Species Distribution of Candidemia and Their Susceptibility in a Single Japanese University Hospital: Prior Micafungin Use Affects the Appearance of Candida parapsilosis and Elevation of Micafungin MICs in Non-parapsilosis Candida Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patients and Episode
2.3. Organism Identification and Susceptibility Testing
2.4. Antifungal Use
2.5. Factorial Analysis
2.6. Statistical Analysis
3. Results
3.1. Species Distribution of the Isolates and Mortality Rate
3.2. Amount of Antifungal Usage and Species Distribution of Blood Isolated Candidemia from 2010 to 2018
3.3. Factorial Analysis for C. parapsilosis Associated with Candidemia
3.4. Antifungal MIC Distribution of Candida Blood Isolates
3.5. Factorial Analysis for Low Micafungin Susceptibility in Non-parapsilosis Candida Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Nagao, M. A multicentre analysis of epidemiology of the nosocomial bloodstream infections in Japanese university hospitals. Clin. Microbiol. Infect. 2013, 19, 852–858. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Gudlaugsson, O.; Gillespie, S.; Lee, K.; Berg, J.V.; Hu, J.; Messer, S.; Herwaldt, L.; Pfaller, M.; Diekema, D. Attributable Mortality of Nosocomial Candidemia, Revisited. Clin. Infect. Dis. 2003, 37, 1172–1177. [Google Scholar] [CrossRef]
- Klingspor, L.; Tortorano, A.M.; Pemán, J.; Willinger, B.; Hamal, P.; Sendid, B.; Velegraki, A.; Kibbler, C.; Meis, J.; Sabino, R.F.P.; et al. Invasive Candida infections in surgical patients in intensive care units: A prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008). Clin. Microbiol. Infect. 2015, 21, 87.e1–87.e10. [Google Scholar] [CrossRef]
- Morgan, J.; Meltzer, M.I.; Plikaytis, B.D.; Sofair, A.N.; Huie-White, S.; Wilcox, S.; Harrison, L.H.; Seaberg, E.C.; Hajjeh, R.A.; Teutsch, S.M. Excess Mortality, Hospital Stay, and Cost Due to Candidemia: A Case-Control Study Using Data From Population-Based Candidemia Surveillance. Infect. Control. Hosp. Epidemiol. 2005, 26, 540–547. [Google Scholar] [CrossRef]
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D. Time to Initiation of Fluconazole Therapy Impacts Mortality in Patients with Candidemia: A Multi-Institutional Study. Clin. Infect. Dis. 2006, 43, 25–31. [Google Scholar] [CrossRef]
- Ben-Abraham, R. Predictors of adverse outcome from candidal infection in a tertiary care hospital. J. Infect. 2004, 49, 317–323. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.; Akova, M.; Herbrecht, R.; Viscoli, C.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID* Guideline for the diagnosis and management of Candida diseases 2012: Adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. 2012, 18, 53–67. [Google Scholar] [CrossRef]
- Kohno, S.; Tamura, K.; Niki, Y.; Izumikawa, K.; Oka, S.; Ogawa, K.; Kadota, J.; Kamei, K.; Kanda, Y.; Kiuchi, T.; et al. Executive Summary of Japanese Domestic Guidelines for Management of Deep-seated Mycosis Med. Mycol. J. 2016, 57, E117–E163. [Google Scholar] [CrossRef]
- Tashiro, S.; Osa, S.; Igarashi, Y.; Watabe, Y.; Liu, X.; Enoki, Y.; Taguchi, K.; Mayumi, T.; Miyazaki, Y.; Takesue, Y.; et al. Echinocandins versus non-echinocandins for the treatment of invasive candidiasis: A meta-analysis of randomized controlled trials. J. Infect. Chemother. 2020, 26, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Japan Society for Hematopoietic Cell Transplantation (JSHCT). Guideline for Prophylaxis and Treatment of Fungal Infection; JSHCT Monograph Volume; September 2017; Available online: https://www.jshct.com/uploads/files/guideline/01_04_shinkin.pdf (accessed on 11 April 2021).
