Cell Immobilization for Erythritol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains
2.2. Description of Grape Must
2.3. Free-Cell Preliminary Tests in Flask Experiments
2.4. Free-Cell Batch Fermentation in the Bioreactor
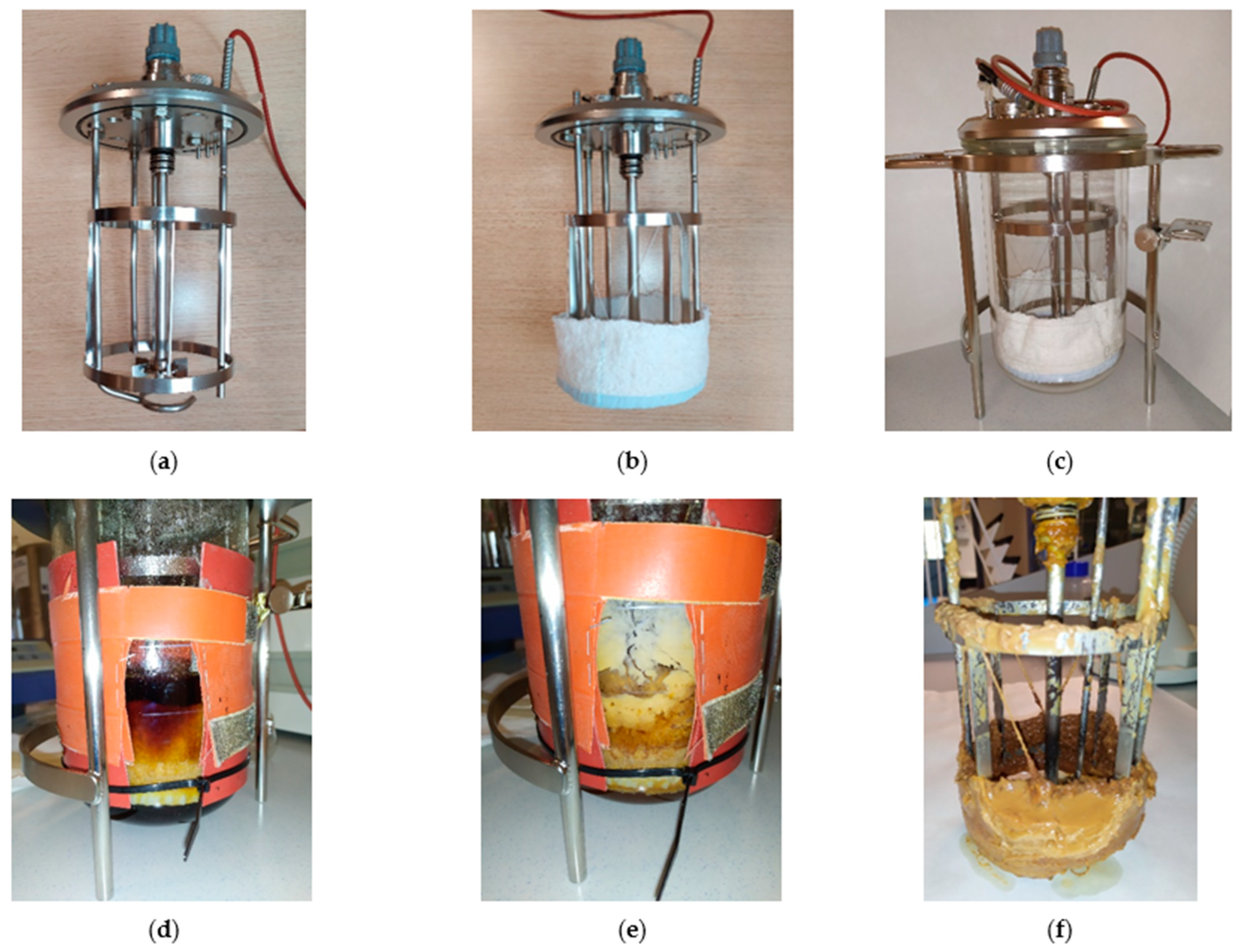
2.5. Cell Immobilization: Repeated Batch Fermentation in the Bioreactor
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results and Discussion
