The Lipid Profile of the Endomyces magnusii Yeast upon the Assimilation of the Substrates of Different Types and upon Calorie Restriction
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
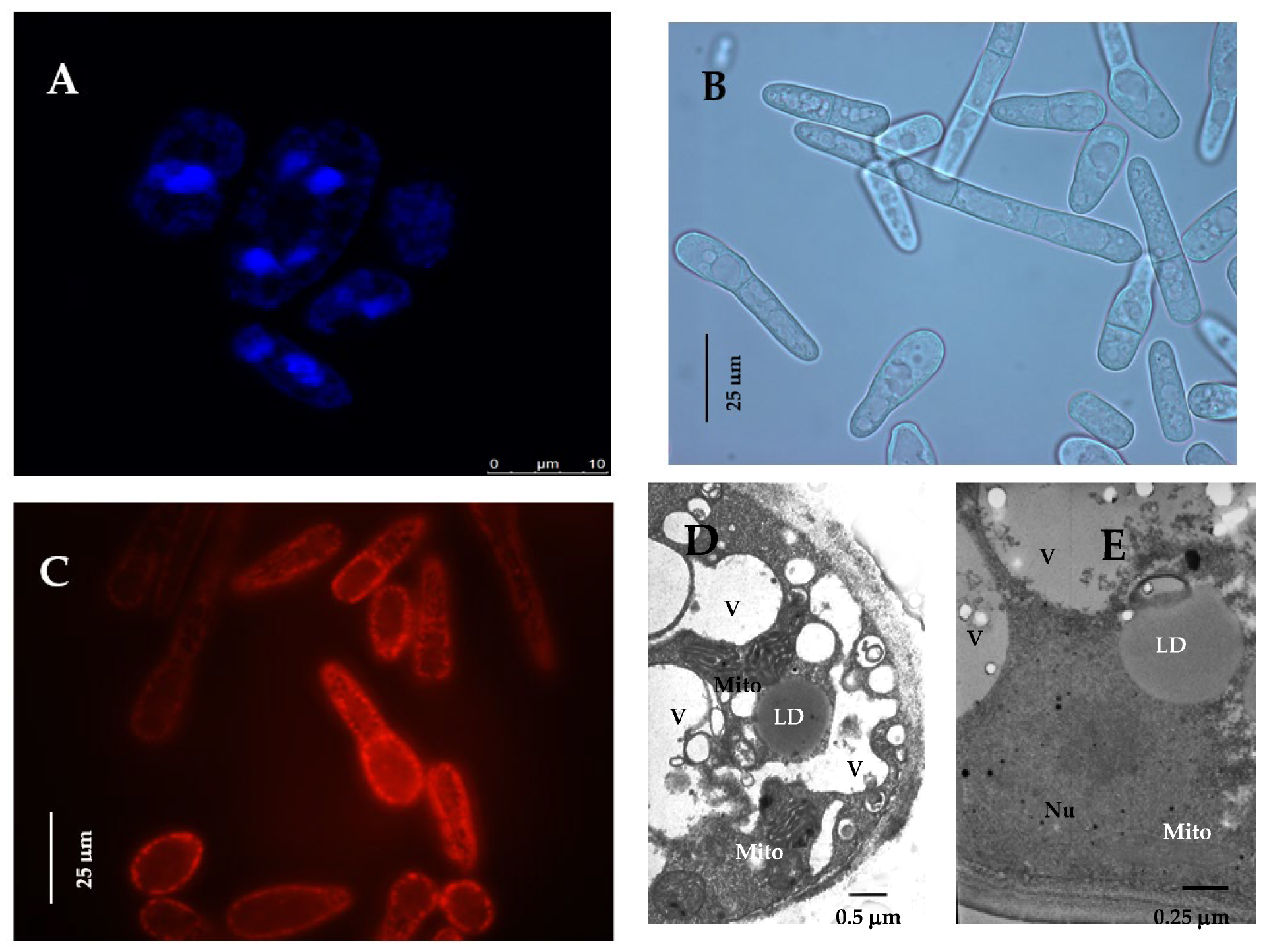
2.2. DAPI Staining
2.3. Potential-Dependent Staining
2.4. Transmission Electron Microscopy (TEM)
2.5. Preparation and Analysis of Lipids
2.6. Detection of the Reactive Oxygen Species ROS
2.7. Statistical Analyses
3. Results
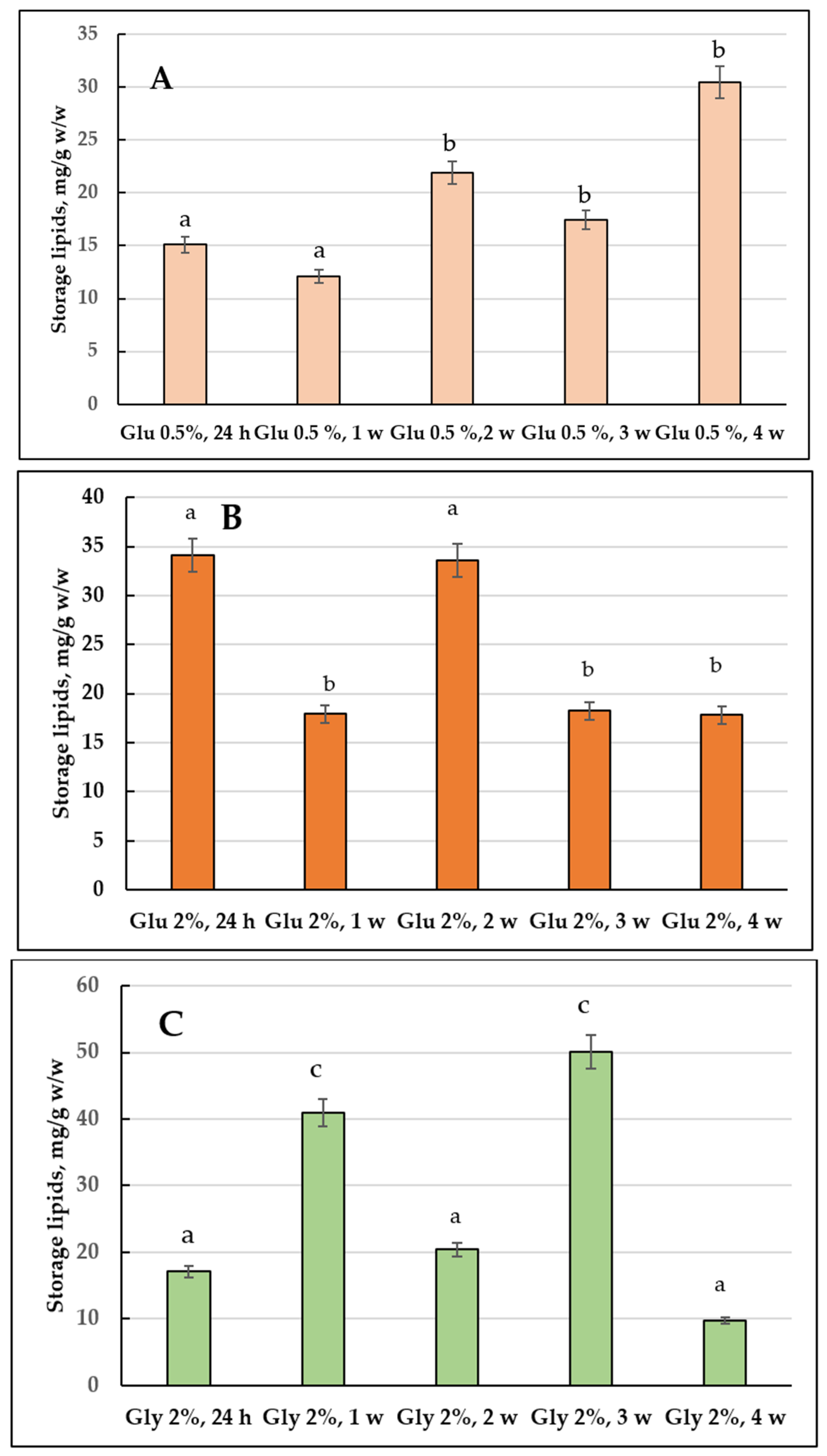
3.1. Storage Lipids Profile
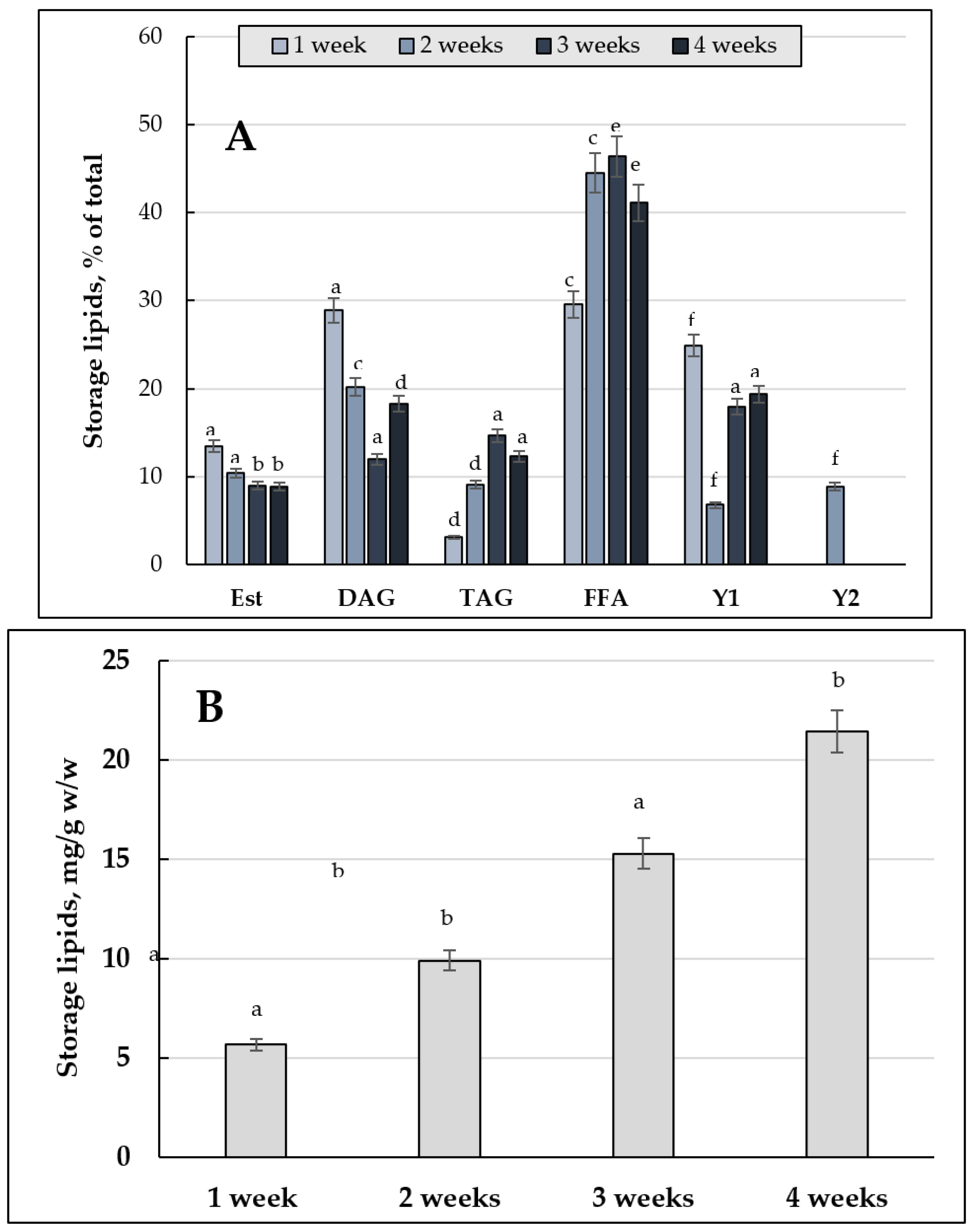
3.1.1. Comparative Analysis of the Storage Lipids upon Culturing the E. magnusii Cells on 2% Glucose and 2% Glycerol (2% Glucose vs. 2% Glycerol)
3.1.2. The Storage Lipid Profile of the E. magnusii upon CR
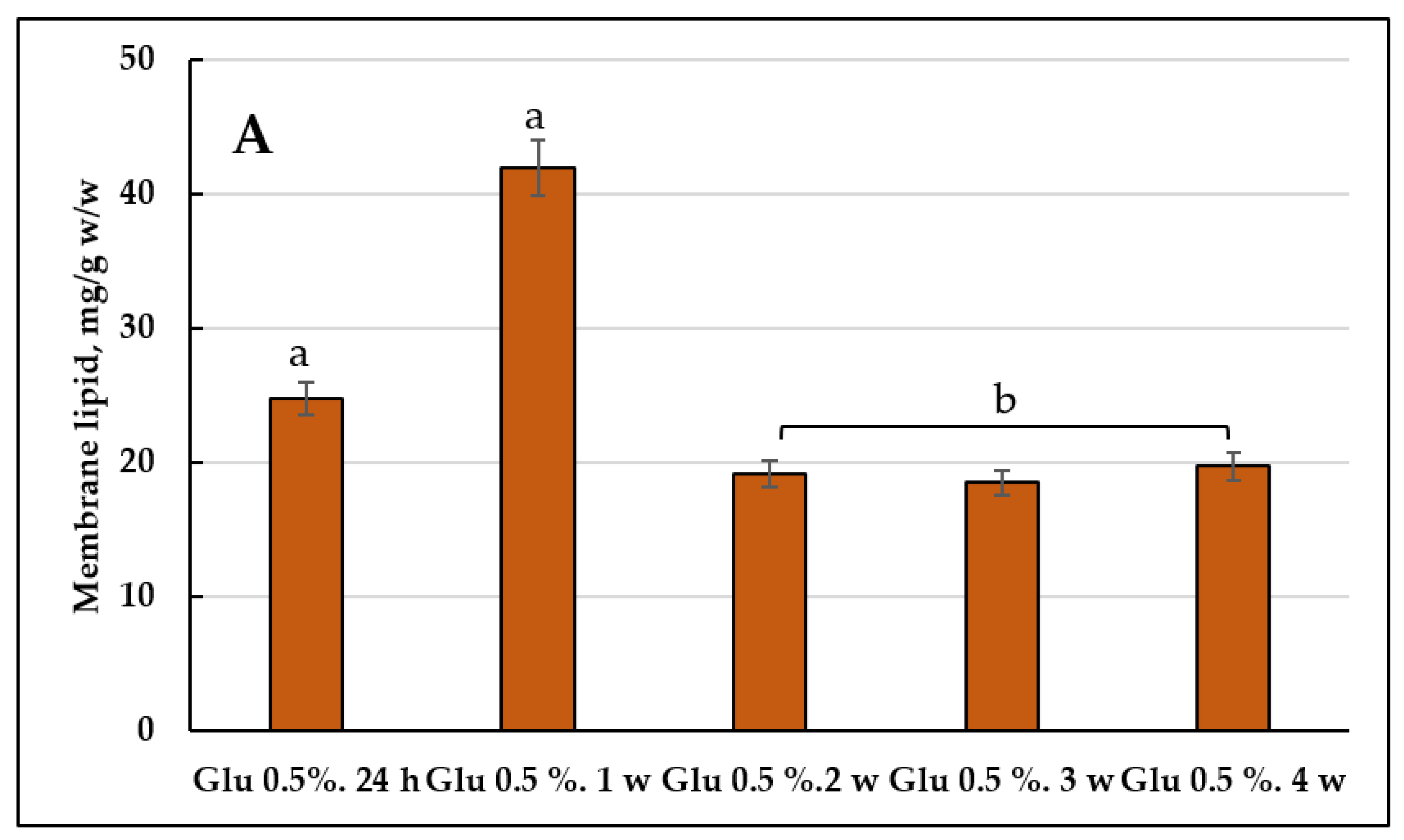
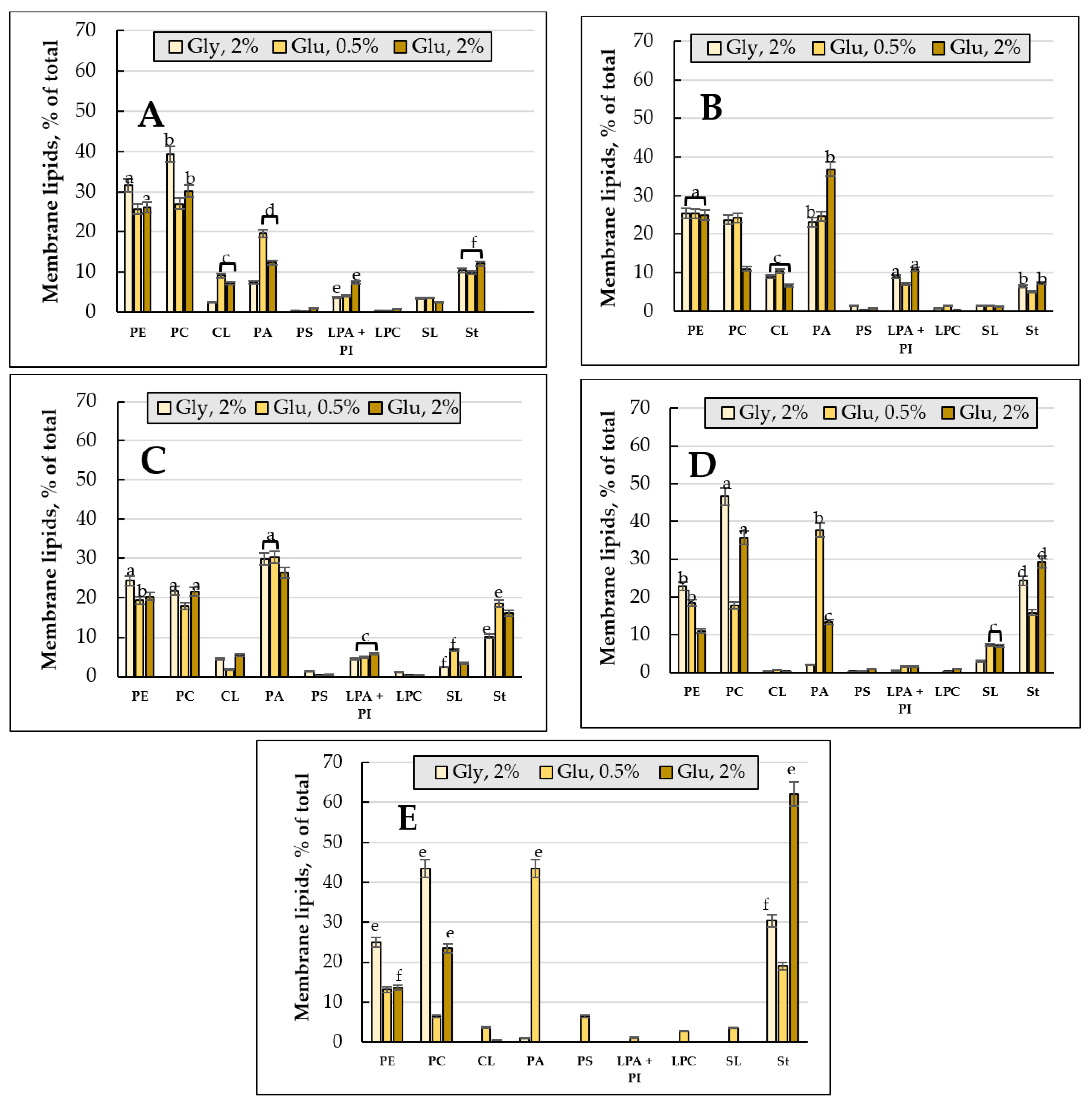
3.2. Membrane Lipids Profile
3.2.1. Comparative Analysis of the Membrane Lipid Profile upon Cultivating the E. magnusii Yeast on 2% Glucose and 2% Glycerol (2% Glucose vs. 2% Glycerol)
3.2.2. The Membrane Lipid Profile of E. magnusii Culture upon CR
3.3. FAs of the Main PLs
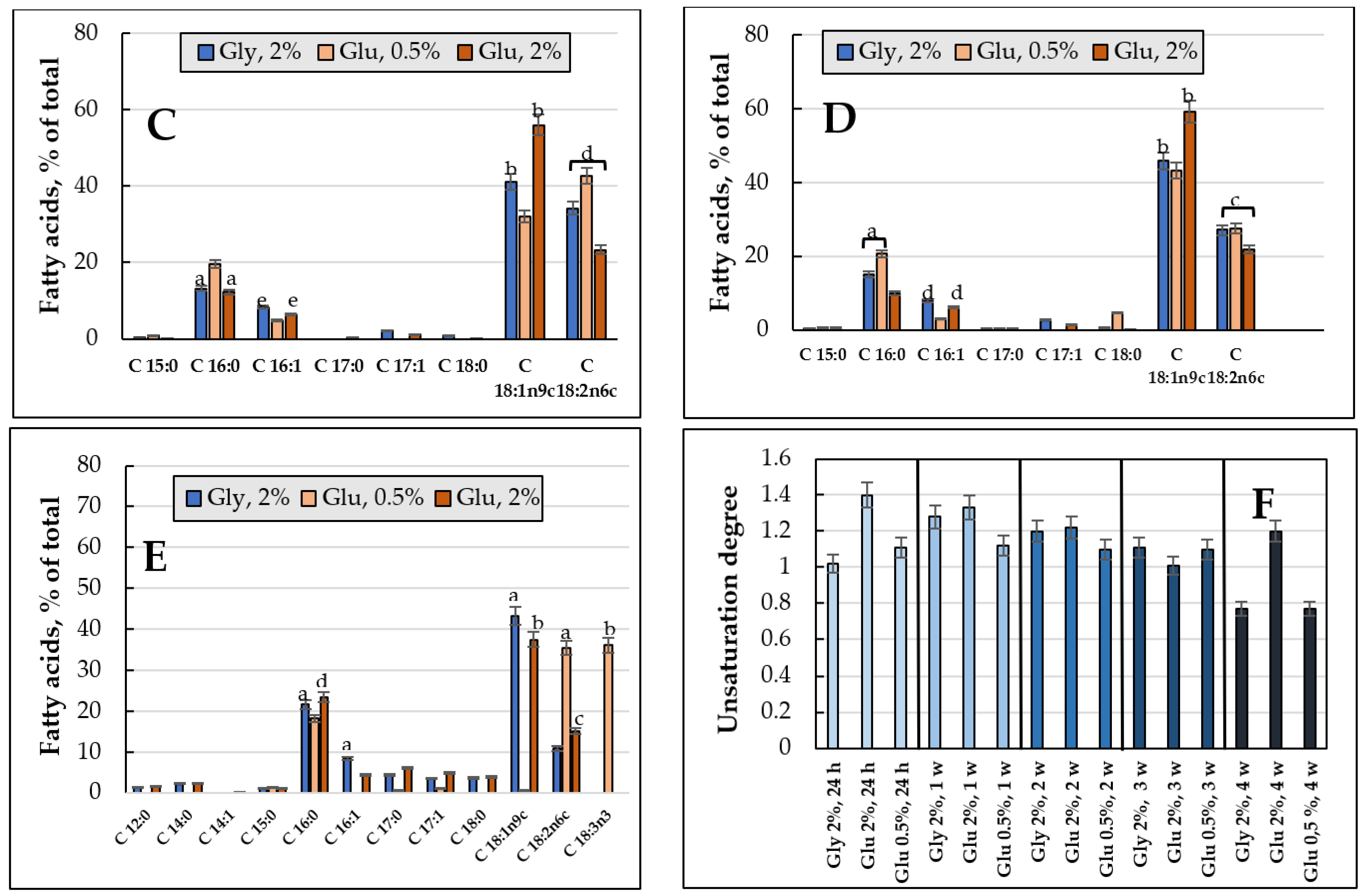
3.3.1. FAs Composition and the Degree of Unsaturation of Total PLs upon Cultivating E. magnusii Cells Using 2% Glucose and 2% Glycerol (2% Glucose vs. 2% Glycerol)
3.3.2. FAs Composition and the Degree of Unsaturation of the PLs upon Cultivating the E. magnusii Cells under the CR Conditions
3.4. Changes in the Lipid Profile upon Carbohydrate Depletion
3.4.1. Storage Lipids Profile
3.4.2. Membrane Lipids Profile
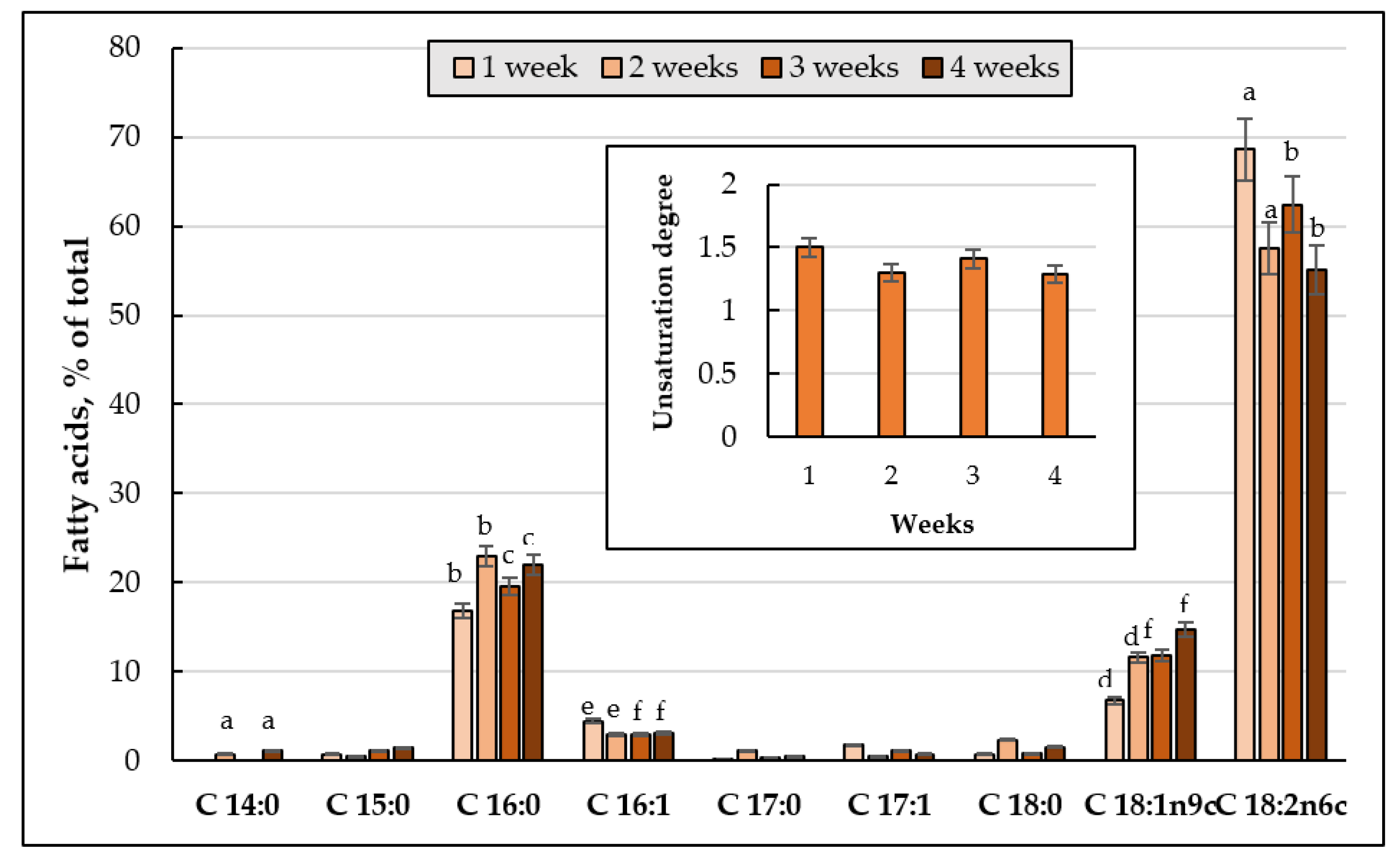
3.4.3. FAs Composition of the Main Phospholipids
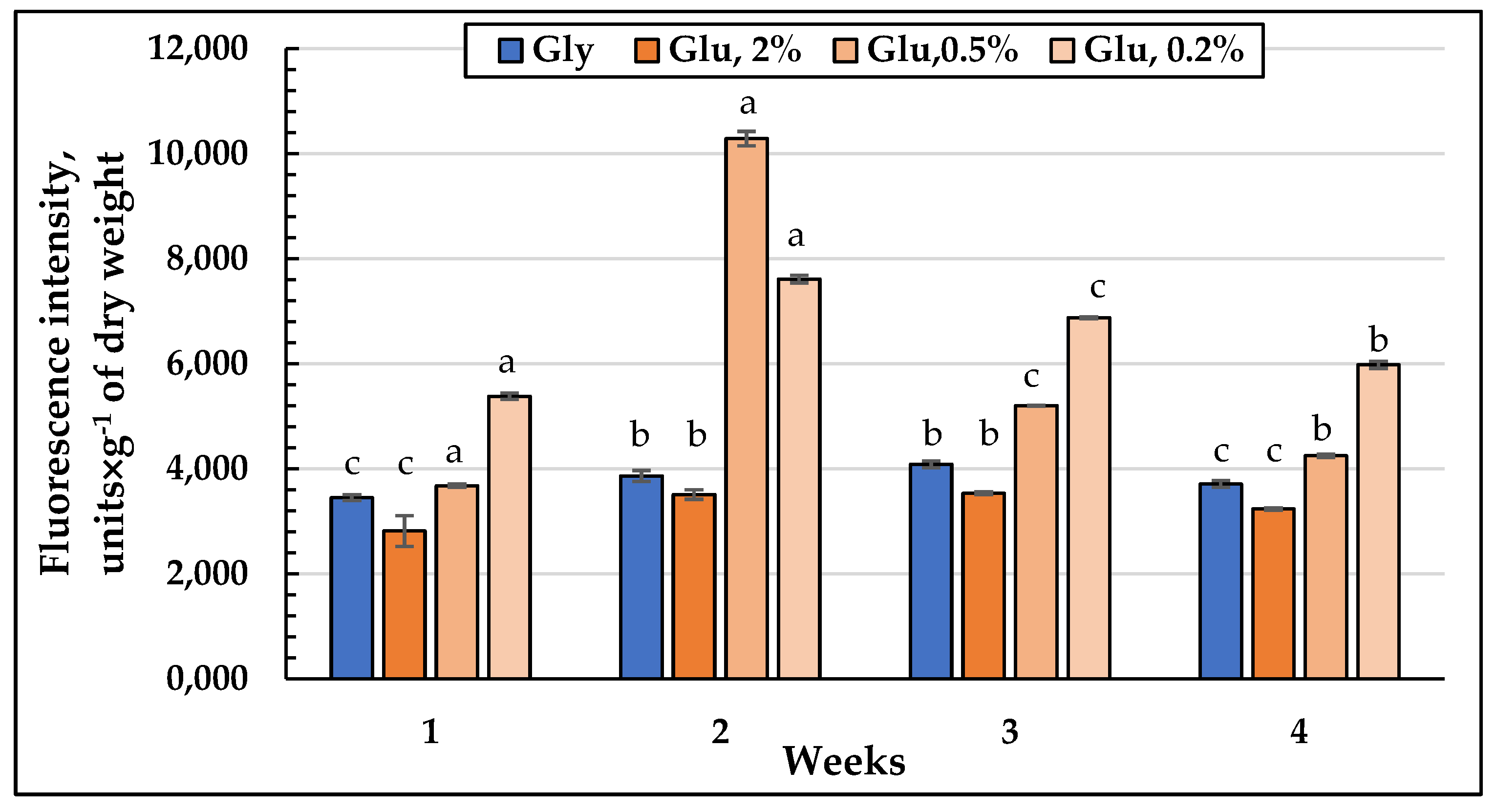
3.5. Visualization of Lysosomes Using E. magnusii LysoTracker Red DND99 Cells
3.6. ROS Level in the Glycerol and Glucose Assimilating E. magnusii Yeasts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajakumari, S.; Grillitsch, K.; Daum, G. Synthesis and turnover of non-polar lipids in yeast. Prog. Lipid Res. 2008, 47, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippoussis, A.; Papanikolaou, S. Lipids from yeasts and fungi: Physiology, production and analytical considerations. J. Appl. Microbiol. 