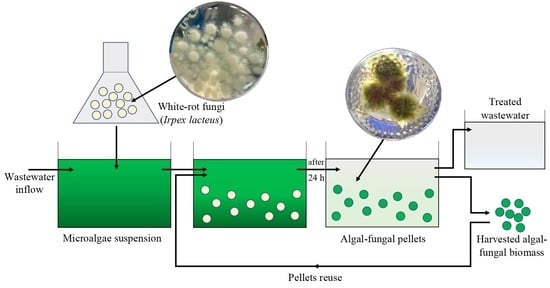
Microalgae Harvesting after Tertiary Wastewater Treatment with White-Rot Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Wastewater Source
2.3. Experimental Setup
2.4. Experimental Harvesting Conditions
2.5. Microalgal Cell Measurements
2.6. Statistical Analysis
3. Results & Discussion
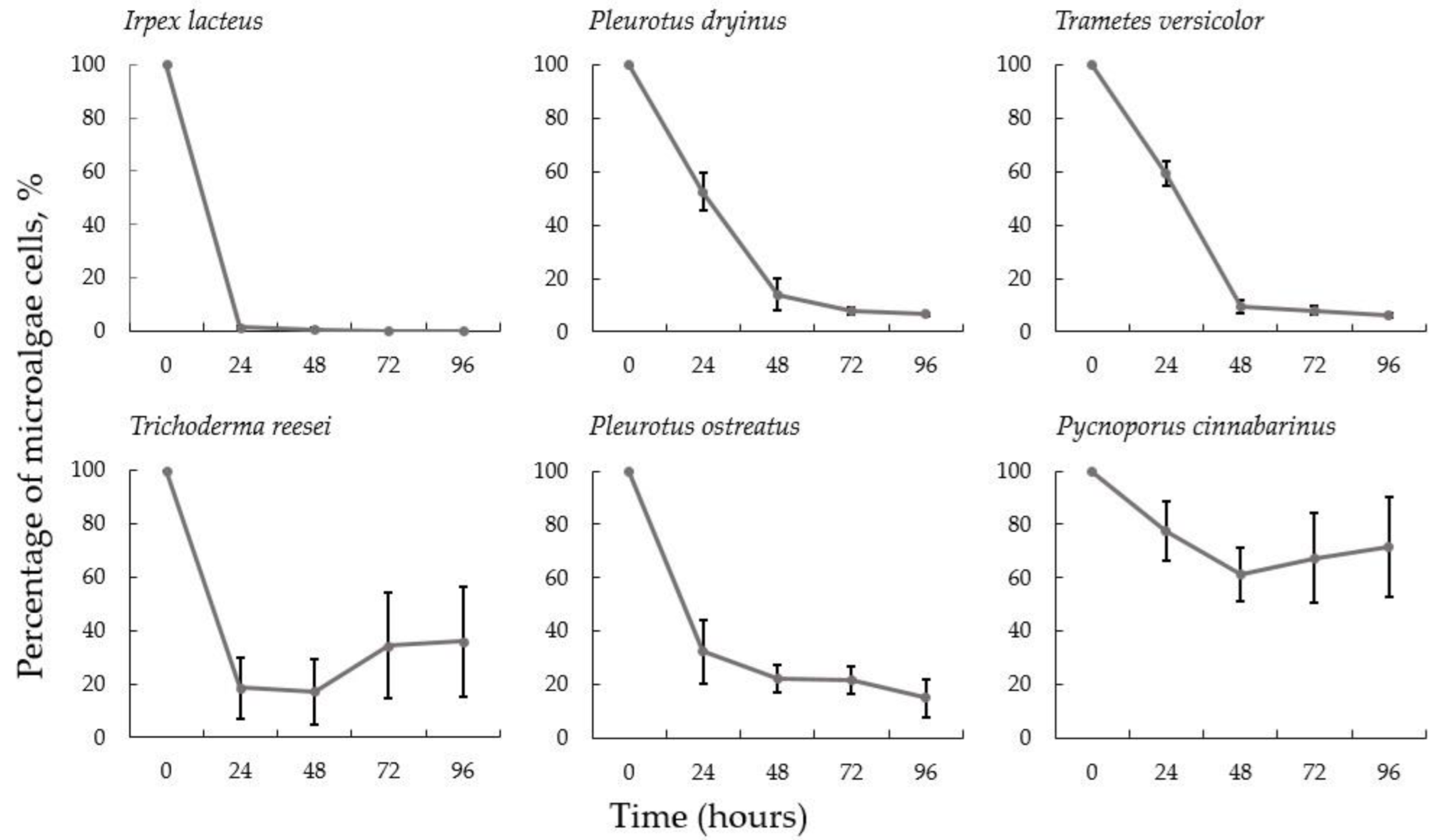
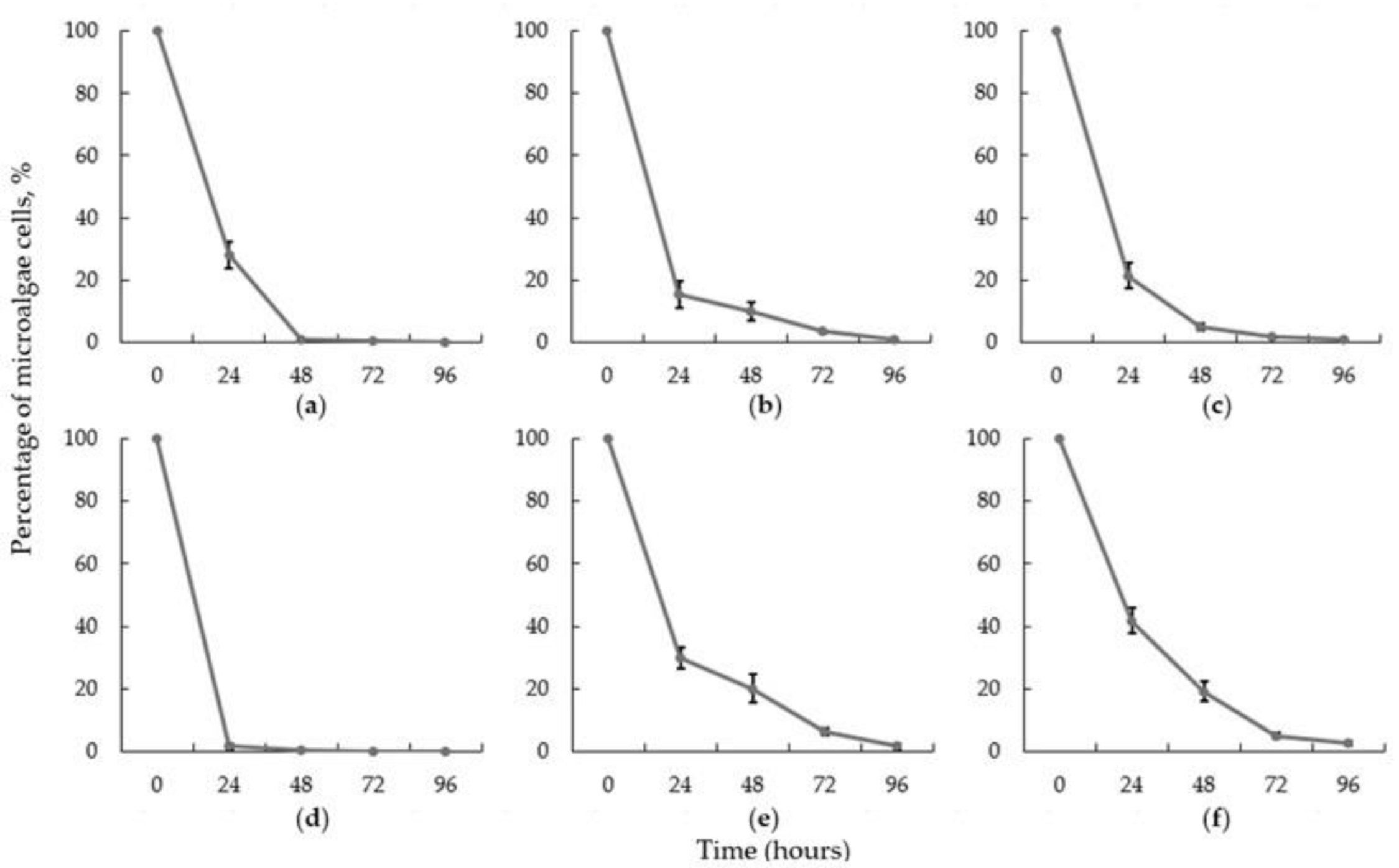
3.1. Microalgae Harvesting with Various Fungal Species
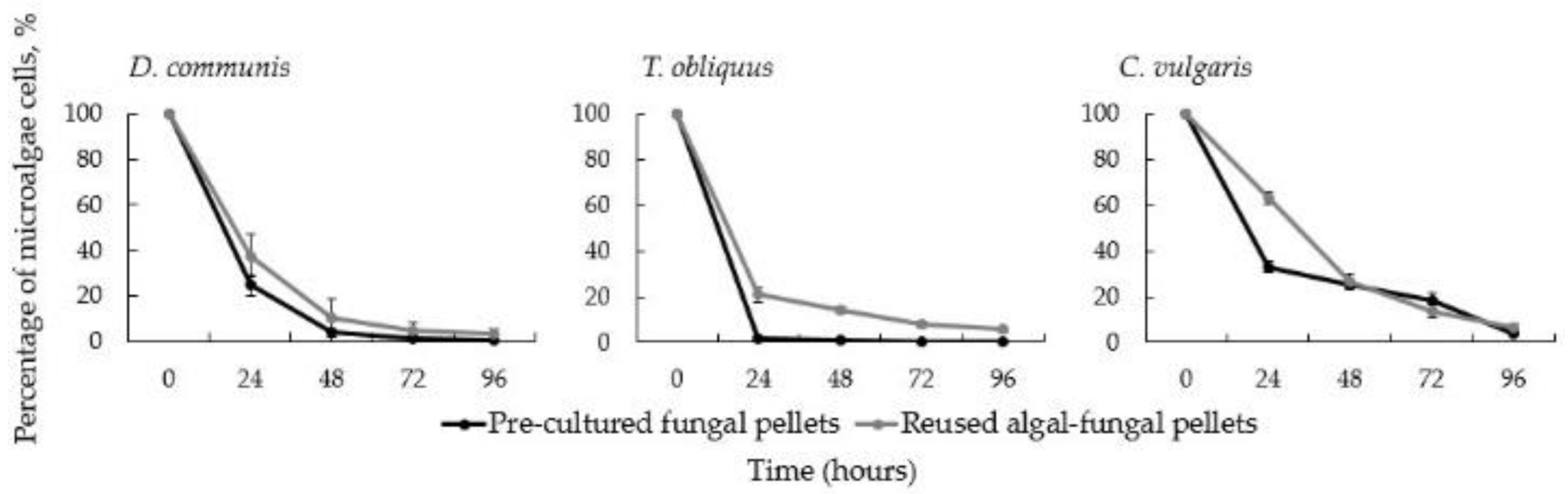
3.2. Effect of the Reuse of Algal-Fungal Pellets
3.3. Impact of Bio-Flocculation Conditions
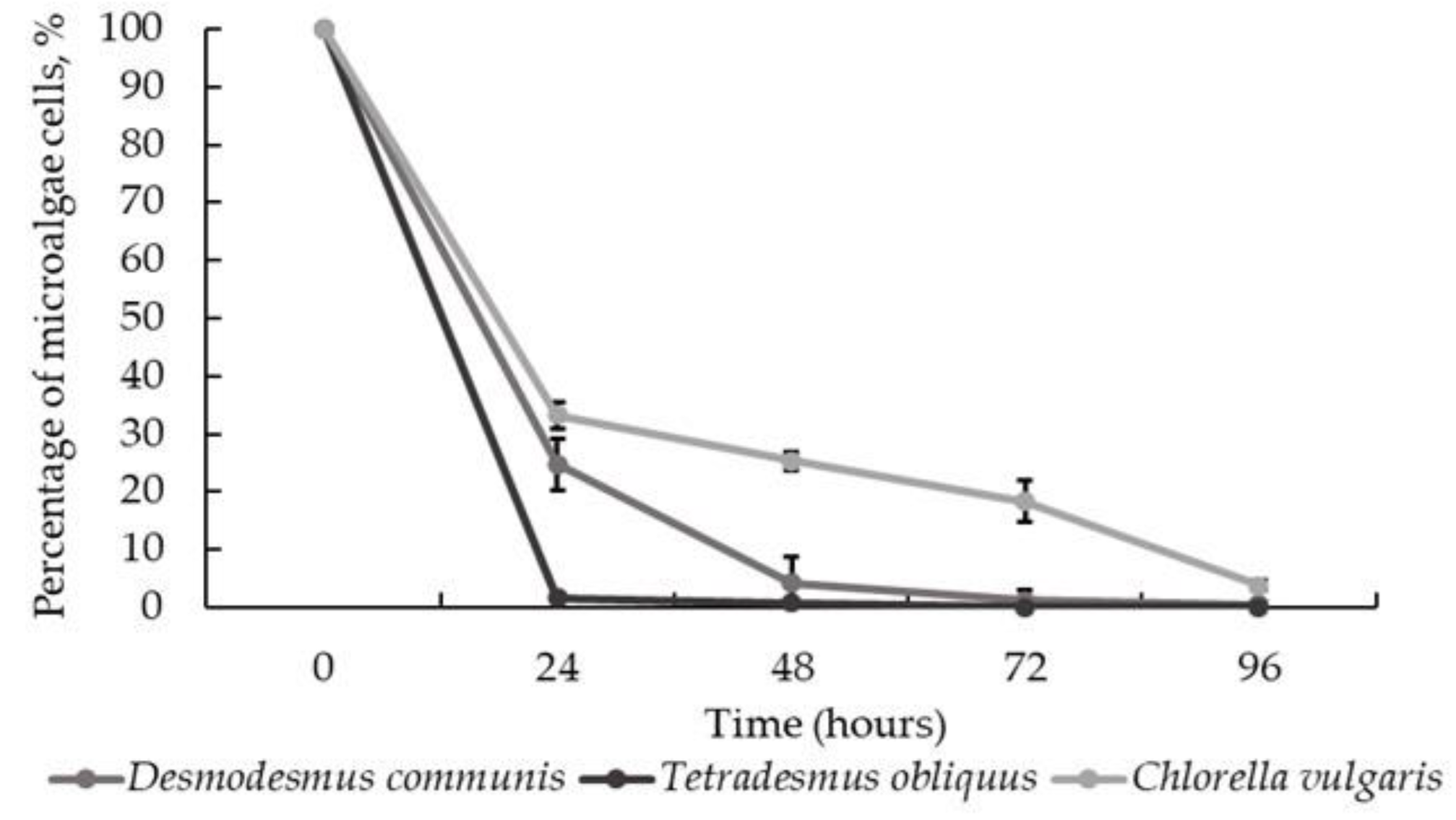
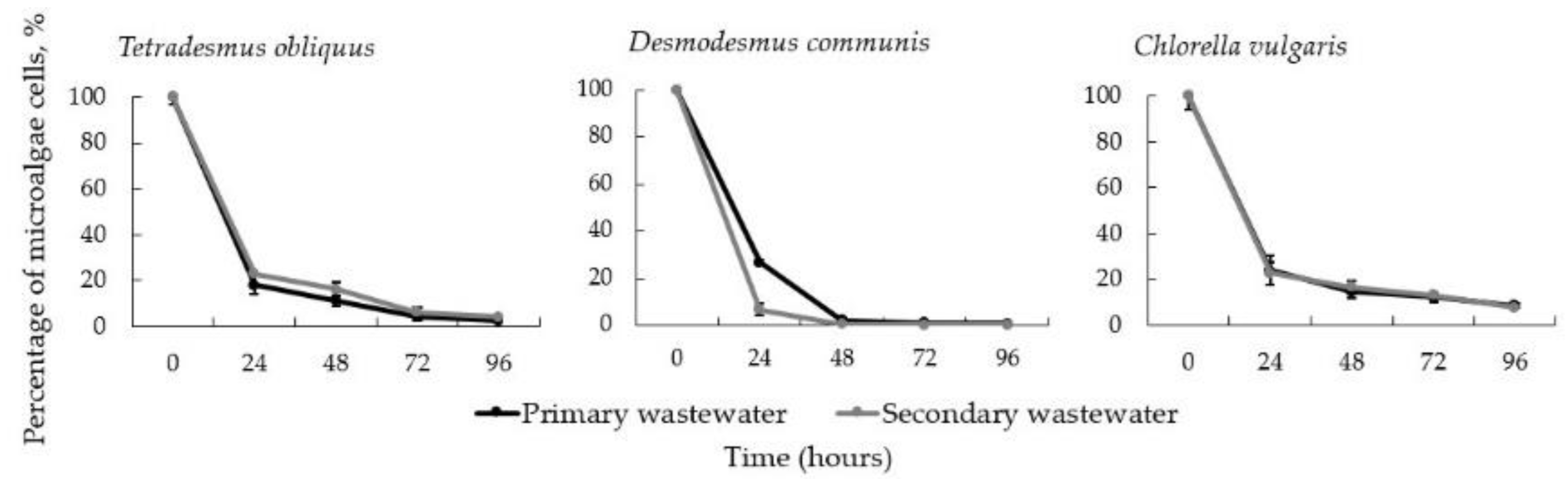
3.4. Algal-Fungal Co-Cultivation in Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Aliyu, A.; Lee, J.G.M.; Harvey, A.P. Microalgae for biofuels: A review of thermochemical conversion processes and associated opportunities and challenges. Bioresour. Technol. Rep. 2021, 15, 100694. [Google Scholar] [CrossRef]
