Dynamics and Predictors of Mortality Due to Candidemia Caused by Different Candida Species: Comparison of Intensive Care Unit-Associated Candidemia (ICUAC) and Non-ICUAC
Abstract
1. Introduction
2. Materials and Methods
2.1. Candida Isolates and Clinical Data Collection
2.2. Species Identification and Antifungal Susceptibility Testing
2.3. Definition
2.4. Statistical Analysis
3. Results
3.1. Species Distribution and Antifungal Resistance
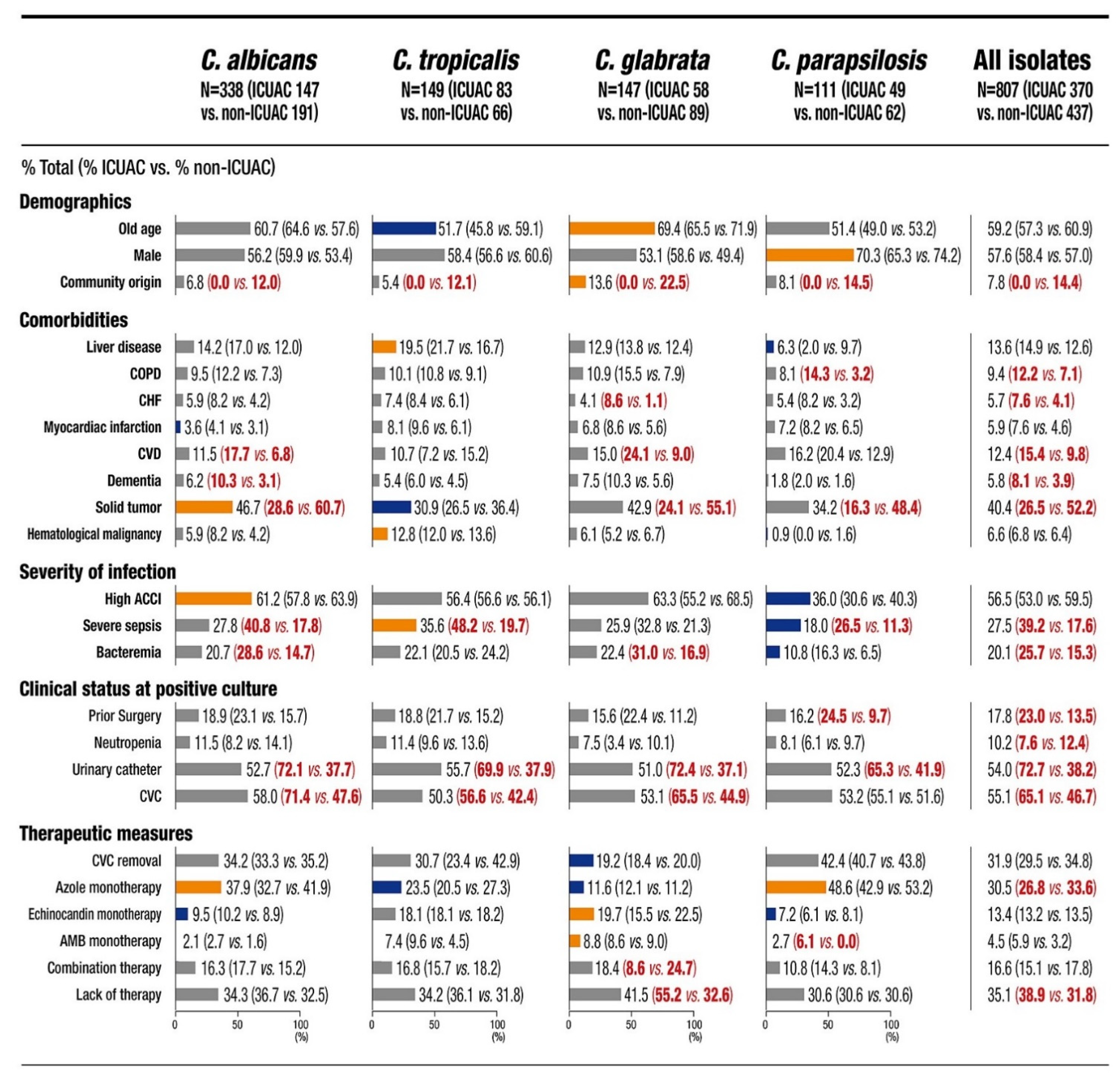
3.2. Baseline Characteristics
3.3. Mortality Rate
3.4. Predictors of Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gudlaugsson, O.; Gillespie, S.; Lee, K.; Vande Berg, J.; Hu, J.; Messer, S.; Herwaldt, L.; Pfaller, M.; Diekema, D. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 2003, 37, 1172–1177. [Google Scholar] [CrossRef]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Pappas, P.G.; Rex, J.H.; Lee, J.; Hamill, R.J.; Larsen, R.A.; Powderly, W.; Kauffman, C.A.; Hyslop, N.; Mangino, J.E.; Chapman, S.; et al. A prospective observational study of candidemia: Epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 2003, 37, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug. Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Garnacho-Montero, J.; Calandra, T.; Kullberg, B.; Dimopoulos, G.; Azoulay, E.; Chakrabarti, A.; Kett, D.; Leon, C.; Ostrosky-Zeichner, L.; et al. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Med. 2017, 43, 1225–1238. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sood, P.; Rudramurthy, S.M.; Chen, S.; Kaur, H.; Capoor, M.; Chhina, D.; Rao, R.; Eshwara, V.K.; Xess, I.; et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015, 41, 285–295. [Google Scholar] [CrossRef]
- Colombo, A.L.; Guimaraes, T.; Sukienik, T.; Pasqualotto, A.C.; Andreotti, R.; Queiroz-Telles, F.; Nouer, S.A.; Nucci, M. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: An analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med. 2014, 40, 1489–1498. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Madec, Y.; Denoeud-Ndam, L.; Wolff, M.; Fontanet, A.; Bretagne, S.; Dromer, F.; The French Mycosis Study Group. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014, 40, 1303–1312. [Google Scholar] [CrossRef]