2018, 124, 336–367. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Preta, G. Lipids in the cell: Organisation regulates function. Cell. Mol. Life Sci. 2018, 75, 1909–1927. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.A.; Gaspar, M.L.; Jesch, S.A. The response to inositol: Regulation of glycerolipid metabolism and stress response signaling in yeast. Chem. Phys. Lipids 2014, 180, 23–43. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Smith, M.T. Chapter 45—Magnusiomyces Zender (1977). In The Yeasts, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 565–574. ISBN 9780444521491. [Google Scholar] [CrossRef]
- Catalogue of Life: 2011 Annual Checklist. 2011. Available online: http://www.catalogueoflife.org/annual-checklist/2011/details/species/id/8526224 (accessed on 15 March 2020).
- Van der Klei, I.; Veenhuis, M.; Brul, S.; Klis, F.M.; De Groot, P.W.J.; Müller, W.H.; van Driel, K.G.A.; Boekhout, T. Chapter 8—Cytology, Cell Walls and Septa: A Summary of Yeast Cell Biology from a Phylogenetic Perspective. In The Yeasts, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 111–128. ISBN 9780444521491. [Google Scholar]
- Van der Walt, J.P.; von Arx, J.A.; Liebenberg, N.V.D.W. Multiperforate septa in Geotrichum and Dipodascus. South Afr. J. Bot. 1983, 2, 184–186. [Google Scholar] [CrossRef][Green Version]
- Zvyagilskaya, R.A.; Korosteleva, N.L.; Kotelnikova, A.V. The properties of the respiratory system of Endomyces magnusii at different growth stages. Microbiology 1977, 46, 605–611. [Google Scholar]
- Kokoreva, A.S.; Isakova, E.P.; Tereshina, V.M.; Klein, O.I.; Gessler, N.N.; Deryabina, Y.I. The Effect of Different Substrates on the Morphological Features and Polyols Production of Endomyces magnusii Yeast during Long-Lasting Cultivation. Microorganisms 2022, 10, 1709. [Google Scholar] [CrossRef]
- Mikova, H.; Rosenberg, M.; Kristofilova, L. Production of L-Malate from Fumarate by the Yeast Dipodascus magnusii. Acta Biotechnol. 1999, 19, 357–363. [Google Scholar] [CrossRef]
- Rosenberg, M.; Mikova, H.; Kristofilova, L. Formation of L-malic acid by yeasts of the genus Dipodascus. Lett. Appl. Microbiol. 1999, 29, 221–223. [Google Scholar] [CrossRef]
