). Crosstalk between Yeast Cell Plasma Membrane Ergosterol Content and Cell Wall Stiffness under Acetic Acid Stress Involving Pdr18. Journal of Fungi, 8(2), 103. https://doi.org/10.3390/jof8020103