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef]
- Kawabe, A.; Muraki, Y.; Inose, R.; Kusama, Y.; Goto, R.; Ebisui, A.; Ishii, S.; Ishikane, M.; Ohge, H.; Ohmagari, N. Trends of Antifungal Use Based on Sales Data in Japan from 2006 to 2015. Biol. Pharm. Bull. 2020, 43, 1248–1252. [Google Scholar] [CrossRef]
- Takakura, S.; Fujihara, N.; Saito, T.; Kudo, T.; Iinuma, Y.; Ichiyama, S. National surveillance of species distribution in blood isolates of Candida species in Japan and their susceptibility to six antifungal agents including voriconazole and micafungin. J. Antimicrob. Chemother. 2004, 53, 283–289. [Google Scholar] [CrossRef]
- Kakeya, H.; Yamada, K.; Kaneko, Y.; Yanagihara, K.; Tateda, K.; Maesaki, S.; Takesue, Y.; Tomono, K.; Kadota, J.-I.; Kaku, M.; et al. National Trends in the Distribution of Candida Species Causing Candidemia in Japan from 2003 to 2014. Med. Mycol. J. 2018, 59, E19–E22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakagami, T.; Kawano, T.; Yamashita, K.; Yamada, E.; Fujino, N.; Kaeriyama, M.; Fukuda, Y.; Nomura, N.; Mitsuyama, J.; Suematsu, H.; et al. Antifungal susceptibility trend and analysis of resistance mechanism for Candida species isolated from bloodstream at a Japanese university hospital. J. Infect. Chemother. 2019, 25, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Forrest, G.N.; Weekes, E.; Johnson, J.K. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J. Infect. 2008, 56, 126–129. [Google Scholar] [CrossRef]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61, S612–S617. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed.; CLSI Supplement, M60; Wayne, P.A., Ed.; Clinical and Laboratory Standards Institute: Wayne, SA, USA, 2017; pp. 1–3. Available online: http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M60%20ED1:2017&xormat=SPDF&src=BB (accessed on 11 April 2021).
- Samura, M.; Hirose, N.; Kurata, T.; Ishii, J.; Nagumo, F.; Takada, K.; Koshioka, S.; Uchida, M.; Yamamoto, S.; Inoue, J.; et al. Support for fungal infection treatment mediated by pharmacist-led antifungal stewardship activities. J. Infect. Chemother. 2020, 26, 272–279. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Baggs, J.; Tsay, S.; Srinivasan, A.R.; A Jernigan, J.; Jackson, B.R. Trends in antifungal use in US hospitals, 2006–12. J. Antimicrob. Chemother. 2018, 73, 2867–2875. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Díaz-Martín, A.; García-Cabrera, E.; de Pipaón, M.R.P.; Hernández-Caballero, C.; Martín, J.A.; Cisneros, J.M.; Ortiz-Leyba, C. Risk Factors for Fluconazole-Resistant Candidemia. Antimicrob. Agents Chemother. 2010, 54, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Taplitz, R.A.; Kennedy, E.B.; Flowers, C.R. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2018, 14, 692–695. [Google Scholar] [CrossRef]
