1. Introduction
After maize, wheat is the second most commonly grown grain in the world. Many diseases affect wheat growth and yield production. Rust diseases are the most prevalent threats that affect wheat plants, such as wheat stem rust, which significantly affects wheat leaves and grain yield [
1,
2]. Stem rust, caused by
Puccinia graminis f. sp.
tritici, is the most destructive disease that seriously threatens wheat grain production [
3,
4,
5]. Stem rust caused severe problems in Africa, the Middle East, Central and West Asia and North Africa (CWANA), Australia, New Zealand, Europe, and the USA [
6].
Ug99 race of
P. graminis f. sp.
tritici was detected in Uganda in 1998 for the first time. Based on North American nomenclature [
7], the initial race was identified as TTKSK, with pathogenicity to the stem rust resistance gene
Sr31. It is also virulent to a wide range of wheat resistance genes, as well as others of unknown origin. The Ug99 lineage extended over the eastern African highlands, including Zimbabwe, South Africa, Sudan, Yemen, and Iran. Because 90 percent of known wheat cultivars are susceptible to Ug99 races, it is often regarded as the most serious danger to global wheat production [
8]. Resistant wheat varieties are uncommon in Kenya and Ethiopia. New sources of race-specific resistance from various wheat relatives must be found and transmitted to improve resistance. Major efforts are required to replace Ug99 susceptible types with those that have diverse race-specific or long-lasting resistances, thereby reducing the Ug99 threat.
The Ug99 (TTKSK) was reported first in Yemen and then throughout the Middle East and Asia [
5]. In March 2008, the Food and Agriculture Organization (FAO) registered Ug99 (race TTKSK) in Iran [
9]. Detection in Iran in 2007 was followed by drought conditions, and no reports of Ug99 were received from Iran in 2008. However, in 2009, Ug99 was found in the southern Iranian province of Khuzestan, where spring wheat is grown, and weather conditions are favorable.
The pathogen is changing rapidly; approximately fifteen known variants have been identified within the Ug99 lineage. All are closely related, having nearly identical DNA fingerprints, but they differ slightly in their avirulence/virulence profiles [
10]. In contrast, these races are very different from other known races in the United States and South Africa. The similarity of Ug99 races’ simple sequence repeats markers indicates that they evolved from a common ancestor. Pathogen surveillance reveals that known variants in the Ug99 lineage are expanding across Africa. The majority of the substantial differences were identified in Kenya. Notable recent developments include the confirmation of coupled virulence to
Sr31 and
Sr24 (race PTKST) from South African isolates collected in 2009 [
11], as well as the same race in Ethiopia from isolates collected in 2007.
New variants are expected to continue spreading, and it is likely that more will be found within the Ug99 race. Currently, race TTKSK is the only known pathotype in the Ug99 lineage that has been reported outside Africa, although the development of more variations outside Africa is thought to be plausible. Reports from various African countries are being verified. TTKST and TTTSK, for example, were reported from Uganda in 2012, TTKSK from Eritrea in 2012, and Egypt and Rwanda in 2014 [
12,
13]. Moreover, Ug99 (TTKSK) of wheat stem rust fungus was reported in Egypt in 2020 [
14]. Additionally, the Ug99 race TTKTT of wheat stem rust was recently reported in Iraq [
15].
Airflows out of Yemen followed the same fundamental pattern as in previous years, with air movement from Yemen to southern Iran during the 2009–2010 winter seasons. One minor change in projected airflows from 2009 to 2010 vs. previous years was a tendency for air to travel northwesterly out of Yemen towards Israel and Jordan. In 2010, Israel and Lebanon reported stem rust. However, it is unknown whether Ug99 was present. Given the revised likely migratory paths provided by Singh et al. [
5], the presence of Ug99 or its variations in this region may have future implications for Egypt’s wheat fields. In the present research, we continued efforts made by researchers throughout the world to fill in a missing piece of the puzzle regarding the emergence and dissemination of novel races of the wheat stem rust fungus, particularly those belonging to the Ug99 lineage. In order to identify the Ug99 race using a molecular marker, we first sought to determine the geographic relationship and genetic diversity of newly generated races of the stem rust pathogen,
P. graminis tritici, throughout Africa, Asia, and Europe. In order to confirm the virulence formula of 14 recently discovered races of the stem rust pathogen, we assessed the most prevalent monogenic lines (
Sr genes).