- Kurylenko, O.O.; Ruchala, J.; Dmytruk, K.V.; Abbas, C.A.; Sibirny, A.A. Multinuclear Yeast Magnusiomyces (Dipodascus, Endomyces) magnusii is a Promising Isobutanol Producer. Biotechnol. J. 2020, 15, 1900490. [Google Scholar] [CrossRef]
- Roberts, G.G.; Hudson, A.P. Rsf1p is required for an efficient metabolic shift from fermentative to glycerol-based respiratory growth in S. cerevisiae. Yeast 2009, 26, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.G.; Hudson, A.P. Transcriptome profiling of Saccharomyces cerevisiae during a transition from fermentative to glycerol-based respiratory growth reveals extensive metabolic and structural remodeling. Mol. Genet. Genom. 2006, 276, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Renvoisé, M.; Bonhomme, L.; Davanture, M.; Valot, B.; Zivy, M.; Lemaire, C. Quantitative variations of the mitochondrial proteome and phosphoproteome during fermentative and respiratory growth in Saccharomyces cerevisiae. J. Proteom. 2014, 106, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Ohlmeier, S.; Hiltunen, J.K.; Bergmann, U. Protein phosphorylation in mitochondria --a study on fermentative and respiratory growth of Saccharomyces cerevisiae. Electrophoresis 2010, 31, 2869–2881. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Sánchez, R.; Gutiérrez, G.; Rincón, A.M.; Codón, A.C.; Benítez, T. Transcriptional regulation of fermentative and respiratory metabolism in Saccharomyces cerevisiae industrial bakers’ strains. FEMS Yeast Res. 2012, 12, 625–636. [Google Scholar] [CrossRef]
- Enriquez-Hesles, E.; Smith, D.L., Jr.; Maqani, N.; Wierman, M.B.; Sutcliffe, M.D.; Fine, R.D.; Kalita, A.; Santos, S.M.; Muehlbauer, M.J.; Bain, J.R.; et al. A cell-nonautonomous mechanism of yeast chronological aging regulated by caloric restriction and one-carbon metabolism. J. Biol. Chem. 2021, 296, 100125. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu. Rev. Nutr. 2020, 40, 105–133. [Google Scholar] [CrossRef]
- Giacomello, E.; Toniolo, L. The Potential of Calorie Restriction and Calorie Restriction Mimetics in Delaying Aging: Focus on Experimental Models. Nutrients 2021, 13, 2346. [Google Scholar] [CrossRef]