- Byun, S.A.; Won, E.J.; Kim, M.N.; Lee, W.G.; Lee, K.; Lee, H.S.; Uh, Y.; Healey, K.R.; Perlin, D.S.; Choi, M.J.; et al. Multilocus sequence typing (MLST) genotypes of Candida glabrata bloodstream isolates in Korea: Association with antifungal resistance, mutations in mismatch repair gene (Msh2), and clinical outcomes. Front. Microbiol. 2018, 9, 1523. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.G.; Trecarichi, E.M.; Montrucchio, C.; Losito, A.R.; Raviolo, S.; Posteraro, B.; Corcione, S.; Di Giambenedetto, S.; Fossati, L.; Sanguinetti, M.; et al. Mortality in patients with early- or late-onset candidaemia. J. Antimicrob. Chemother. 2013, 68, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, E.J.; Kim, D.; Jeong, S.H.; Shin, J.H.; Shin, J.H.; Shin, K.S.; Kim, Y.A.; Uh, Y.; Park, C.; et al. Establishment of the South Korean national antimicrobial resistance surveillance system, Kor-GLASS, in 2016. Eurosurveillance 2018, 23, 1700734. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, E.J.; Kim, D.; Jeong, S.H.; Won, E.J.; Shin, J.H.; Kim, S.H.; Shin, J.H.; Shin, K.S.; Kim, Y.A.; et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: First one-year report from Kor-GLASS. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Won, E.J.; Choi, M.J.; Kim, M.N.; Yong, D.; Lee, W.G.; Uh, Y.; Kim, T.S.; Byeon, S.A.; Lee, S.Y.; Kim, S.H.; et al. Fluconazole-Resistant Candida glabrata bloodstream isolates, South Korea, 2008–2018. Emerg. Infect. Dis. 2021, 27, 779–788. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Progress in antifungal susceptibility testing of Candida spp. by use of clinical and laboratory standards institute broth microdilution methods, 2010 to 2012. J. Clin. Microbiol. 2012, 50, 2846–2856. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Executive summary: Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, 409–417. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeast, 2nd ed.; CLSI M60; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 2nd ed.; CLSI Suppplement M59; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Almirante, B.; Rodriguez, D.; Park, B.J.; Cuenca-Estrella, M.; Planes, A.M.; Almela, M.; Mensa, J.; Sanchez, F.; Ayats, J.; Gimenez, M.; et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: Results from population-based surveillance, barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2005, 43, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Ghrenassia, E.; Mokart, D.; Mayaux, J.; Demoule, A.; Rezine, I.; Kerhuel, L.; Calvet, L.; De Jong, A.; Azoulay, E.; Darmon, M. Candidemia in critically ill immunocompromised patients: Report of a retrospective multicenter cohort study. Ann. Intensive Care 2019, 9, 62. [Google Scholar] [CrossRef]
- Munoz, P.; Giannella, M.; Fanciulli, C.; Guinea, J.; Valerio, M.; Rojas, L.; Rodriguez-Creixems, M.; Bouza, E. Candida tropicalis fungaemia: Incidence, risk factors and mortality in a general hospital. Clin. Microbiol. Infect. 2011, 17, 1538–1545. [Google Scholar] [CrossRef]