- Ali, S.; Paul Peter, A.; Chew, K.W.; Munawaroh, H.; Show, P.L. Resource recovery from industrial effluents through the cultivation of microalgae: A review. Bioresour. Technol. 2021, 337, 125461. [Google Scholar] [CrossRef] [PubMed]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.C.; Tian, Y.; Liang, H.; Zuo, W.; Wang, Z.W.; Zhang, J.; He, Z.W. Enhanced nitrogen and phosphorus removal from domestic wastewater via algae-assisted sequencing batch biofilm reactor. Bioresour. Technol. 2018, 250, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Shahid, M.K.; Dash, R.R.; Bhunia, P.; Liu, D.; Varjani, S.; Zhang, T.C.; Surampalli, R.Y. Nutrient removal from domestic wastewater: A comprehensive review on conventional and advanced technologies. J. Environ. Manag. 2021, 296, 113246. [Google Scholar] [CrossRef]
- Tiwari, A.; Kiran, T.; Pandey, A. Chapter 14-Algal cultivation for biofuel production. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 383–403. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Chu, R.; Li, S.; Zhu, L.; Yin, Z.; Hu, D.; Liu, C.; Mo, F. A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment and biofuel production. Renew. Sustain. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Smith, B.; Davis, R. Sedimentation of algae flocculated using naturally-available, magnesium-based flocculants. Algal Res. 2012, 1, 32–39. [Google Scholar] [CrossRef]
- Papazi, A.; Makridis, P.; Divanach, P. Harvesting Chlorella minutissima using cell coagulants. J. Appl. Phycol. 2010, 22, 349–355. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.L.; Smith, R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Barros, A.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of microalgae by centrifugation for biodiesel production: A review. Algal Res. 2020, 51, 02046. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Sommerfeld, M.; Puruhito, E.; Chen, Y. Harvesting algal biomass for biofuels using ultrafiltration membranes. Bioresour. Technol. 2010, 101, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae-An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, G.; Sun, S.; Hu, C.; Liu, J. Co-pelletization of microalgae and fungi for efficient nutrient purification and biogas upgrading. Bioresour. Technol. 