- Isakova, E.P.; Matushkina, I.N.; Popova, T.N.; Dergacheva, D.I.; Gessler, N.N.; Klein, O.I.; Semenikhina, A.V.; Deryabina, Y.I.; La Porta, N.; Saris, N.L. Metabolic Remodeling during Long-Lasting Cultivation of the Endomyces magnusii Yeast on Oxidative and Fermentative Substrates. Microorganisms 2020, 8, 91. [Google Scholar] [CrossRef]
- Deryabina, Y.I.; Isakova, E.P.; Sekova, V.; Antipov, A.; Saris, N.L. Inhibition of free radical scavenging enzymes affects mitochondrial membrane permeability transition during growth and aging of yeast cells. J. Bioenerg. Biomembr. 2014, 46, 479–492. [Google Scholar] [CrossRef]
- Nichols, B.W. Separation of the lipids of photosynthetic tissues: Improvements in analysis by thin-layer chromatography. Biochim. Biophys. Acta 1963, 70, 417–422. [Google Scholar] [CrossRef]
- Kates, M. Techniques of Lipidology: Isolation, Analysis, and Identification of Lipids, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1986; 464p. [Google Scholar]
- Benning, C.; Huang, Z.H.; Gage, D.A. Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 1995, 317, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, K.M.E.; Bisinski, D.D.; Shashkova, S.; Paine, K.M.; Veillon, V.A.; Leake, M.C.; MacDonald, C. A glucose-starvation response governs endocytic trafficking and eisosomal retention of surface cargoes in budding yeast. J. Cell Sci. 2021, 134, 257733. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, J.; Du, J.; Peng, X. Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Martins, W.K.; Santos, N.F.; Rocha, C.S.; Bacellar, I.O.L.; Tsubone, T.M. Parallel damage in mitochondria and lysosomes is an efficient way to photoinduce cell death. Autophagy 2019, 15, 259–279. [Google Scholar] [CrossRef]
- Cao, L.; Tang, Y.; Quan, Z.; Zhang, Z.; Oliver, S.G.; Zhang, N. Chronological lifespan in yeast is dependent on the accumulation of storage carbohydrates mediated by Yak1, Mck1 and Rim15 kinases. PLoS Genet. 2016, 12, e1006458. [Google Scholar] [CrossRef]
- Zhang, N.; Cao, L. Starvation signals in yeast are integrated to coordinate metabolic reprogramming and stress response to ensure longevity. Curr. Genet. 2017, 63, 839–843. [Google Scholar] [CrossRef]
- Schafer, D. Aging, longevity, and diet: Historical remarks on calorie intake reduction. Gerontology 2005, 51, 126–130. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef]