- Paiva, J.A.; Pereira, J.M.; Tabah, A.; Mikstacki, A.; de Carvalho, F.B.; Koulenti, D.; Ruckly, S.; Cakar, N.; Misset, B.; Dimopoulos, G.; et al. Characteristics and risk factors for 28-day mortality of hospital acquired fungemias in ICUs: Data from the EUROBACT study. Crit. Care 2016, 20, 53. [Google Scholar] [CrossRef]
- Poissy, J.; Damonti, L.; Bignon, A.; Khanna, N.; Von Kietzell, M.; Boggian, K.; Neofytos, D.; Vuotto, F.; Coiteux, V.; Artru, F.; et al. Risk factors for candidemia: A prospective matched case-control study. Crit. Care 2020, 24, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, H.; Yin, M.; Han, H.; Yue, J.F.; Zhang, F.; Shan, T.C.; Guo, H.P.; Wu, D.W. The differences in the epidemiology and predictors of death between candidemia acquired in intensive care units and other hospital settings. Intern. Med. 2015, 54, 3009–3016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pfaller, M.A.; Messer, S.A.; Moet, G.J.; Jones, R.N.; Castanheira, M. Candida bloodstream infections: Comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int. J. Antimicrob. Agents 2011, 38, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Jung, D.S.; Lee, J.Y.; Kim, H.A.; Ryu, S.Y.; Jung, S.I.; Joo, E.J.; Cheon, S.; Kim, Y.S.; Kim, S.W.; et al. Changing epidemiology of non-albicans candidemia in Korea. J. Infect. Chemother. 2019, 25, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Jung, D.S.; Lee, J.Y.; Kim, H.A.; Ryu, S.Y.; Jung, S.I.; Joo, E.J.; Cheon, S.; Kim, Y.S.; Kim, S.W.; et al. Poor prognosis of Candida tropicalis among non-albicans candidemia: A retrospective multicenter cohort study, Korea. Diagn. Microbiol. Infect. Dis. 2019, 95, 195–200. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Desnos-Ollivier, M.; Bretagne, S.; Dromer, F.; The French Mycoses Study. The risk and clinical outcome of candidemia depending on underlying malignancy. Intensive Care Med. 2017, 43, 652–662. [Google Scholar] [CrossRef]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J.; Mycoses Study, G. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Silva, S.; Henriques, M.; Oliveira, R. Insights into Candida tropicalis nosocomial infections and virulence factors. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1399–1412. [Google Scholar] [CrossRef]
- Tang, H.J.; Liu, W.L.; Lin, H.L.; Lai, C.C. Epidemiology and prognostic factors of candidemia in cancer patients. PLoS ONE 2014, 9, e99103. [Google Scholar] [CrossRef]