2019, 289, 121656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, B. A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour. Technol. 2012, 114, 529–535. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, L.; Li, P.; Yan, Y.; Chen, T.; Zheng, T.; Wang, H. Flocculation mechanism of Aspergillus niger on harvesting of Chlorella vulgaris biomass. Algal Res. 2017, 25, 402–412. [Google Scholar] [CrossRef]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Min, M.; Hu, B.; Ma, X.; Liu, Y.; Wang, Q.; Shi, J.; Chen, P.; Ruan, R. Filamentous fungi assisted bio-flocculation: A novel alternative technique for harvesting heterotrophic and autotrophic microalgal cells. Sep. Purif. Technol. 2013, 107, 158–165. [Google Scholar] [CrossRef]
- Alrubaie, G.; Al-Shammari, R.H.H. Microalgae Chlorella Vulgaris Harvesting Via Co-Pelletization with Filamentous Fungus. Baghdad Sci. J. 2018, 15, 31–36. [Google Scholar] [CrossRef]
- Al-Hothaly, K.A.; Adetutu, E.M.; Taha, M.; Fabbri, D.; Lorenzetti, C.; Conti, R.; May, B.H.; Shar, S.S.; Bayoumi, R.A.; Ball, A.S. Bio-harvesting and pyrolysis of the microalgae Botryococcus braunii. Bioresour. Technol. 2015, 191, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Li, W.; Chen, J.; Leng, S.; Chen, J.; Wei, L.; Peng, H.; Li, J.; Zhou, W.; Huang, H. Co-culture of fungi-microalgae consortium for wastewater treatment: A review. Bioresour. Technol. 2021, 330, 125008. [Google Scholar] [CrossRef] [PubMed]
- Dalecka, B.; Juhna, T.; Rajarao, G.K. Constructive use of filamentous fungi to remove pharmaceutical substances from wastewater. J. Water Process Eng. 2020, 33, 100992. [Google Scholar] [CrossRef]
- Mezule, L.; Civzele, A. Bioprospecting White-Rot Basidiomycete Irpex lacteus for Improved Extraction of Lignocellulose-Degrading Enzymes and Their Further Application. J. Fungi 2020, 6, 256. [Google Scholar] [CrossRef]
- Mezule, L.; Berzina, I.; Strods, M. The Impact of Substrate–Enzyme Proportion for Efficient Hydrolysis of Hay. Energies 2019, 12, 3526. [Google Scholar] [CrossRef]
- Laezza, C.; Salbitani, G.; Carfagna, S. Fungal Contamination in Microalgal Cultivation: Biological and Biotechnological Aspects of Fungi-Microalgae Interaction. J. Fungi 2022, 8, 1099. [Google Scholar] [CrossRef]
- Dagenais, T.R.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef]
- Paulussen, C.; Hallsworth, J.E.; Álvarez-Pérez, S.; Nierman, W.C.; Hamill, P.G.; Blain, D.; Rediers, H.; Lievens, B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb. Biotechnol. 2017, 10, 296–322. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Wannemuehler, K.A.; Marr, K.A.; Hadley, S.; Kontoyiannis, D.P.; Walsh, T.J.; Fridkin, S.K.; Pappas, P.G.; Warnock, D.W. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: Interim results of a prospective multicenter surveillance program. Med. Mycol. 2005, 43 (Suppl. 1), S49–S58. [Google Scholar] [CrossRef] [PubMed]
- ISO 5815-1:2019; Water Quality—Determination of Biochemical Oxygen Demand after n Days (BODn)—Part 1: Dilution and Seeding Method with Allylthiourea Addition. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/69058.html (accessed on 2 November 2022).