- Maertens, J.A.; Girmenia, C.; Brüggemann, R.J.; Duarte, R.F.; Kibbler, C.C.; Ljungman, P.; Racil, Z.; Ribaud, P.; Slavin, M.; A Cornely, O.; et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 2018, 73, 3221–3230. [Google Scholar] [CrossRef]
- Fleming, S.; Yannakou, C.K.; Haeusler, G.; Clark, J.; Grigg, A.; Heath, C.H.; Bajel, A.; Van Hal, S.J.; Chen, S.C.; Milliken, S.T.; et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, Intern. Med. J. 2014, 44, 1283–1297. [Google Scholar] [CrossRef]
- Zonios, D.I.; Bennett, J.E. Update on Azole Antifungals. Semin. Respir. Crit. Care Med. 2008, 29, 198–210. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ikawa, K.; Abematsu, K.; Fukunaga, N.; Nishida, K.; Fukamizu, T.; Shimodozono, Y.; Morikawa, N.; Takeda, Y.; Yamada, K. Correlation between voriconazole trough plasma concentration and hepatotoxicity in patients with different CYP2C19 genotypes. Int. J. Antimicrob. Agents 2009, 34, 91–94. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Sulim, S.; Holm, A.; Nielsen, L.; Nielsen, S.D.; Knudsen, J.D.; Drenck, N.E.; Christensen, J.J.; Johansen, H.K. Diagnostic Issues, Clinical Characteristics, and Outcomes for Patients with Fungemia. J. Clin. Microbiol. 2011, 49, 3300–3308. [Google Scholar] [CrossRef] [PubMed]
- Bizerra, F.C.; Jimenez-Ortigosa, C.; Souza, A.C.R.; Breda, G.L.; Queiroz-Telles, F.; Perlin, D.; Colombo, A.L. Breakthrough Candidemia Due to Multidrug-Resistant Candida glabrata during Prophylaxis with a Low Dose of Micafungin. Antimicrob. Agents Chemother. 2014, 58, 2438–2440. [Google Scholar] [CrossRef]
- Andes, D.R.; Diekema, D.; Pfaller, M.; Marchillo, K.; Bohrmueller, J. In Vivo Pharmacodynamic Target Investigation for Micafungin against Candida albicans and C. glabrata in a Neutropenic Murine Candidiasis Model. Antimicrob. Agents Chemother. 2008, 52, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Almirante, B.; Rodríguez, D.; Cuenca-Estrella, M.; Almela, M.; Sanchez-Reus, F.; Ayats, J.; Alonso-Tarres, C.; Rodriguez-Tudela, J.L.; Pahissa, A.; The Barcelona Candidemia Project Study Group. Epidemiology, Risk Factors, and Prognosis of Candida parapsilosis Bloodstream Infections: Case-Control Population-Based Surveillance Study of Patients in Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2006, 44, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Yoshimura, Y.; Suido, Y.; Shimizu, H.; Ide, K.; Sugiyama, Y.; Matsuno, K.; Nakajima, H. Mortality and risk factor analysis for Candida blood stream infection: A multicenter study. J. Infect. Chemother. 2019, 25, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Ambrose, P.G.; Hammel, J.P.; Van Wart, S.A.; Iyer, V.; Reynolds, D.K.; Buell, D.N.; Kovanda, L.L.; Bhavnani, S.M. Use of Pharmacokinetic-Pharmacodynamic Analyses To Optimize Therapy with the Systemic Antifungal Micafungin for Invasive Candidiasis or Candidemia. Antimicrob. Agents Chemother. 2011, 55, 2113–2121. [Google Scholar] [CrossRef]