- Cobos-Trigueros, N.; Kaasch, A.J.; Soriano, A.; Torres, J.L.; Vergara, A.; Morata, L.; Zboromyrska, Y.; De La Calle, C.; Alejo, I.; Hernandez, C.; et al. Time to positivity and detection of growth in anaerobic blood culture vials predict the presence of Candida glabrata in candidemia: A two-center European cohort study. J. Clin. Microbiol. 2014, 52, 3082–3084. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Weinberger, M.; Orni-Wasserlauff, R.; Schwartz, D.; Itzhaki, A.; Lazarovitch, T.; Bash, E.; Aharoni, Y.; Moroz, I.; Giladi, M. Time to blood culture positivity as a marker for catheter-related candidemia. J. Clin. Microbiol. 2008, 46, 2222–2226. [Google Scholar] [CrossRef]
- Nunes, C.Z.; Marra, A.R.; Edmond, M.B.; da Silva Victor, E.; Pereira, C.A. Time to blood culture positivity as a predictor of clinical outcome in patients with Candida albicans bloodstream infection. BMC Infect. Dis. 2013, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin. Infect. Dis. 2006, 43, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, O.; Chasan, R. Candidemia in the intensive care unit. Clin. Chest. Med. 2017, 38, 493–509. [Google Scholar] [CrossRef]
- Paiva, J.A.; Charles, P.E. Biomarker-guided antifungal therapy in patients with suspected invasive candidiasis: Ready for prime time? Intensive Care Med. 2017, 43, 1889–1891. [Google Scholar] [CrossRef][Green Version]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef] [PubMed]

| Species | No (%) of Isolates | No. (%) Fluconazole Resistance 1 | No. (%) Micafungin Resistance 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | ICU | Non-ICU | Total | ICU | Non-ICU | Total | ICU | Non-ICU | |
| C. albicans | 353 (42.6) | 156 (40.7) | 197 (44.2) | 1 (0.3) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. glabrata | 159 (19.2) | 65 (17.0) | 94 (21.1) | 10 (6.3) | 6 (9.2) | 4 (4.3) | 1 (0.6) | 1 (1.5) | 0 (0.0) |
| C. tropicalis | 156 (18.8) | 89 (23.2) 2 | 67 (15.0) | 2 (1.3) | 1 (1.1) | 1 (1.5) | 1 (0.6) | 0 (0.0) | 1 (1.5) |
| C. parapsilosis | 112 (13.5) | 49 (12.8) | 63 (14.1) | 4 (3.6) | 4 (8.2) 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. krusei | 15 (1.8) | 10 (2.6) | 5 (1.1) | 15 (100) | 10 (100) | 5 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. lusitaniae | 9 (1.1) | 5 (1.3) | 4 (0.9) | 1 (11.1) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. guilliermondii | 8 (1.0) | 1 (0.3) | 7 (1.6) | 1 (12.5) | 1 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. dubliniensis | 1 (0.1) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - |
| Others 3 | 16 (1.9) | 7 (1.8) | 9 (2.0) | NA | NA | NA | NA | NA | NA |
| Total | 829 (100) 4 | 383 (100) | 446 (100) | 34 (4.2) | 23 (6.1) 2 | 11 (2.5) | 2 (0.2) | 1 (0.3) | 1 (0.2) |