- Gabandé-Rodríguez, E.; Gómez de Las Heras, M.M.; Mittelbrunn, M. Control of Inflammation by Calorie Restriction Mimetics: On the Crossroad of Autophagy and Mitochondria. Cells 2019, 28, 82. [Google Scholar] [CrossRef]
- Bluher, M.; Kahn, B.B.; Kahn, C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 2003, 299, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.K.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007, 4, e76. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Klopp, R.G.; Weindruch, R.; Prolla, T.A. Gene expression profile of aging and its retardation by caloric restriction. Science 1999, 285, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Handee, W.; Li, X.; Hall, K.W.; Deng, X.; Li, P.; Benning, C.; Williams, B.L.; Kuo, M.H. An energy-independent pro-longevity function of triacylglycerol in yeast. PLoS Genet. 2016, 12, e1005878. [Google Scholar] [CrossRef]
- Li, H.; Handee, W.; Kuo, M.H. The slim, the fat, and the obese: Guess who lives the longest? Curr. Genet. 2017, 63, 43–49. [Google Scholar] [CrossRef]
- Klug, L.; Daum, G. Yeast lipid metabolism at a glance. FEMS Yeast Res. 2014, 14, 369–388. [Google Scholar] [CrossRef]
- Tuller, G.; Nemec, T.; Hrastnik, C.; Daum, G. Lipid composition of subcellular membranes of an FY1679-derived haploid yeast wild-type strain grown on different carbon sources. Yeast 1999, 15, 1555–1564. [Google Scholar] [CrossRef]
- Zinser, E.; Sperka-Gottlieb, C.D.; Fasch, E.V.; Kohlwein, S.D.; Paltauf, F.; Daum, G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol. 1991, 173, 2026–2034. [Google Scholar] [CrossRef]
- Horvath, S.E.; Wagner, A.; Steyrer, E.; Daum, G. Metabolic link between phosphatidylethanolamine and triacylglycerol metabolism in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 2011, 1811, 1030–1037. [Google Scholar] [CrossRef]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Han, G.S.; Carman, G.M. The Saccharomyces cerevisiae actin patch protein App1p is a phosphatidate phosphatase enzyme. J. Biol. Chem. 2012, 287, 40186–40196. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Heacock, P.N.; Clancey, C.J.; Dowhan, W. The CDS1 gene encoding CDP-diacylglycerol synthase in Saccharomyces cerevisiae is essential for cell growth. J. Biol. Chem. 1996, 271, 789–795. [Google Scholar] [CrossRef]