4. Discussion
Historically, stem rust disease has been a serious threat in several wheat-producing regions [
9]. More than 20 years ago, race Ug99 of
P. graminis f. sp. tritici was first detected in Uganda and identified as TTKSK based on the North American nomenclature [
7]. Up to today, approximately 15 variants were identified within the Ug99 lineage. These variants are spreading to several wheat-producing countries in the Eastern African highlands, as well as Zimbabwe, South Africa, Sudan, Yemen, and Iran. Races of the Ug99 lineage pose a threat to wheat productivity and food security [
11] due to their capacity to infect almost 90% of the kinds of wheat grown globally. Unfortunately, we know very little regarding the presence of the Ug99 race in Egypt. Although Patpour et al. [
13] and Shahin et al. [
14] reported the Ug99 race in Egypt, some Egyptian varieties, such as Misr-1 and Misr-2, were chosen from CIMMYT wheat genotypes and tested in Uganda, Kenya, and Ethiopia, and they proved their resistance to stem rust disease, especially for this race [
24]. Then, these two varieties were imported for cultivation in these countries to overcome this race (Ug99). As a result, most countries are attempting to speak one language in the field of wheat stem rust disease. As a result, it was critical to use a molecular marker to analyze the geographical connection and genetic diversity of newly generated races of the stem rust pathogen,
P. graminis f. sp.
tritici, across Africa, Asia, and Europe, as well as the Ug99 race.
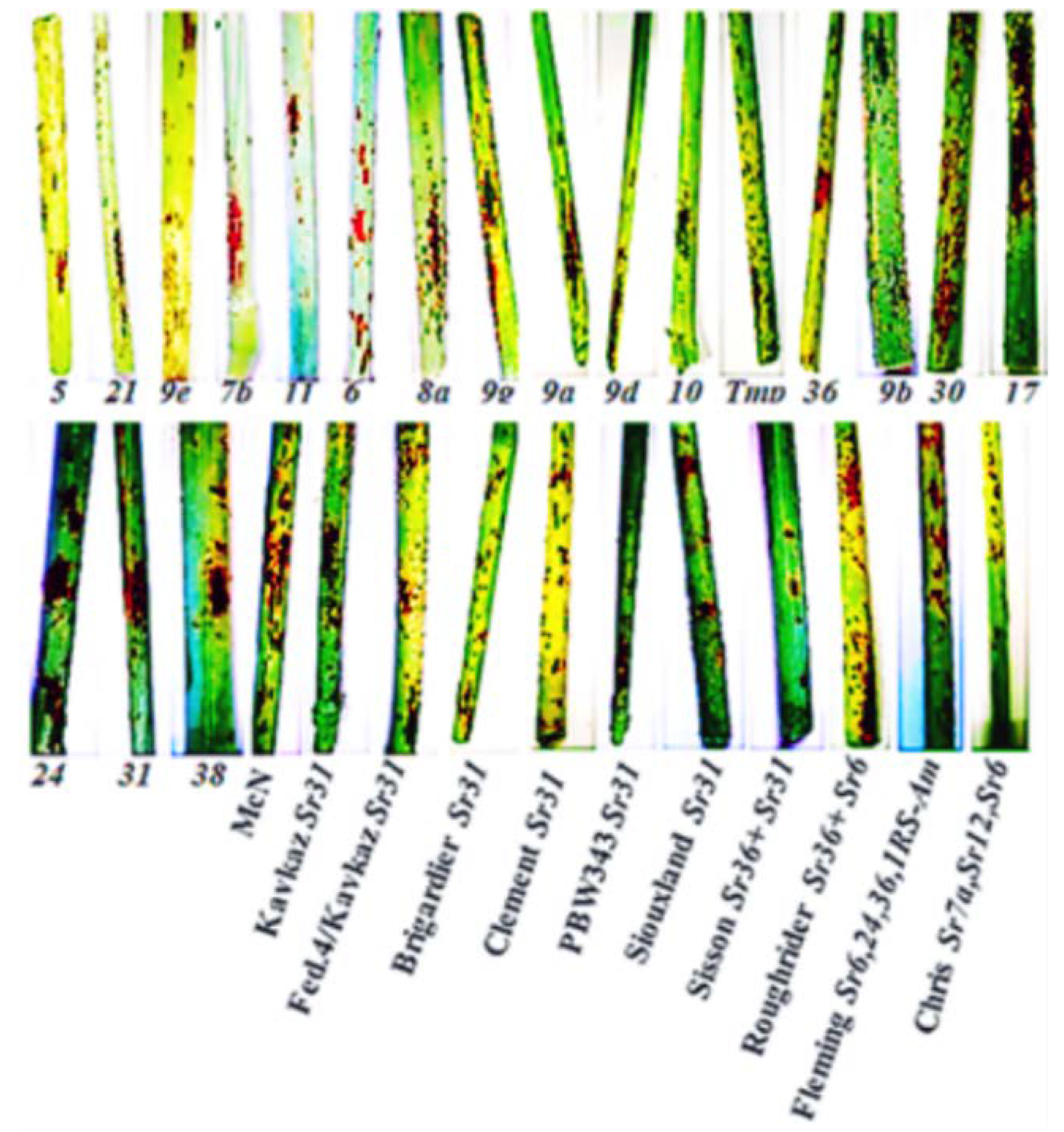
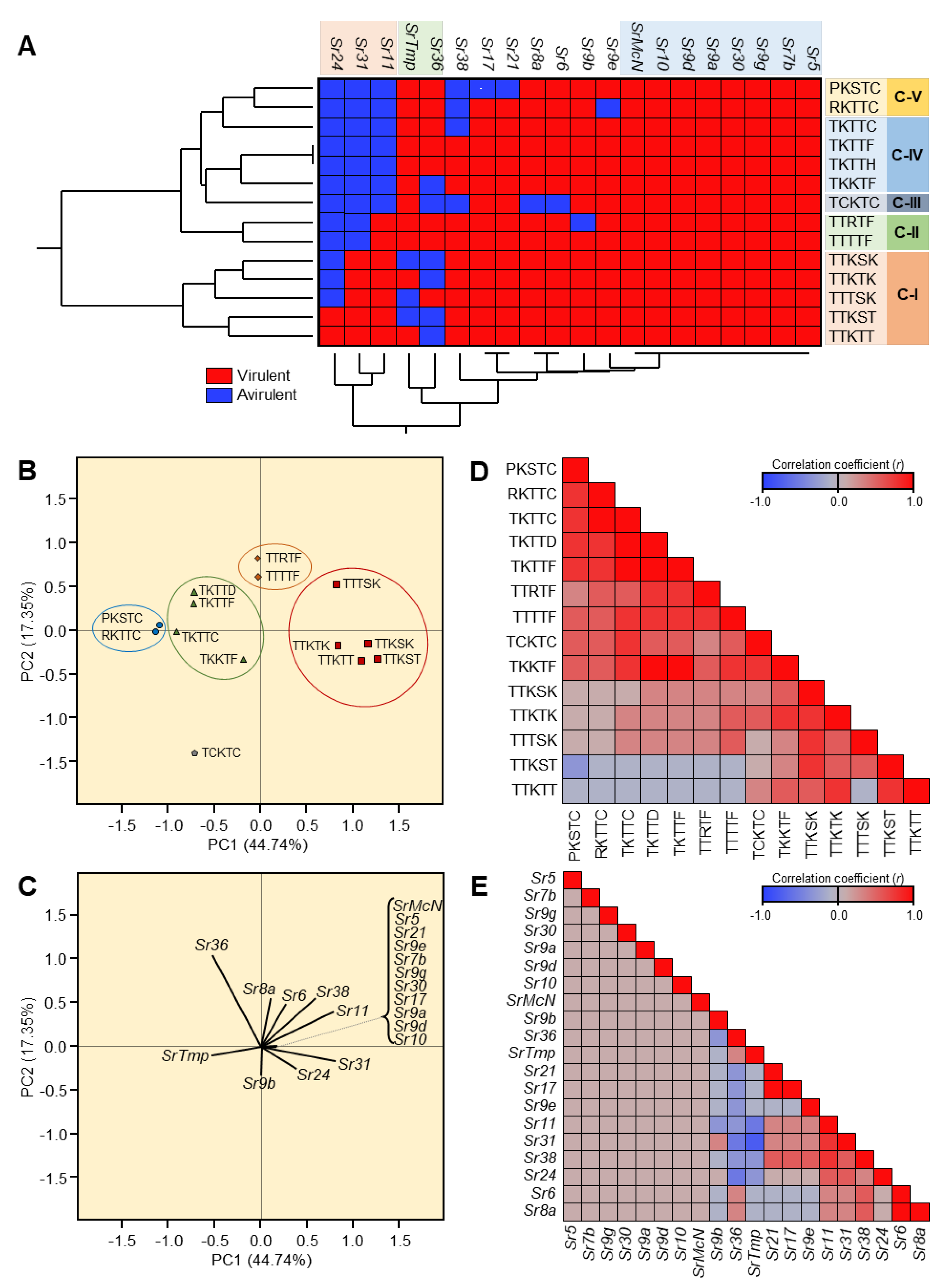
Our findings showed that disease severity of stem rust disease varied from one location to another and from one season to another. For instance, in the Kafrelsheikh governorate, the monogenic lines Kavkaz (Sr31), Federation4/Kavkaz (Sr31), Brigardier (Sr31), PBW343 (Sr31), Fleming (Sr6,24,36,1RS-Am), and Chris (Sr7a, Sr12, Sr6) were resistant during the three seasons under study. On the other hand, wheat lines and varieties with Sr genes, namely, lines Sr24, Sr31, Sr38, Federation4/Kavkaz (31), Brigardier (31), Clement (31), Siouxland (24 + 31), Roughrider (Sr36 + 6), Fleming (Sr6, 24, 36, 1RS-Am), and Chris (Sr7a, Sr12, Sr6) were susceptible to stem rust at the Sharqia location in the third season. Accordingly, it can be concluded that the presence of virulent races of P. graminis was able to break the resistance of Sr genes, which we noticed early in the field.