- Kapralos, I.; Mainas, E.; Neroutsos, E.; Apostolidi, S.; Siopi, M.; Apostolopoulou, O.; Dimopoulos, G.; Sambatakou, H.; Valsami, G.; Meletiadis, J.; et al. Population pharmacokinetics of micafungin over repeated doses in critically ill patients: A need for a loading dose? J. Pharm. Pharmacol. 2020, 72, 1750–1760. [Google Scholar] [CrossRef]
- Burton, M.J.; Swiatlo, E.; Shah, P. Misidentification of Candida parapsilosis as C. famata in a Clinical Case of Vertebral Osteomyelitis. Am. J. Med. Sci. 2011, 341, 71–73. [Google Scholar] [CrossRef]
- Castanheira, M.; Woosley, L.; Diekema, D.; Jones, R.N.; Pfaller, M. Candida guilliermondii and Other Species of Candida Misidentified as Candida famata: Assessment by Vitek 2, DNA Sequencing Analysis, and Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry in Two Global Antifungal Surveillance Programs. J. Clin. Microbiol. 2012, 51, 117–124. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, J.H.; Mok, J.H.; Kim, S.Y.; Song, S.A.; Kim, H.R.; Kook, J.-K.; Chang, Y.-H.; Bae, I.K.; Lee, K. Misidentification of Candida guilliermondii as C. famata among Strains Isolated from Blood Cultures by the VITEK 2 System. Biomed. Res. Int. 2014, 2014, 250408. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Wang, F.-D.; Chen, Y.-C.; Hsieh, M.-H.; Hii, I.-M.; Lee, Y.-L.; Ho, M.-W.; Liu, C.-E.; Chen, Y.-H.; Liu, W.-L. High rates of misidentification of uncommon Candida species causing bloodstream infections using conventional phenotypic methods. J. Formos. Med. Assoc. 2021, 120, 1179–1187. [Google Scholar] [CrossRef]
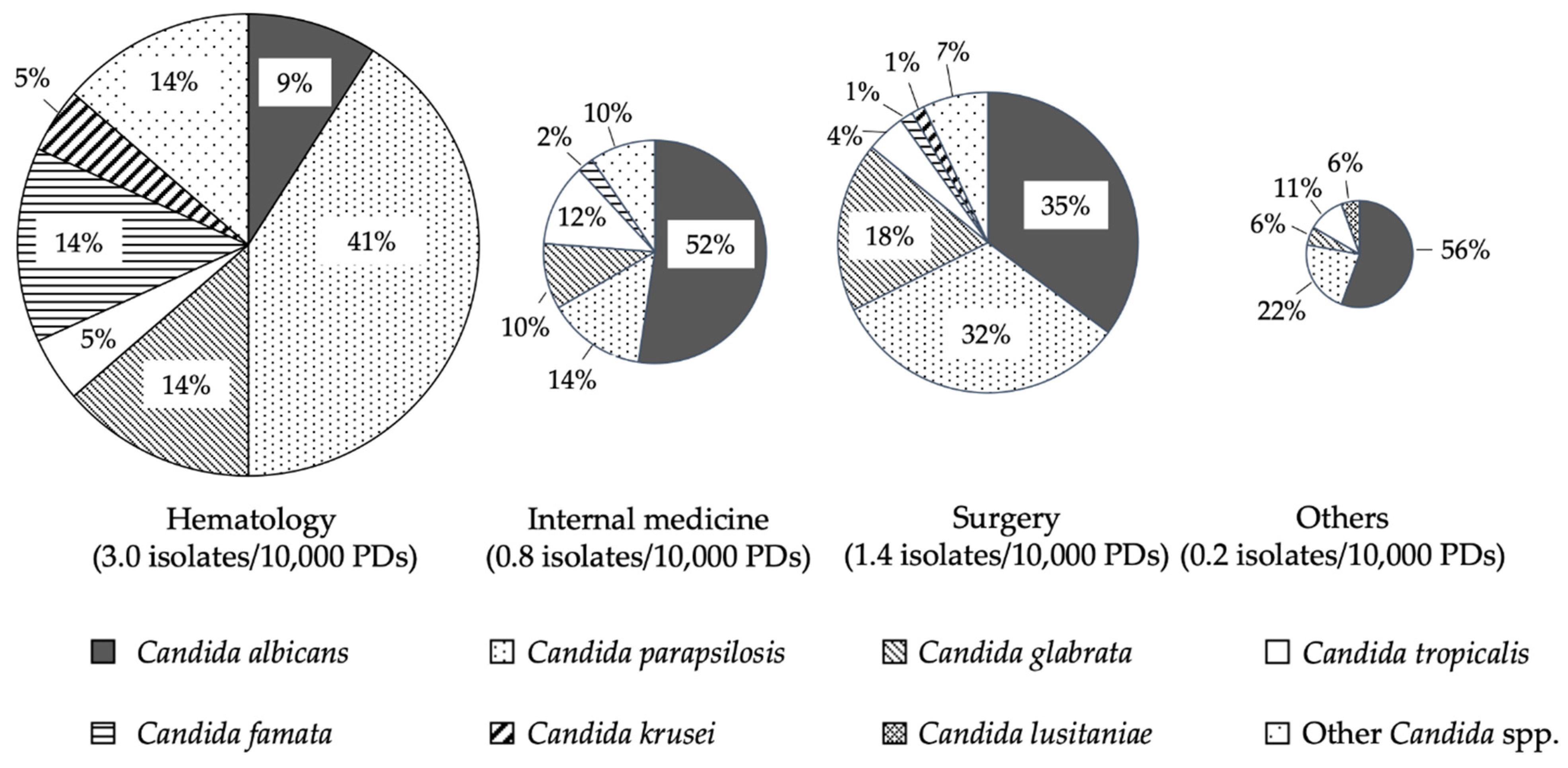
| Species | No. (%) of Isolates | 30-Day Mortality Rates |
|---|---|---|
| Candida albicans | 59 (38.6) | 23.7 |
| Candida parapsilosis | 42 (27.5) | 19.0 |
| Candida glabrata | 21 (13.7) | 23.8 |
| Candida tropicalis | 11 (7.2) | 36.4 |
| Candida famata | 5 (3.3) | 60.0 |
| Candida krusei | 2 (1.3) | 50.0 |
| Candida lusitaniae | 1 (0.7) | 0 |
| Other † | 12 (7.8) | 8.3 |
| Total | 153 (100) | 23.5 |
| Hematology | Internal Medicine | Surgery | Others | |
|---|---|---|---|---|
| Micafungin | 181.9 | 4.3 | 10.7 | 9.2 |
| Caspofungin | 5.9 | 0.1 | 0.2 | 0.2 |
| Fluconazole | 104.1 | 5.1 | 7.1 | 42.7 |
| Fosfluconazole | 0.3 | 0.1 | 0.1 | 0.0 |
| Itraconazole | 332.6 | 2.0 | 0.3 | 4.1 |
| Voriconazole | 38.8 | 2.6 | 0.9 | 6.6 |
| Liposomal | 9.3 | 0.6 | 1.1 | 2.0 |
| Amphotericin B | ||||
| Amphotericin B | 0.0 | 0.0 | 0.0 | 0.1 |
| Flucytosine | 0.0 | 0.4 | 0.0 | 0.1 |