- Tamura, Y.; Harada, Y.; Nishikawa, S.; Yamano, K.; Kamiya, M.; Shiota, T.; Kuroda, T.; Kuge, O.; Sesaki, H. Tam41 is a CDP-diacylglycerol synthase required for cardiolipin biosynthesis in mitochondria. Cell Metab. 2013, 17, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Ianutsevich, E.A.; Danilova, O.A.; Tereshina, V.M. Combinatorial action of different stress factors on the composition of membrane lipids and osmolytes of aspergillus niger. Microbiology 2020, 89, 405–412. [Google Scholar] [CrossRef]
- Bondarenko, S.A.; Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimaylo, A.A.; Kotlova, E.R.; Kamzolkina, O.V.; Bilanenko, E.N.; Tereshina, V.M. Membrane lipids and soluble sugars dynamics of the alkaliphilic fungus Sodiomyces tronii in response to ambient pH. Extremophiles 2017, 21, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, S.A.; Ianutsevich, E.A.; Sinitsyna, N.A.; Georgieva, M.L.; Bilanenko, E.N.; Tereshina, B.M. Dynamics of the cytosol soluble carbohydrates and membrane lipids in response to ambient pH in alkaliphilic and alkalitolerant fungi. Microbiology 2018, 87, 21–32. [Google Scholar] [CrossRef]
- Kodedová, M.; Sychrová, H. Changes in the Sterol Composition of the Plasma Membrane A_ect Membrane Potential, Salt Tolerance and the Activity of Multidrug Resistance Pumps in Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0139306. [Google Scholar] [CrossRef]
- Acehan, D.; Khuchua, Z.; Houtkooper, R.H.; Malhotra, A.; Kaufman, J.; Vaz, F.M.; Ren, M.; Rockman, H.A.; Stokes, D.L.; Schlame, M. Distinct effects of tafazzin deletion in differentiated and undifferentiated mitochondria. Mitochondrion 2009, 9, 86–95. [Google Scholar] [CrossRef]
- Wenz, T.; Hielscher, R.; Hellwig, P.; Schagger, H.; Richers, S.; Hunte, C. Role of phospholipids in respiratory cytochrome bc(1) complex catalysis and supercomplex formation. Biochim. Biophys. Acta 2009, 1787, 609–616. [Google Scholar] [CrossRef]
- Joshi, A.S.; Zhou, J.; Gohil, V.M.; Chen, S.; Greenberg, M.L. Cellular functions of cardiolipin in yeast. Biochim. Biophys. Acta 2009, 1793, 212–218. [Google Scholar] [CrossRef][Green Version]