Previously, Hasan and Abou-Zeid [
25] reported that lines
Sr31, Sr24, Sr38, and
Sr36 were the most resistant genes, and Abebe et al. [
26] reported that lines
Sr24 and
SrTmp were effective for all tested isolates; however, our findings do not support this point of view. Taken together, we suggest that changes in the behavior of some lines, i.e.,
Sr24,
Sr31, Sr36, and
Sr38, are responsible for resistance and had to be interpreted, especially with the first identification of the Ug99 [
15].
As summarized by Singh et al. [
12], Ug99 had been confirmed in Uganda (1998), Kenya (2001), Ethiopia (2003), Sudan (2006), Yemen (2006), Iran (2007), and Tanzania (2009). This pathotype/race can overcome many
Sr genes in which virulence had not previously been detected. For instance, race Ug99 is the first known race of
P. graminis f. sp.
tritici that carries virulence to gene
Sr31 located on 1BL. 1RS translocation from rye (
Secale cereale) [
27] as well as virulent to the majority of
Sr resistance genes of wheat origin such as gene
Sr38, introduced into wheat from
Triticum ventricosum [
20]. This strong virulence combination in Ug99 explains why wheat cultivars and germplasm are susceptible all across the world. Because of the breakdown of these critical genes, it is necessary to better understand the geographical association and genetic diversity of newly formed races within the Ug99 lineage of the stem rust pathogen in various wheat-producing locations in general, and Egypt in particular.
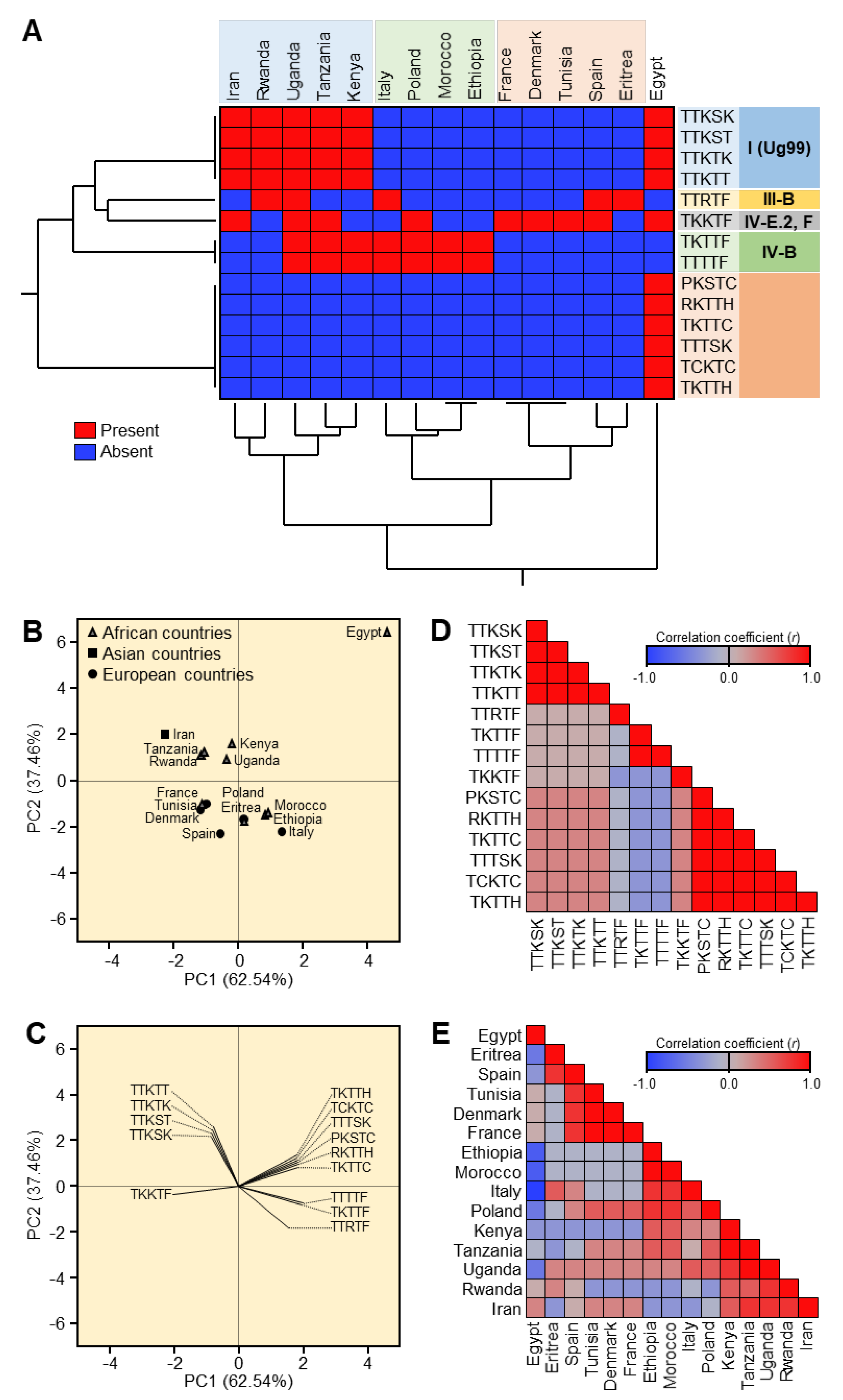
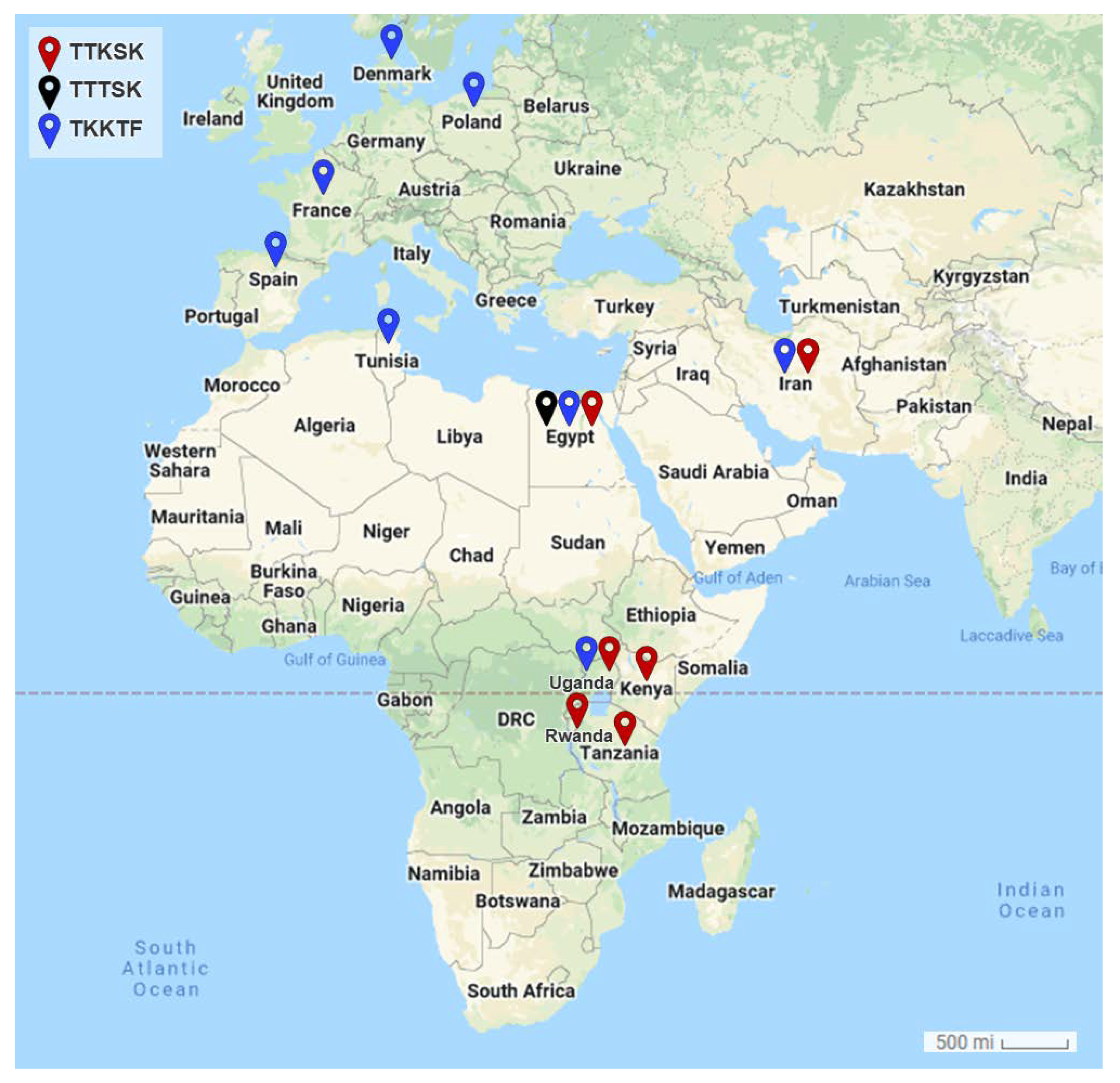
Fourteen stem rust races have been identified in Egypt and other countries in the stem rust greenhouse in Egypt and GRRC in Denmark. The geographical distribution of the most virulent races: TTKTT, TTKST, and TTKTK are found in Iran, Kenya, Rwanda, Tanzania, and Uganda), TTTSK is only found in Egypt, and TTKSK is found in Egypt, Iran, Kenya, Rwanda, Tanzania, and Uganda). The HCA and PCA-associated loading verified the substantial correlation between Kenya, Tanzania, Uganda, Rwanda, and Iran. Egypt, on the other hand, was adversely connected with the majority of the analyzed locations, including Eritrea, Spain, Ethiopia, Morocco, Italy, Poland, Kenya, Tanzania, and Uganda, or had no significant