- ISO 6060:1989; Water Quality—Determination of the Chemical Oxygen Demand. International Organization for Standardization: Geneva, Switzerland, 1989. Available online: https://www.iso.org/standard/12260.html (accessed on 2 November 2022).
- BS EN 872:2005; Water Quality. Determination of Suspended Solids. Method by Filtration through Glass Fibre Filters. European Standards: Pilsen, Czech Republic, 2005. Available online: https://www.en-standard.eu/bs-en-872-2005-water-quality-determination-of-suspended-solids-method-by-filtration-through-glass-fibre-filters/ (accessed on 2 November 2022).
- ISO 7150-1:1984; Water Quality—Determination of Ammonium—Part 1: Manual Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 1984. Available online: https://www.iso.org/standard/13742.html (accessed on 2 November 2022).
- ISO 6777:1984; Water Quality—Water Quality—Determination of Nitrite—Molecular Absorption Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 1984. Available online: https://www.iso.org/standard/13273.html (accessed on 2 November 2022).
- ISO 7890-3:1988; Water Quality—Determination of Nitrate—Part 3: Spectrometric Method Using Sulfosalicylic Acid. International Organization for Standardization: Geneva, Switzerland, 1988. Available online: https://www.iso.org/standard/14842.html (accessed on 2 November 2022).
- ISO 6878:2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 2004. Available online: https://www.iso.org/standard/36917.html (accessed on 2 November 2022).
- ISO 0523:2008; Water Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/51994.html (accessed on 2 November 2022).
- Lavrinovičs, A.; Mežule, L.; Juhna, T. Microalgae starvation for enhanced phosphorus uptake from municipal wastewater. Algal Res. 2020, 52. [Google Scholar] [CrossRef]
- Denisova, V.; Mezule, L.; Juhna, T. The effect of chitosan nanoparticles on Escherichia coli viability in drinking water disinfection. Water Pract. Technol. 2022, 17, 537–543. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K. Insights into lignin degradation and its potential industrial applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar] [CrossRef]
- Saraswat, P.; Yadav, K.; Gupta, A.; Prasad, M.; Ranjan, R. Chapter 34-Physiological and molecular basis for remediation of polyaromatic hydrocarbons. In Handbook of Bioremediation; Hasanuzzaman, M., Prasad, M.N.V., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 535–550. [Google Scholar] [CrossRef]
- Kalinoski, R.M. White-Rot Fungi as Pretreatment Agents for Wood Destined for Biofuel Applications. Masters Theses, Eastern Illinois University, Charleston, IL, USA, 2016; p. 2450. [Google Scholar]
- Canam, T.; Dumonceaux, T.J.; Record, E.; Li, Y. White-rot fungi: The key to sustainable biofuel production? Biofuels 2013, 4, 247–250. [Google Scholar] [CrossRef]
- Jurado, M.; Martinèz, À.T.; Martinez, M.J.; Saparrat, M.C.N. 6.45-Application of White-Rot Fungi in Transformation, Detoxification, or Revalorization of Agriculture Wastes: Role of Laccase in the Processes. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 595–603. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Huang, Z.; Guo, Z.; Huang, T.; Peng, M.; Shi, J.; Hu, L. Applications of white rot fungi in bioremediation with nanoparticles and biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 4853–4862. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef]