- Joshi, A.S.; Thompson, M.N.; Fei, N.; Huttemann, M.; Greenberg, M.L. Cardiolipin and mitochondrial phosphatidylethanolamine have overlapping functions in mitochondrial fusion in Saccharomyces cerevisiae. J. Biol. Chem. 2012, 287, 17589–17597. [Google Scholar] [CrossRef] [PubMed]
- Mileykovskaya, E.; Dowhan, W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim. Biophys. Acta 2009, 1788, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- You, K.M.; Rosenfield, C.L.; Knipple, D.C. Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl. Environ. Microbiol. 2003, 69, 1499–1503. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, S.; Sánchez-García, A.; Martínez-Rivas, J.M.; Prieto, J.A.; Randez-Gil, F. Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl. Environ. Microbiol. 2007, 73, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Daum, G.; Wagner, A.; Czabany, T.; Athenstaedt, K. Dynamics of neutral lipid storage and mobilization in yeast. Biochimie 2007, 89, 243–248. [Google Scholar] [CrossRef]
- Baile, M.G.; Lu, Y.W.; Claypool, S.M. The topology and regulation of cardiolipin biosynthesis and remodeling in yeast. Chem. Phys. Lipids 2014, 179, 25–31. [Google Scholar] [CrossRef][Green Version]
- Shene, C.; Leyton, A.; Esparza, Y.; Flores, L.; Quilodra´n, B.; Hinzpeter, I. Microbial oils and fatty acids: Effect of carbon source on docosahexaenoic acid (c22:6 n-3, DHA) production by thraustochytrid strains. J. Soil Sci. Plant Nutr. 2010, 10, 207–216. [Google Scholar] [CrossRef]
- Gupta, A.; Barrow, C.J.; Puri, M. Omega-3 biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol. Adv. 2012, 30, 1733–1745. [Google Scholar] [CrossRef]
- Kamlangdee, N.; Fan, K.W. Polyunsaturated fatty acids production by Schizochytrium sp. isolated from mangrove. Songklanakarin J. Sci. Technol. 2003, 25, 643–650. [Google Scholar]
- Manjoo, R.; Deepa, S.; Yadav Alok, K.; Singh Nand, K. Isolation and Characterization of Fusarium verticillioides NKF1 for Unsaturated Fatty Acid Production. Curr. Microbiol. 2017, 74, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Chen, H.; Gu, Z.; Zhang, H.; Chen, W.; Chen, Y.Q. Metabolic engineering of Mortierella alpina for enhanced arachidonic acid production through the NADPHsupplying strategy. Appl. Environ. Microbiol. 2016, 82, 3280–3288. [Google Scholar] [CrossRef] [PubMed]