| Species (No. of Isolates) and Variables 1 | 7-Day | 30-Day | 90-Day | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| All (807) | ||||||
| Lack of antifungal therapy | 14.58 (9.77–21.76) | <0.001 | 4.71 (3.71–5.98) | <0.001 | 3.74 (3.00–4.65) | <0.001 |
| CVC placement | 2.03 (1.40–2.95) | <0.001 | 1.59 (1.21–2.08) | 0.001 | 1.54 (1.20–1.98) | 0.001 |
| Urinary catheter placement | 1.87 (1.29–2.70) | 0.001 | 1.97 (1.47–2.63) | <0.001 | 2.10 (1.61–2.74) | <0.001 |
| Candidemia due to C. tropicalis | 1.82 (1.28–2.57) | 0.001 | 1.45 (1.10–1.91) | 0.008 | 1.43 (1.11–1.84) | 0.006 |
| Severe sepsis | 1.78 (1.30–2.45) | <0.001 | 1.92 (1.49–2.46) | <0.001 | 2.04 (1.62–2.57) | <0.001 |
| Prior surgery | 0.55 (0.36–0.86) | 0.009 | - | - | - | - |
| Azole monotherapy | 0.17 (0.10–0.30) | <0.001 | 0.45 (0.33–0.60) | <0.001 | 0.50 (0.39–0.66) | <0.001 |
| Hematologic malignancies | - | - | 1.52 (1.01–2.29) | 0.04 | - | - |
| ICU admission | - | - | 1.43 (1.09–1.86) | 0.009 | 1.39 (1.09–1.76) | 0.008 |
| CVC removal | - | - | 0.64 (0.45–0.92) | 0.01 | 0.58 (0.41–0.80) | 0.001 |
| Candidemia due to C. parapsilosis | - | - | - | - | 0.62 (0.42–0.93) | 0.01 |
| C. albicans (338) | ||||||
| Lack of antifungal therapy | 17.82 (9.32–34.08) | <0.001 | 5.87 (4.02–8.56) | <0.001 | 4.76 (3.37–6.70) | <0.001 |
| CVC placement | 2.44 (1.38–4.34) | 0.002 | 2.14 (1.39–3.29) | 0.001 | 2.22 (1.50–3.28) | <0.001 |
| Urine catheter use | 1.94 (1.13–3.33) | 0.01 | 2.26 (1.48–3.44) | <0.001 | 2.45 (1.66–3.60) | <0.001 |
| Severe sepsis | - | - | 1.80 (1.22–2.67) | 0.003 | 1.95 (1.36–2.78) | <0.001 |
| Prior surgery | 0.39 (0.18–0.85) | 0.01 | - | - | - | - |
| Azole monotherapy | 0.13 (0.06–0.29) | <0.001 | 0.35 (0.23–0.55) | <0.001 | 0.42 (0.29–0.60) | <0.001 |
| CVC removal | - | - | 0.55 (0.33–0.92) | 0.02 | 0.49 (0.30–0.81) | 0.006 |
| C. tropicalis (149) | ||||||
| Lack of antifungal therapy | 8.64 (4.52–16.53) | <0.001 | 4.19 (2.54–6.89) | <0.001 | 3.65 (2.32–5.75) | <0.001 |
| CVC placement | 2.29 (1.10–4.75) | 0.02 | - | - | - | - |
| Urinary catheter placement | 2.21 (1.02–4.81) | 0.04 | 2.59 (1.45–4.61) | 0.001 | 2.95 (1.73–5.03) | <0.001 |
| Severe sepsis | - | - | 2.18 (1.29–3.67) | 0.003 | 2.39 (1.48–3.85) | <0.001 |
| Azole monotherapy | 0.35 (0.14–0.90) | 0.02 | - | - | 0.48 (0.27–0.86) | 0.01 |
| Congestive heart failure | - | - | 2.48 (1.20–5.12) | 0.01 | 2.10 (1.03–4.28) | 0.04 |
| Neutropenia | - | - | 2.17 (1.10–4.26) | 0.02 | 2.22 (1.17–4.23) | 0.01 |
| C. glabrata (147) | ||||||
| Lack of antifungal therapy | 45.86 (6.19–339.57) | <0.001 | 3.26 (1.84–5.80) | <0.001 | 2.40 (1.43–4.01) | 0.001 |