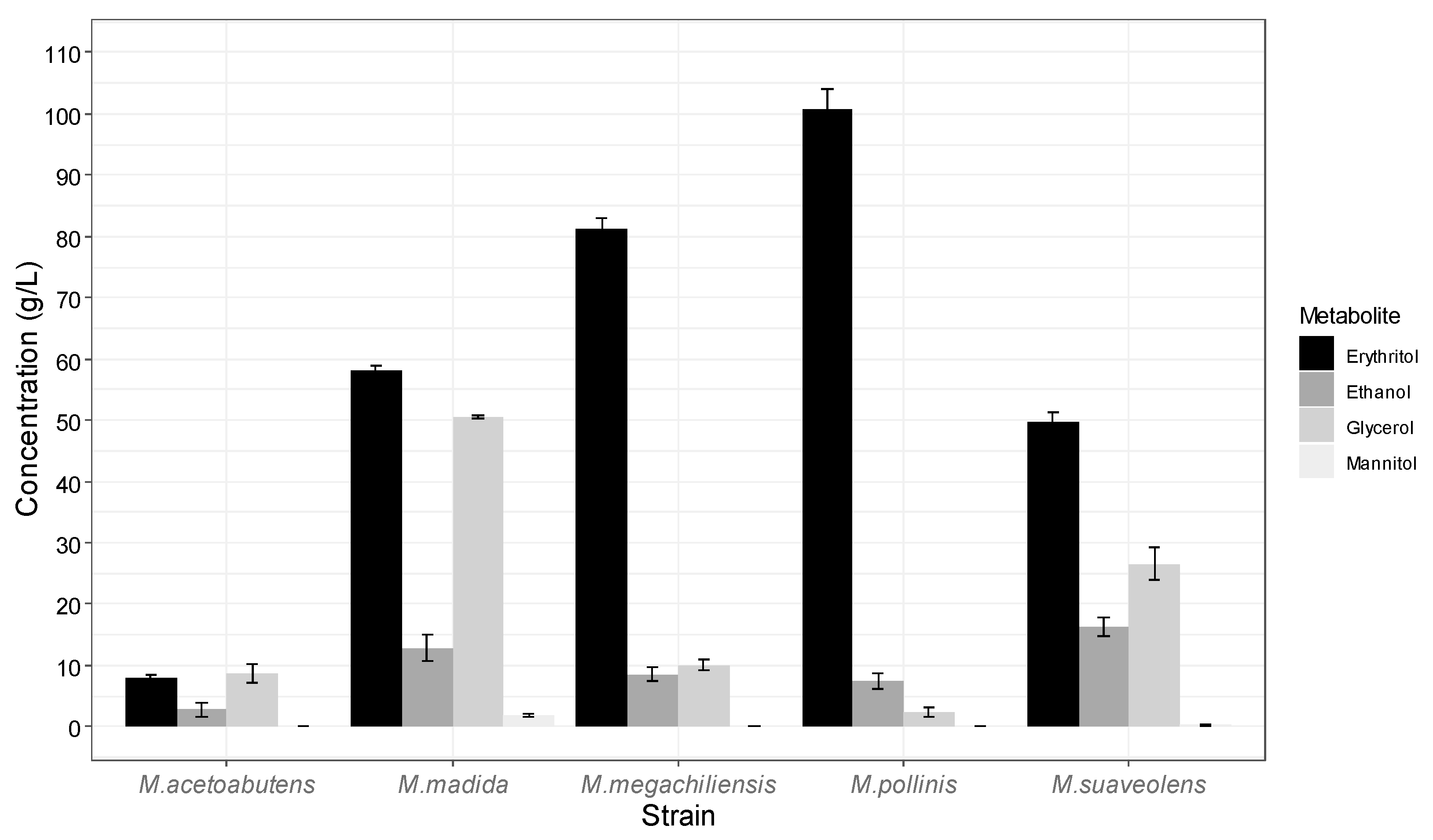
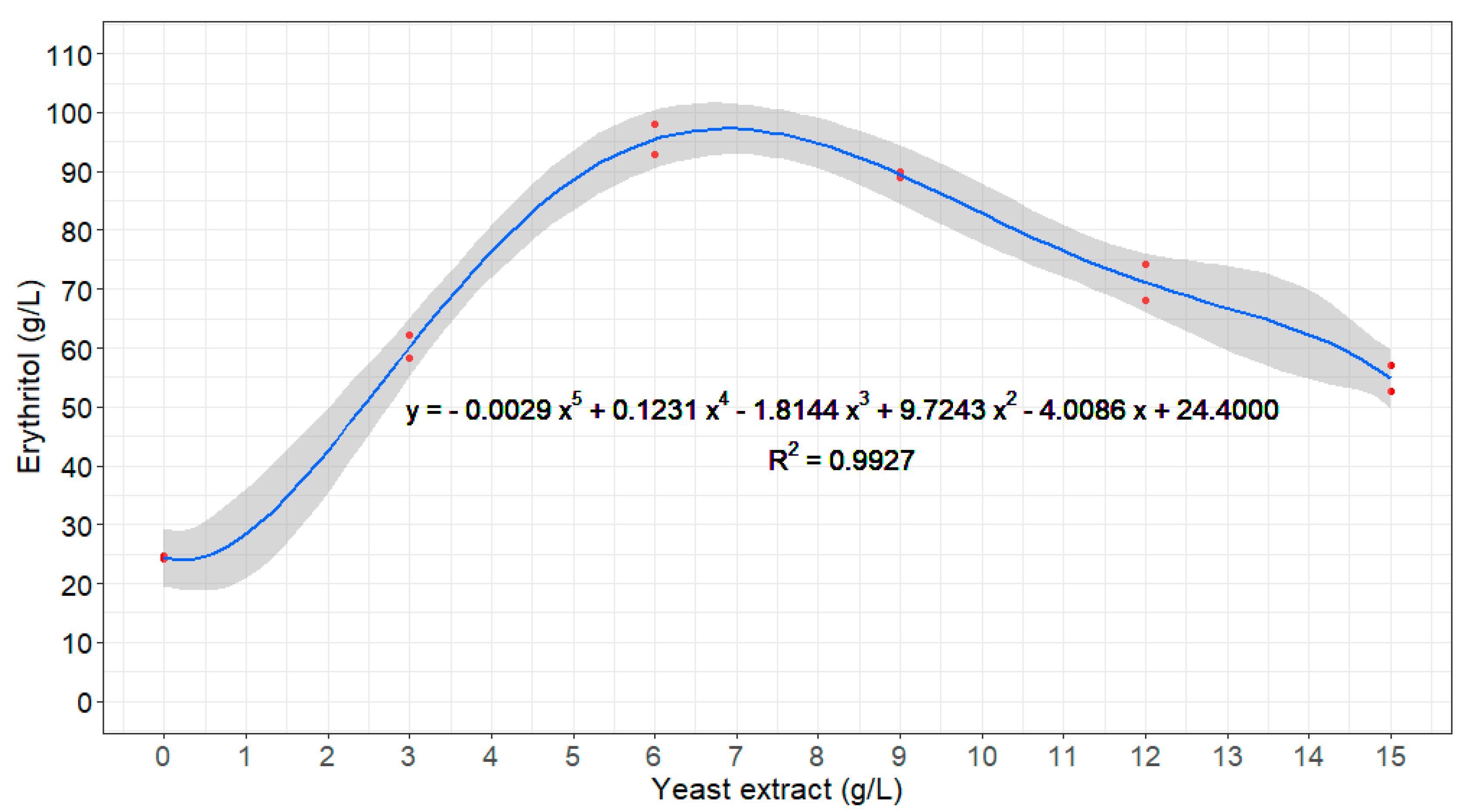
3.1. Optimization of Strain and Nutrient Conditions in Flask Experiments: Free-Cell Fermentation
3.2. Free-Cell Fermentation in the Bioreactor
3.3. Cell Immobilization in the Reactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moon, H.-J.; Jeya, M.; Kim, I.-W.; Lee, J.-K. Biotechnological production of erythritol and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Coman, V.; Vodnar, D.C. Recent advances in the biotechnological production of erythritol and mannitol. Crit. Rev. Biotechnol. 2020, 40, 608–622. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). Erythritol. GRAS Notice 789. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=789&sort=GRN_No&order=DESC&startrow=1&type=basic&search=erythritol (accessed on 17 December 2021).
- EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific Opinion on the safety of the proposed extension of use of erythritol (E 968) as a food additive. EFSA J. 2015, 13, 4033. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Kasumi, T.; Ogihara, J.; Tamura, M.; Arai, T.; Tomishige, K. Erythritol: Another C4 platform chemical in biomass refinery. ACS Omega 2020, 5, 2520–2530. [Google Scholar] [CrossRef]
- Daza-Serna, L.; Serna-Loaiza, S.; Masi, A.; Mach, R.L.; Mach-Aigner, A.S.; Friedl, A. From the culture broth to the erythritol crystals: An opportunity for circular economy. Appl. Microbiol. Biotechnol. 2021, 105, 4467–4486. [Google Scholar] [CrossRef] [PubMed]
- Hajny, G.J.; Smith, J.H.; Garver, J.C. Erythritol production by a yeastlike fungus. Appl. Microbiol. 1964, 12, 240–246. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mironczuk, A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; Wang, Z.; Xia, J.; Yan, Y.; Hu, L.; Wang, X.; Xu, J.; He, A.; Zhao, P. Enhanced erythritol production by a Snf1-deficient Yarrowia lipolytica strain under nitrogen-enriched fermentation condition. Food Bioprod. Process. 2020, 119, 306–316. [Google Scholar] [CrossRef]
- Teleky, B.E.; Martău, G.A.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Biofunctional soy-based sourdough for improved rheological properties during storage. Sci. Rep. 2022, 12, 17535. [Google Scholar] [CrossRef]
- Veiga-da-Cunha, M.; Santos, H.; Van Schaftingen, E. Pathway and regulation of erythritol formation in Leuconostoc oenos. J. Bacteriol. 1993, 175, 3941–3948. [Google Scholar] [CrossRef]
- Cho, H.; Yamagishi, K.; Mikawa, T.; Mitsubishi Chemical Corporation. Erythritol-producing Microorganism and Process for Producing the Same. EUR Patent EP0922757A1, 16 June 1999. [Google Scholar]
- Cho, H.; Yamagishi, K.; Abe, S.; Morioka, S. Mitsubishi Chemical Corporation. Method of producing erythritol. U.S. Patent 5,981,241, 9 November 1999. [Google Scholar]
- Abe, S.; Morioka, S. Mitsubishi Chemical Corporation. Method of producing erythritol. U.S. Patent 5,902,739, 11 May 1999. [Google Scholar]
- Commission Regulation (EU) No 231/2012 of 9 March 2012 Laying Down Specifications for Food Additives Listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32012R0231 (accessed on 8 April 2022).
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. By-products of sugar factories and wineries as feedstocks for erythritol generation. Food Bioprod. Process. 2021, 126, 345–355. [Google Scholar] [CrossRef]
- Qiu, X.; Gu, Y.; Du, G.; Zhang, J.; Xu, P.; Li, J. Conferring thermotolerant phenotype to wild type Yarrowia lipolytica improves cell growth and erythritol production. Biotechnol. Bioeng. 2021, 118, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Seshadrinathan, S.; Chakraborty, S. Fermentative production of erythritol from cane molasses using Candida magnoliae: Media optimization, purification, and characterization. Sustainability 2022, 14, 10342. [Google Scholar] [CrossRef]
- Tomaszewska-Hetman, L.; Rymowicz, W.; Rywińska, A. Waste conversion into a sweetener—Development of an innovative strategy for erythritol production by Yarrowia lipolytica. Sustainability 2020, 12, 7122. [Google Scholar] [CrossRef]
- Zhang, L.; Nie, M.-Y.; Liu, F.; Chen, J.; Wei, L.-J.; Hua, Q. Multiple gene integration to promote erythritol production on glycerol in Yarrowia lipolytica. Biotechnol. Lett. 2021, 43, 1277–1287. [Google Scholar] [CrossRef]
- Deshpande, M.S.; Kulkarni, P.P.; Kumbhar, P.S.; Ghosalkar, A.R. Erythritol production from sugar based feedstocks by Moniliella pollinis using lysate of recycled cells as nutrients source. Process Biochem. 2022, 112, 45–52. [Google Scholar] [CrossRef]