relationship, including Tunisia, Denmark, France, and Rwanda. Through this, it became clear that there is a relationship between the most virulent races (TTKSK, TTKTK, TTTSK, TTKST, TTKTT, TTRTF, and TTTTF) and the resistant monogenic lines (Sr11, Sr31, and Sr24) that fell into the same cluster through HCA and the PCA-associated loading.
Based on the North American naming system [
28], isolates of Ug99 obtained in Kenya were identified as TTKS and were renamed TTKSK after adding a fifth set of differentials to the nomenclature system [
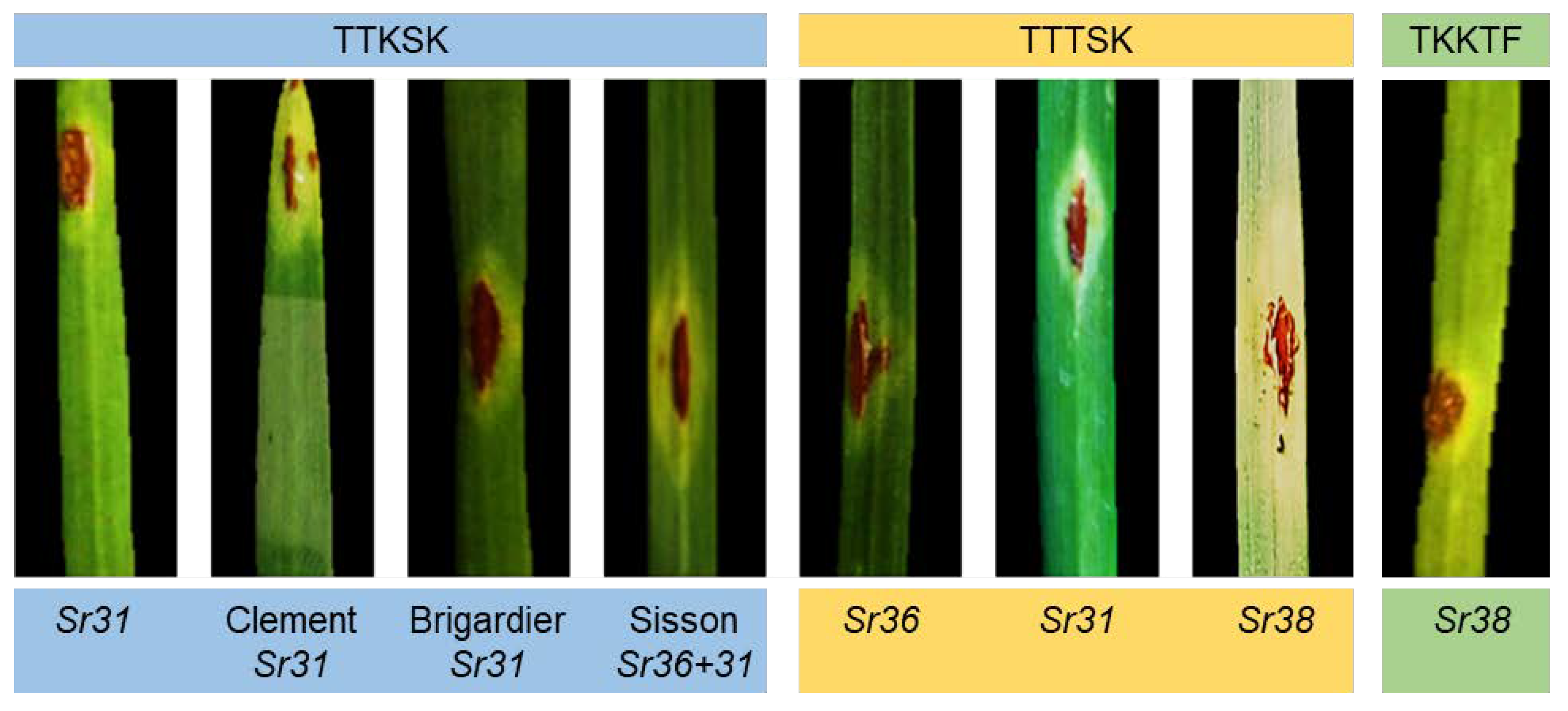
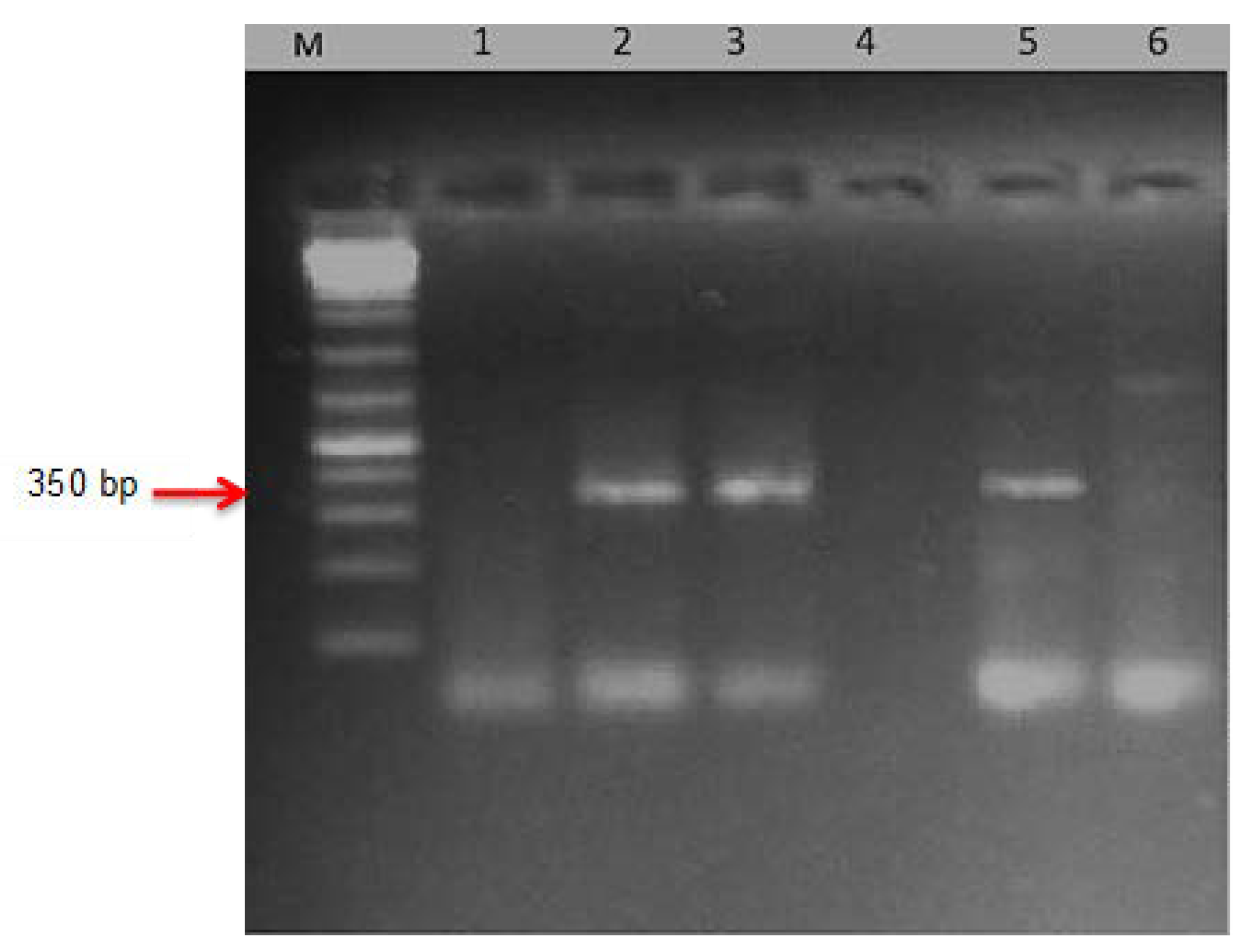
20]. Given the gravity of this race, TTKSK (Ug99), it was vital to prove its existence using molecular markers in the majority of the nations where it was defined. The diagnostic PCR fragments indicate virulent TTKSK (Ug99) to
Sr31, Clement (
Sr31), and Brigardier (
Sr31) as a fragment of 350 bp. This was in agreement with what was defined in the greenhouse (TTKSK) in Egypt and other countries based on the North American nomenclature [
7,
24].