| Total | 673.0 | 15.2 | 20.2 | 65.0 |
| Factor | Non-parapsilosis Candida Species (n = 108) | C. parapsilosis (n = 39) | p | Unadjusted OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|---|---|---|
| No. (%) male patients | 69 (64) | 27 (69) | 0.695 | ||||
| Hospitalization in prior 90 days | 56 (52) | 16 (55) | 0.267 | ||||
| ICU stay | 38 (35) | 6 (15) | 0.025 | 0.307 (0.102–0.923) | 0.036 | 0.276 (0.094–0.809) | 0.019 |
| No. (%) of patient department: | |||||||
| Hematology | 13 (12) | 7 (18) | 0.415 | ||||
| Internal medicine | 35 (32) | 5 (13) | 0.021 | ||||
| Surgery | 46 (43) | 23 (56) | 0.094 | ||||
| Others | 14 (13) | 4 (10) | 0.781 | ||||
| No. (%) of patients with: | |||||||
| Diabetes | 23 (21) | 6 (15) | 0.490 | ||||
| Chronic renal disease | 54 (50) | 12 (31) | 0.041 | 0.443 (0.187–1.05) | 0.063 | 0.441 (0.190–1.027) | 0.058 |
| HIV infection | 0 (0) | 0 (0) | - | ||||
| Solid organ transplantation | 2 (2) | 0 (0) | 1.00 | ||||
| Bone marrow transplantation | 4 (4) | 4 (10) | 0.209 | ||||
| Neutropenia (< 500/μL) | 9 (8) | 4 (10) | 0.746 | ||||
| No. (%) of patients who underwent invasive procedures | |||||||
| Gastrointestinal surgery | 13 (12) | 5 (13) | 1.00 | ||||
| Renal replacement therapy | 17 (16) | 3 (8) | 0.281 | ||||
| Tunneled catheter | 3 (3) | 4 (10) | 0.081 | 2.17 (0.383–12.2) | 0.382 | ||
| Nontunneled catheter | 84 (78) | 26 (67) | 0.198 | 0.6 (0.228–1.58) | 0.302 | ||
| No. (%) of patients with previous: | |||||||
| Antibiotic treatment | 78 (72) | 24 (62) | 0.229 | ||||
| Fluconazole exposure | 2 (2) | 0 (0) | 1.00 | ||||
| Itraconazole exposure | 2 (2) | 1 (3) | 1.00 | ||||
| Voriconazole exposure | 1 (1) | 0 (0) | 1.00 | ||||
| Micafungin exposure | 13 (12) | 9 (23) | 0.118 | 4.97 (1.56–15.5) | 0.006 | 4.22 (1.39–12.8) | 0.011 |
| Liposomal Amphotericin B exposure | 1 (1) | 0 (0) | 1.00 | ||||
| Corticosteroid treatment | 29 (27) | 11 (28) | 1.00 | ||||
| Immunosuppression medications | 11 (10) | 6 (15) | 0.391 | ||||
| Species and Antifungal Agent (No. of Isolates) | No. of Isolates with MIC (mg/mL) of: | MIC50 (μg/mL) | MIC90 (μg/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | >64 | |||
| C. albicans (57) | ||||||||||||||||
| Fluconazole | 19 | 18 | 6 | 8 | 3 | 1 | 1 | 1 | 0.25 | 2 | ||||||
| Itraconazole | 5 | 14 | 21 | 11 | 2 | 3 | 1 † | 0.06 | 0.25 | |||||||
| Voriconazole | 35 | 10 | 6 | 2 | 1 | 2 | 1 † | ≤ 0.01 | 0.12 | |||||||
| Amphotericin B | 1 | 1 | 4 | 24 | 22 | 4 | 1 | 0.25 | 0.5 | |||||||
| Caspofungin ‡ | 2 | 12 | 3 | 0.25 | 0.5 | |||||||||||
| Micafungin | 49 | 7 | 1 | ≤ 0.01 | 0.03 | |||||||||||
| C. parapsilosis (39) | ||||||||||||||||
| Fluconazole | 1 | 5 | 20 | 12 | 1 | 0.5 | 1 | |||||||||
| Itraconazole | 3 | 14 | 19 | 2 | 1 | 0.12 | 0.12 | |||||||||