- Liu, X.; Dong, X.; Chen, S.; Yan, Y.; He, J.; Xu, J.; Xu, J. Enhancing erythritol production by wheat straw biochar-incorporated solid-state fermentation of agricultural wastes using defatted Schizochytrium sp. biomass as supplementary feedstock. Ind. Crops Prod. 2021, 170, 113703. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; He, A.; Xia, J.; He, J.; Deng, Y.; Xu, N.; Qiu, Z.; Wang, X.; Zhao, P. One-pot fermentation for erythritol production from distillers grains by the co-cultivation of Yarrowia lipolytica and Trichoderma reesei. Bioresour. Technol. 2022, 351, 127053. [Google Scholar] [CrossRef]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef]
- Gao, H.; Lu, J.; Jiang, Y.; Fang, Y.; Tang, Y.; Yu, Z.; Zhang, W.; Xin, F.; Jiang, M. Material-mediated cell immobilization technology in the biological fermentation process. Biofuels Bioprod. Biorefin. 2012, 15, 1160–1173. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Jeya, M.; Lee, K.-M.; Tiwari, M.K.; Kim, J.-S.; Gunasekaran, P.; Kim, S.-Y.; Kim, I.-W.; Lee, J.-K. Isolation of a novel high erythritol-producing Pseudozyma tsukubaensis and scale-up of erythritol fermentation to industrial level. Appl. Microbiol. Biotechnol. 2009, 83, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Corry, J.E.L.; Curtis, G.D.W.; Baird, R.M. (Eds.) Handbook of Culture Media for Food and Water Microbiology, 3rd ed.; RSC Publishing: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Edouard, S.; Pagnier, I.; Mediannikov, O.; Drancourt, M.; Raoult, D. Current and past strategies for bacterial culture in clinical microbiology. Clin. Microbiol. Rev. 2015, 28, 208–236. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R. Assessment of vine shoots and surplus grape must for succinic acid bioproduction. Appl. Microbiol. Biotechnol. 2022, 106, 4977–4994. [Google Scholar] [CrossRef]
- 2022–2029 Global Polyol Sweeteners Professional Market Research Report, Analysis from Perspective of Segmentation (Competitor Landscape, Type, Application and Geography). Maia Research. 2022. Available online: https://www.marketresearch.com/Maia-Research-v4212/Global-Polyol-Sweeteners-Professional-Research-31082221/ (accessed on 19 September 2022).

| Experiment | Yeast Extract (%) | (NH4)2SO4 (%) | Yeast Extract (g/L) | (NH4)2SO4 (g/L) |
|---|---|---|---|---|
| 1 | 100 | 0 | 6.88 | 0 |
| 2 | 80 | 20 | 5.50 | 0.71 |
| 3 | 60 | 40 | 4.13 | 1.43 |
| 4 | 40 | 60 | 2.75 | 2.14 |
| 5 | 20 | 80 | 1.38 | 2.86 |
| 6 | 0 | 100 | 0 | 3.57 |
| Type | CX (× 108 cells/mL) * | CETH (g/L) | CERY (g/L) | CGLY (g/L) | ΔG (%) | ΔF (%) | ΔS (%) | YETH (g/g) | YERY (g/g) | YGLY (g/g) | Evaporation (%, Vi) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Free | 1.18 ± 0.67 | 4.84 ± 3.87 | 41.88 ± 5.18 | 9.16 ± 4.21 | 95.67 ± 3.24 | 88.02 ± 7.73 | 91.72 ± 5.27 | 0.02 ± 0.02 | 0.17 ± 0.01 | 0.04 ± 0.02 | 22.05 ± 5.26 |
| Immobilized | 0.97 ± 0.57 | 10.37 ± 9.38 | 47.03 ± 6.16 | 13.27 ± 1.89 | 96.97 ± 2.60 | 91.54 ± 4.42 | 94.27 ± 3.20 | 0.04 ± 0.04 | 0.18 ± 0.04 | 0.05 ± 0.01 | 25.18 ± 3.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R. Cell Immobilization for Erythritol Production. J. Fungi 2022, 8, 1286. https://doi.org/10.3390/jof8121286
Hijosa-Valsero M, Paniagua-García AI, Díez-Antolínez R. Cell Immobilization for Erythritol Production. Journal of Fungi. 2022; 8(12):1286. https://doi.org/10.3390/jof8121286
Chicago/Turabian StyleHijosa-Valsero, María, Ana I. Paniagua-García, and Rebeca Díez-Antolínez. 2022. "Cell Immobilization for Erythritol Production" Journal of Fungi 8, no. 12: 1286. https://doi.org/10.3390/jof8121286
APA StyleHijosa-Valsero, M., Paniagua-García, A. I., & Díez-Antolínez, R. (2022). Cell Immobilization for Erythritol Production. Journal of Fungi, 8(12), 1286. https://doi.org/10.3390/jof8121286