- Rødkær, S.V.; Færgeman, N.G. Glucose- and nitrogen sensing and regulatory mechanisms in Saccharomyces cerevisiae. FEMS Yeast Res. 2014, 14, 683–696. [Google Scholar] [CrossRef]
- Pan, Y.; Schroeder, E.A.; Ocampo, A.; Barrientos, A.; Shadel, G.S. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011, 13, 668–678. [Google Scholar] [CrossRef]
- Mesquita, A.; Weinberger, M.; Silva, A.; Sampaio-Marques, B.; Almeida, B.; Leao, C.; Costa, V.; Rodrigues, F.; Burhans, W.C.; Ludovico, P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc. Natl. Acad. Sci. USA 2010, 107, 15123–15128. [Google Scholar] [CrossRef]
- Kolouchová, I.; Maťátková, O.; Sigler, K.; Masák, J.; Řezanka, T. Production of Palmitoleic and Linoleic Acid in Oleaginous and Nonoleaginous Yeast Biomass. Int. J. Anal. Chem. 2016, 2016, 7583684. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deryabina, Y.I.; Kokoreva, A.S.; Klein, O.I.; Gessler, N.N.; Isakova, E.P. The Lipid Profile of the Endomyces magnusii Yeast upon the Assimilation of the Substrates of Different Types and upon Calorie Restriction. J. Fungi 2022, 8, 1233. https://doi.org/10.3390/jof8111233
Deryabina YI, Kokoreva AS, Klein OI, Gessler NN, Isakova EP. The Lipid Profile of the Endomyces magnusii Yeast upon the Assimilation of the Substrates of Different Types and upon Calorie Restriction. Journal of Fungi. 2022; 8(11):1233. https://doi.org/10.3390/jof8111233
Chicago/Turabian StyleDeryabina, Yulia I., Anastasia S. Kokoreva, Olga I. Klein, Natalya N. Gessler, and Elena P. Isakova. 2022. "The Lipid Profile of the Endomyces magnusii Yeast upon the Assimilation of the Substrates of Different Types and upon Calorie Restriction" Journal of Fungi 8, no. 11: 1233. https://doi.org/10.3390/jof8111233
APA StyleDeryabina, Y. I., Kokoreva, A. S., Klein, O. I., Gessler, N. N., & Isakova, E. P. (2022). The Lipid Profile of the Endomyces magnusii Yeast upon the Assimilation of the Substrates of Different Types and upon Calorie Restriction. Journal of Fungi, 8(11), 1233. https://doi.org/10.3390/jof8111233