- Göçenoğlu, A.; Pazarlioglu, N. Cinnabarinic acid: Enhanced production from Pycnoporus cinnabarinus, characterization, structural and functional properties. Hacet. J. Biol. Chem. 2014, 42, 281–290. [Google Scholar] [CrossRef]
- Nasir, N.M.; Bakar, N.S.; Lananan, F.; Abdul Hamid, S.H.; Lam, S.S.; Jusoh, A. Treatment of African catfish, Clarias gariepinus wastewater utilizing phytoremediation of microalgae, Chlorella sp. with Aspergillus niger bio-harvesting. Bioresour. Technol. 2015, 190, 492–498. [Google Scholar] [CrossRef]
- Oliveira, H.R.; Bassin, I.D.; Cammarota, M.C. Bioflocculation of cyanobacteria with pellets of Aspergillus niger: Effects of carbon supplementation, pellet diameter, and other factors in biomass densification. Bioresour. Technol. 2019, 294, 122167. [Google Scholar] [CrossRef] [PubMed]
- Tejido-Nuñez, Y.; Aymerich, E.; Sancho, L.; Refardt, D. Treatment of aquaculture effluent with Chlorella vulgaris and Tetradesmus obliquus: The effect of pretreatment on microalgae growth and nutrient removal efficiency. Ecol. Eng. 2019, 136, 1–9. [Google Scholar] [CrossRef]
- Rugnini, L.; Ellwood, N.; Costa, G.; Falsetti, A.; Congestri, R.; Bruno, L. Scaling-up of wastewater bioremediation by Tetradesmus obliquus, sequential bio-treatments of nutrients and metals. Ecotoxicol. Environ. Saf. 2019, 172, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Rugnini, L.; Costa, G.; Congestri, R.; Antonaroli, S.; Sanità di Toppi, L.; Bruno, L. Phosphorus and metal removal combined with lipid production by the green microalga Desmodesmus sp.: An integrated approach. Plant Physiol. Biochem. 2018, 125, 45–51. [Google Scholar] [CrossRef]
- Kong, W.; Kong, J.; Ma, J.; Lyu, H.; Feng, S.; Wang, Z.; Yuan, P.; Shen, B. Chlorella vulgaris cultivation in simulated wastewater for the biomass production, nutrients removal and CO2 fixation simultaneously. J. Environ. Manag. 2021, 284, 112070. [Google Scholar] [CrossRef]
- Al hattab, M.; Ghaly, A.; Hammouda, A. Microalgae Harvesting Methods for Industrial Production of Biodiesel: Critical Review and Comparative Analysis. J. Fundam Renew. Energy Appl. 2015, 5, 154. [Google Scholar] [CrossRef]


| Parameter | Method | Primary Wastewater | Secondary Wastewater | |
|---|---|---|---|---|
| BOD5 (biochemical oxygen demand) | ISO 5815-1 | 160 | 6 | mg/L |
| COD (chemical oxygen demand) | ISO 6060 | 480 | 39 | mg/L |
| SS (suspended solids) | EN 872 | 210 | 6 | mg/L |
| NH4-N (dissolved ammonium) | ISO 7150-1 | 45 | 2.02 | mg/L |
| NO2-N (dissolved nitrite) | ISO 6777:1984 + AC:2001 | 0.054 | mg/L | |
| NO3-N (dissolved nitrate) | ISO 7890-3 | 3.22 | mg/L | |
| PO4-P (dissolved phosphate) | ISO 6878 | 3.9 | 0.29 | mg/L |
| pH | ISO 10523 | 6.9 | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Civzele, A.; Mezule, L. Microalgae Harvesting after Tertiary Wastewater Treatment with White-Rot Fungi. J. Fungi 2022, 8, 1232. https://doi.org/10.3390/jof8111232
Civzele A, Mezule L. Microalgae Harvesting after Tertiary Wastewater Treatment with White-Rot Fungi. Journal of Fungi. 2022; 8(11):1232. https://doi.org/10.3390/jof8111232
Chicago/Turabian StyleCivzele, Anna, and Linda Mezule. 2022. "Microalgae Harvesting after Tertiary Wastewater Treatment with White-Rot Fungi" Journal of Fungi 8, no. 11: 1232. https://doi.org/10.3390/jof8111232
APA StyleCivzele, A., & Mezule, L. (2022). Microalgae Harvesting after Tertiary Wastewater Treatment with White-Rot Fungi. Journal of Fungi, 8(11), 1232. https://doi.org/10.3390/jof8111232