| Severe sepsis | - | - | 1.93 (1.08–3.43) | 0.02 | 2.30 (1.37–3.86) | 0.002 |
| ICU admission | 2.48 (1.11–5.51) | 0.02 | 2.15 (1.21–3.83) | 0.009 | 2.07 (1.22–3.49) | 0.007 |
| Fluconazole resistance | - | - | 2.80 (1.17–6.66) | 0.02 | 2.84 (1.28–6.33) | 0.01 |
| C. parapsilosis (111) | ||||||
| Lack of antifungal therapy | 36.30 (4.28–307.86) | 0.001 | 9.18 (2.75–30.61) | <0.001 | 4.72 (1.51–14.76) | 0.008 |
| Urinary catheter placement | - | - | - | - | 4.35 (1.13–16.71) | 0.03 |
| Azole monotherapy | 0.05 (0.01–0.43) | 0.006 | 0.28 (0.10–0.84) | 0.02 | - | - |
| ICU admission | 11.54 (1.38–96.67) | 0.02 | 6.93 (1.84–26.09) | 0.004 | 6.06 (1.92–19.17) | 0.002 |
| CVC removal | - | - | - | - | 0.27 (0.08–0.88) | 0.02 |
| Species (No. of Isolates) and Variables 1 | Setting | 7-Day | 30-Day | 90-Day | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| C. albicans (ICU 147; non-ICU 191) | |||||||
| Lack of antifungal therapy | ICUAC | 18.33 (7.65–43.92) | <0.001 | 6.90 (4.12–11.57) | <0.001 | 5.47 (3.42–8.75) | <0.001 |
| Non-ICUAC 2 | 34.64 (7.86–152.68) | <0.001 | 10.49 (4.68–23.51) | <0.001 | 3.97 (2.32–6.79) | <0.001 | |
| CVC placement | ICUAC | - | - | - | - | 1.86 (1.01–3.43) | 0.04 |
| Non-ICUAC | - | - | - | - | 2.05 (1.20–3.51) | 0.009 | |
| Urinary catheter placement | ICUAC | - | - | 2.56 (1.25–5.24) | 0.01 | 3.28 (1.65–6.51) | 0.001 |
| Non-ICUAC | - | - | 2.71 (1.25–5.84) | 0.03 | 1.79 (1.07–2.99) | 0.02 | |
| Severe sepsis | ICUAC | - | - | 1.69 (1.04–2.75) | 0.03 | 1.62 (1.03–2.56) | 0.03 |
| Non-ICUAC | - | - | - | - | 2.57 (1.49–4.43) | 0.001 | |
| Prior surgery | ICUAC | 0.40 (0.17–0.96) | 0.04 | - | - | - | - |
| Azole monotherapy | ICUAC | 0.11 (0.03–0.35) | <0.001 | 0.27 (0.14–0.52) | <0.001 | 0.33 (0.19–0.59) | <0.001 |
| Non-ICUAC | 0.06 (0.01–0.44) | 0.006 | 0.26 (0.11–0.62) | 0.002 | 0.46 (0.27–0.77) | 0.004 | |
| CVC removal | ICUAC | - | - | - | - | 0.49 (0.27–0.88) | 0.01 |
| Non-ICUAC | - | - | - | - | 0.34 (0.15–0.80) | 0.01 | |
| C. tropicalis (ICU 83; non-ICU 66) | |||||||
| Lack of antifungal therapy | ICUAC | 10.52 (3.91–28.25) | <0.001 | 4.01 (2.15–7.47) | <0.001 | 3.79 (2.14–6.74) | <0.001 |
| Non-ICUAC | 7.74 (2.59–23.17) | <0.001 | 4.54 (1.87–11.00) | 0.001 | 6.02 (2.45–14.81) | <0.001 | |
| Severe sepsis | ICUAC | - | - | 2.71 (1.42–5.19) | 0.004 | 2.16 (1.14–4.11) | 0.002 |
| Congestive heart failure | ICUAC | 3.93 (1.17–13.15) | 0.02 | 3.53 (1.43–8.69) | 0.006 | 3.16 (1.31–7.63) | 0.01 |
| Neutropenia | ICUAC | - | - | 2.50 (1.06–5.88) | 0.03 | 2.50 (1.09–5.73) | 0.01 |
| Azole monotherapy | ICUAC | - | - | 0.21 (0.08–0.59) | 0.003 | 0.20 (0.08–0.46) | 0.03 |