| Voriconazole | 23 | 15 | 1 | 0.015 | 0.03 | |||||||||||
| Amphotericin B | 6 | 13 | 18 | 2 | 0.5 | 0.5 | ||||||||||
| Caspofungin ‡ | 2 | 7 | 1 | 1 | ||||||||||||
| Micafungin | 1 | 1 | 4 | 23 | 10 | 0.5 | 1 | |||||||||
| C. glabarata (20) | ||||||||||||||||
| Fluconazole | 1 | 4 | 7 | 6 | 2 | 4 | 16 | |||||||||
| Itraconazole | 1 | 3 | 6 | 6 | 4 | 0.25 | 1 | |||||||||
| Voriconazole | 3 | 1 | 4 | 5 | 6 | 1 | 0.12 | 0.25 | ||||||||
| Amphotericin B | 1 | 5 | 11 | 3 | 0.5 | 1 | ||||||||||
| Caspofungin ‡ | 3 | 3 | 1 | 1 | 4 | |||||||||||
| Micafungin | 8 | 3 | 1 | 1 | 2 | 2 | 3 | 0.03 | 1 | |||||||
| C. tropicalis (11) | ||||||||||||||||
| Fluconazole | 4 | 2 | 1 | 1 | 1 | 2 | 0.5 | > 64 | ||||||||
| Itraconazole | 1 | 5 | 1 | 1 | 1 | 1 | 1 † | 0.06 | 2 | |||||||
| Voriconazole | 2 | 4 | 1 | 1 | 1 | 1 | 1 † | 0.03 | 4 | |||||||
| Amphotericin B | 3 | 8 | 0.5 | 0.5 | ||||||||||||
| Caspofungin ‡ | 2 | 5 | 0.5 | 0.5 | ||||||||||||
| Micafungin | 4 | 7 | 0.03 | 0.03 | ||||||||||||
| C. famata (5) | ||||||||||||||||
| Fluconazole | 1 | 1 | 3 | 2 | 2 | |||||||||||
| Itraconazole | 1 | 4 | 0.25 | 0.25 | ||||||||||||
| Voriconazole | 1 | 1 | 3 | 0.06 | 0.06 | |||||||||||
| Amphotericin B | 3 | 2 | 0.25 | 0.5 | ||||||||||||
| Caspofungin ‡ | 2 | 1 | 2 | 0.5 | 1 | |||||||||||
| Micafungin | 2 | 2 | 1 | 0.25 | 0.5 | |||||||||||
| C. krusei (2) | ||||||||||||||||
| Fluconazole § | 1 | 1 | 16 | 32 | ||||||||||||
| Itraconazole | 2 | 0.25 | 0.25 | |||||||||||||
| Voriconazole | 2 | 0.25 | 0.25 | |||||||||||||
| Amphotericin B | 1 | 1 | 0.5 | 1 | ||||||||||||
| Caspofungin ‡ | 2 | 1 | 1 | |||||||||||||
| Micafungin | 2 | 0.12 | 0.12 | |||||||||||||
| Other Candida spp. (13) | ||||||||||||||||
| Fluconazole | 3 | 4 | 1 | 3 | 2 | 0.5 | 4 | |||||||||
| Itraconazole | 4 | 4 | 4 | 1 | 0.12 | 0.25 | ||||||||||
| Voriconazole | 6 | 1 | 4 | 1 | 1 | 0.03 | 0.12 | |||||||||
| Amphotericin B | 1 | 8 | 3 | 1 | 0.25 | 0.5 | ||||||||||
| Caspofungin ‡ | 3 | 5 | 1 | 1 | ||||||||||||
| Micafungin | 1 | 3 | 3 | 4 | 2 | 0.25 | 1 | |||||||||
| Factor | MIC ≤ 0.06 μg/mL (n = 80) | MIC > 0.06 μg/mL (n = 28) | p | Unadjusted OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|---|---|---|
| No. (%) male patients | 51 (64) | 18 (64) | 1.00 | ||||
| Hospitalization in prior 90 days | 38 (48) | 16 (57) | 0.511 | ||||
| ICU stay | 26 (33) | 12 (43) | 0.362 | ||||
| No. (%) of patient department: | |||||||
| Hematology | 4 (5) | 9 (32) | < 0.001 | ||||
| Internal medicine | 30 (38) | 5 (18) | 0.064 | ||||
| Surgery | 33 (41) | 13 (46) | 0.662 | ||||
| Others | 13 (16) | 1 (4) | 0.109 | ||||
| No. (%) of patients with: | |||||||
| Diabetes | 15 (19) | 8 (29) | 0.292 | ||||
| Chronic renal disease | 39 (49) | 15 (54) | 0.669 | ||||
| HIV infection | 0 (0) | 0 (0) | - | ||||