Collectively, the findings of this study highlight the emergence of more aggressive races in Egypt, such as TTKSK, that break the resistance of wheat lines carrying Sr31, Sr24, Sr36, and Sr38 resistance. These aggressive races also appeared in some other countries such as Iran, Kenya, Rwanda, Tanzania, and Uganda. The TTTKS race is discovered for the first time in Egypt. As a result, the findings caution against breaking the resistance of Egyptian and international types bearing these genes. Because they were prone to infection with this disease, particularly the fungal races, it is vital in breeding efforts for rusts, particularly stem rust, to look for more efficient resistance genes and to incorporate more than one gene in one variety.
More research is needed to determine the genetic basis of existing wheat stem rust resistance in Egyptian wheat breeding materials (genotypes) and other nations. It is critical to understand the stem rust resistance genes that are now deployed in wheat cultivars in order to establish which genes provide long-lasting protection and more durable resistance in these wheat cultivars. This understanding will help alert pathologists and breeders to wheat cultivars’ genetic vulnerability in addition to assisting us in developing disease-resistant varieties and how to trade these types with one another in order to ensure global food security.
5. Conclusions
Due to their migration to countries with the bulk of the stem rust resistance genes (Sr) and the complex virulence combinations they carry, the Ug99 group of wheat stem rust fungus races continue to pose a threat to the global supply of wheat. In Central West Asia (CWANA), 10 African nations, 10 European nations, and at least 14 stem rust races have been recognized. Four races—TTKSK, TTKST, TTKTK, and TTKTT—came from five African nations—Egypt, Kenya, Rwanda, Tanzania, and Uganda—as well as Iran—but none from Europe—in the Ug99 group. On the other hand, TTRTF, TKTTF, and TTTTF were not discovered in Egypt. Kenya, Tanzania, Uganda, Rwanda, and Iran were all closely associated with one another, according to a correlation analysis. Contrarily, Egypt had a negative correlation with the majority of the countries examined, including Eritrea, Spain, Ethiopia, Morocco, Italy, Poland, Kenya, Tanzania, and Uganda, or at least no significant correlation was found, including Tunisia, Denmark, France, and Rwanda. By using molecular markers, Ug99 (TTKSK) was validated in Egypt. Only in Egypt did the TTTSK race become known.