| C. glabrata (ICU 58; non-ICU 89) | |||||||
| Lack of antifungal therapy | ICUAC | 21.30 (2.79–162.83) | 0.003 | 2.72 (1.12–6.62) | 0.02 | 3.41 (1.59–7.32) | 0.002 |
| Non-ICUAC | - | - | 5.02 (1.82–13.88) | 0.002 | - | - | |
| Urinary catheter placement | ICUAC | - | - | 6.81 (1.71–27.06) | 0.006 | - | - |
| Solid tumor | ICUAC | 3.64 (1.38–9.57) | 0.009 | 2.86 (1.23–6.70) | 0.01 | - | - |
| Chronic kidney disease | ICUAC | - | - | 4.05 (1.45–11.31) | 0.007 | - | - |
| Fluconazole resistance | ICUAC | - | - | - | - | 5.14 (1.80–14.65) | 0.002 |
| C. parapsilosis (ICU 49; non-ICU 62) | |||||||
| Lack of antifungal therapy | ICUAC | - | - | 22.77 (4.17–124.26) | <0.001 | 12.90 (4.16–39.98) | <0.001 |
| Urinary catheter placement | ICUAC | - | - | - | - | 4.81 (1.25–18.48) | 0.02 |
| Azole monotherapy | ICUAC | - | - | 0.13 (0.03–0.50) | 0.003 | 0.19 (0.06–0.55) | 0.002 |
| Male | ICUAC | 0.26 (0.07–0.95) | 0.04 | - | - | - | - |
| Old age (≥65 yrs) | ICUAC | 9.96 (1.25–79.60) | 0.03 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.J.; Won, E.J.; Jeong, S.H.; Shin, K.S.; Shin, J.H.; Kim, Y.R.; Kim, H.S.; Kim, Y.A.; Uh, Y.; Kim, T.S.; et al. Dynamics and Predictors of Mortality Due to Candidemia Caused by Different Candida Species: Comparison of Intensive Care Unit-Associated Candidemia (ICUAC) and Non-ICUAC. J. Fungi 2021, 7, 597. https://doi.org/10.3390/jof7080597
Kwon YJ, Won EJ, Jeong SH, Shin KS, Shin JH, Kim YR, Kim HS, Kim YA, Uh Y, Kim TS, et al. Dynamics and Predictors of Mortality Due to Candidemia Caused by Different Candida Species: Comparison of Intensive Care Unit-Associated Candidemia (ICUAC) and Non-ICUAC. Journal of Fungi. 2021; 7(8):597. https://doi.org/10.3390/jof7080597
Chicago/Turabian StyleKwon, Yong Jun, Eun Jeong Won, Seok Hoon Jeong, Kyeong Seob Shin, Jeong Hwan Shin, Young Ree Kim, Hyun Soo Kim, Young Ah Kim, Young Uh, Taek Soo Kim, and et al. 2021. "Dynamics and Predictors of Mortality Due to Candidemia Caused by Different Candida Species: Comparison of Intensive Care Unit-Associated Candidemia (ICUAC) and Non-ICUAC" Journal of Fungi 7, no. 8: 597. https://doi.org/10.3390/jof7080597
APA StyleKwon, Y. J., Won, E. J., Jeong, S. H., Shin, K. S., Shin, J. H., Kim, Y. R., Kim, H. S., Kim, Y. A., Uh, Y., Kim, T. S., Park, J. H., Lee, J., Choi, M. J., Byun, S. A., Kim, S. H., & Shin, J. H. (2021). Dynamics and Predictors of Mortality Due to Candidemia Caused by Different Candida Species: Comparison of Intensive Care Unit-Associated Candidemia (ICUAC) and Non-ICUAC. Journal of Fungi, 7(8), 597. https://doi.org/10.3390/jof7080597