| Solid organ transplantation | 1 (1) | 1 (4) | 0.453 | ||||
| Bone marrow transplantation | 2 (3) | 2 (7) | 0.276 | ||||
| Neutropenia(< 500/μL) | 3 (4) | 6 (21) | 0.009 | 0.762 (0.094–6.18) | 0.799 | ||
| No. (%) of patients who underwent invasive procedures | |||||||
| Gastrointestinal surgery | 11 (14) | 2 (7) | 0.508 | ||||
| Renal replacement therapy | 12 (15) | 5 (18) | 0.766 | ||||
| Tunneled catheter | 3 (4) | 0 (0) | 0.567 | ||||
| Nontunneled catheter | 58 (73) | 26 (93) | 0.033 | 4.61 (0.846–25.1) | 0.077 | ||
| No. (%) of patients with previous: | |||||||
| Antibiotic treatment | 55 (69) | 23 (82) | 0.224 | ||||
| Micafungin exposure | 3 (4) | 10 (36) | < 0.001 | 11.5 (1.91–69.1) | 0.008 | 13.2 (3.23–54.2) | < 0.01 |
| Corticosteroid treatment | 20 (25) | 9 (32) | 0.467 | ||||
| Immunosuppression medications | 5 (6) | 6 (21) | 0.032 | 5.44 (1.05–28.1) | 0.043 | 3.44 (0.831–14.2) | 0.088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, Y.; Kawabe, K.; Suzuki, T.; Sano, K.; Ide, K.; Nishigaki, T.; Enoki, Y.; Taguchi, K.; Koike, H.; Kato, H.; et al. Species Distribution of Candidemia and Their Susceptibility in a Single Japanese University Hospital: Prior Micafungin Use Affects the Appearance of Candida parapsilosis and Elevation of Micafungin MICs in Non-parapsilosis Candida Species. J. Fungi 2021, 7, 596. https://doi.org/10.3390/jof7080596
Sakamoto Y, Kawabe K, Suzuki T, Sano K, Ide K, Nishigaki T, Enoki Y, Taguchi K, Koike H, Kato H, et al. Species Distribution of Candidemia and Their Susceptibility in a Single Japanese University Hospital: Prior Micafungin Use Affects the Appearance of Candida parapsilosis and Elevation of Micafungin MICs in Non-parapsilosis Candida Species. Journal of Fungi. 2021; 7(8):596. https://doi.org/10.3390/jof7080596
Chicago/Turabian StyleSakamoto, Yasutaka, Kazuhiro Kawabe, Tomoyo Suzuki, Kayoko Sano, Kazuo Ide, Tetsuta Nishigaki, Yuki Enoki, Kazuaki Taguchi, Hirofumi Koike, Hideaki Kato, and et al. 2021. "Species Distribution of Candidemia and Their Susceptibility in a Single Japanese University Hospital: Prior Micafungin Use Affects the Appearance of Candida parapsilosis and Elevation of Micafungin MICs in Non-parapsilosis Candida Species" Journal of Fungi 7, no. 8: 596. https://doi.org/10.3390/jof7080596
APA StyleSakamoto, Y., Kawabe, K., Suzuki, T., Sano, K., Ide, K., Nishigaki, T., Enoki, Y., Taguchi, K., Koike, H., Kato, H., Sahashi, Y., & Matsumoto, K. (2021). Species Distribution of Candidemia and Their Susceptibility in a Single Japanese University Hospital: Prior Micafungin Use Affects the Appearance of Candida parapsilosis and Elevation of Micafungin MICs in Non-parapsilosis Candida Species. Journal of Fungi, 7(8), 596. https://doi.org/10.3390/jof7080596






