Epidemiology of Systemic Mycoses in the COVID-19 Pandemic
Abstract
1. Introduction
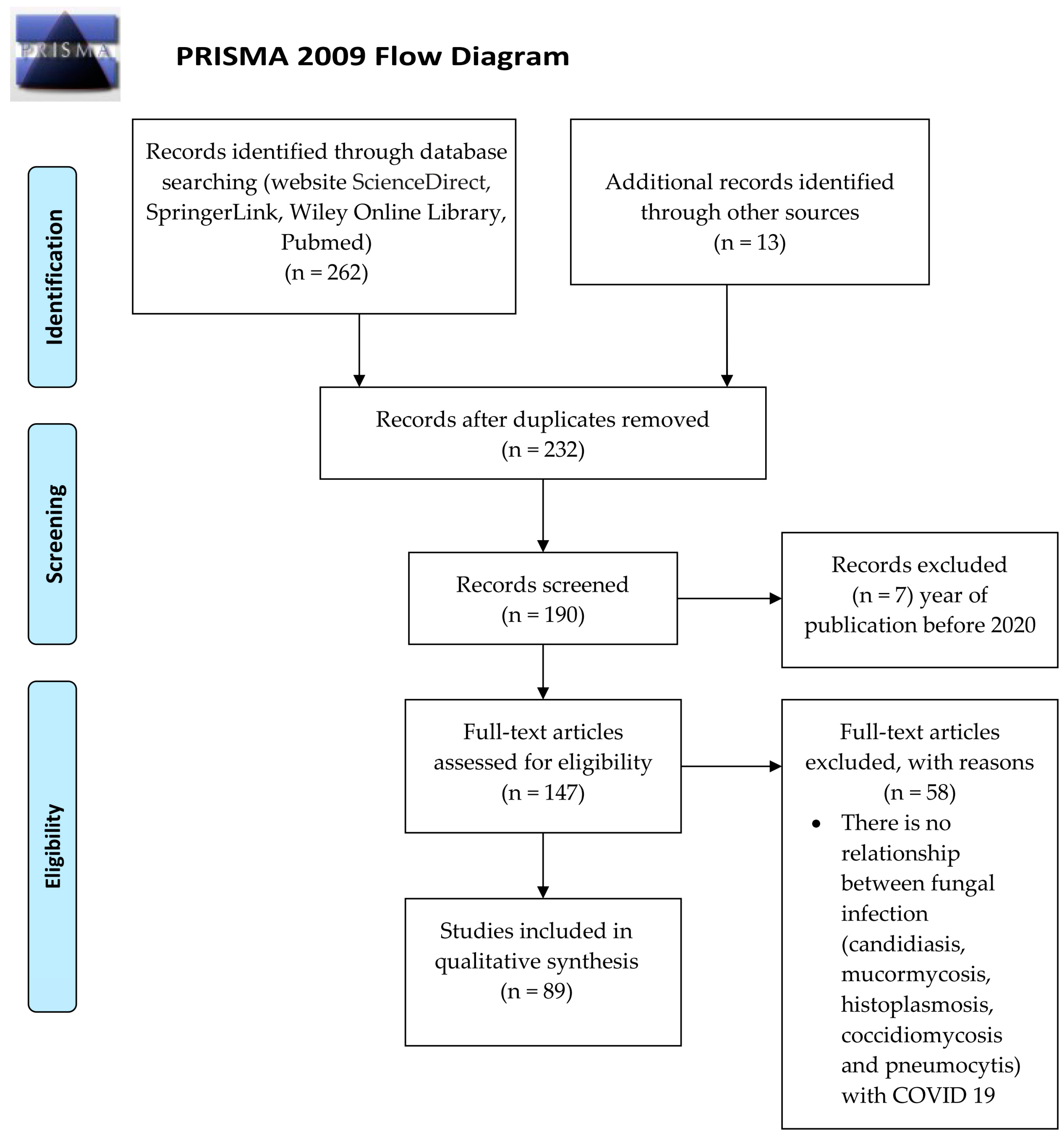
2. Materials and Methods
3. Pneumonia by Pneumocystis jirovecii and COVID-19
4. Candidiasis and COVID-19
5. Aspergillosis and COVID-19
6. Mucormycosis and COVID-19
7. Endemic Mycoses and COVID-19
8. Future Challenges of Systemic Mycoses Co-Infections and COVID-19
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 21 March 2021).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Seyed Hosseini, E.; Riahi Kashani, N.; Nikzad, H.; Azadbakht, J.; Hassani Bafrani, H.; Haddad Kashani, H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tavakolpour, S.; Rakhshandehroo, T.; Wei, E.X.; Rashidian, M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020, 225, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Pemán, J.; Ruiz-Gaitán, A.; García-Vidal, C.; Salavert, M.; Ramírez, P.; Puchades, F.; García-Hita, M.; Alastruey-Izquierdo, A.; Quindós, G. Fungal co-infection in COVID-19 patients: Should we be concerned? Rev. Iberoam. Micol. 2020, 37, 41–46. [Google Scholar] [CrossRef]
- Segrelles-Calvo, G.; Araujo, G.R.S.; Frases, S. Systemic mycoses: A potential alert for complications in COVID-19 patients. Future Microbiol. 2020, 15, 1405–1413. [Google Scholar] [CrossRef]
- Adalja, A.A.; Sappington, P.L.; Harris, S.P.; Rimmele, T.; Kreit, J.W.; Kellum, J.A.; Boujoukos, A.J. Isolation of Aspergillus in three 2009 H1N1 influenza patients. Influenza Other Resp. Viruses 2011, 5, 225–229. [Google Scholar] [CrossRef][Green Version]
- García-Vidal, C.; Barba, P.; Arnan, M.; Moreno, A.; Ruiz-Camps, I.; Gudiol, C.; Ayats, J.; Ortí, G.; Carratala, J. Invasive aspergillosis complicating pandemic influenza A (H1N1) infection in severely immunocompromised patients. Clin. Infect. Dis. 2011, 53, e16–e19. [Google Scholar] [CrossRef]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: A retrospective study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef]
- Crum-Cianflone, N. Invasive aspergillosis associated with severe influenza infection. Open Forum. Infect. Dis. 2016, 3, ofw171. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Kolwijck, E.; Lestrade, P.P.A.; Hodiamont, C.J.; Rijnders, B.J.A.; van Paassen, J.; Haas, P.J.; Oliveira Dos Santos, C.; Kampinga, G.A.; Bergmans, D.C.; et al. Influenza-associated aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2017. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Vanderbeke, L.; Spriet, I.; Breynaert, C.; Rijnders, B.J.A.; Verweij, P.E.; Wauters, J. Invasive pulmonary aspergillosis complicating severe influenza: Epidemiology, diagnosis and treatment. Curr. Opin. Infect. Dis. 2018, 31, 471–480. [Google Scholar] [CrossRef]
- Immel, S.; Yu, E. Case report: Disseminated aspergillosis complicating influenza. Med. Mycol. Case Rep. 2019, 24, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Kochanek, M.; Shimabukuro-Vornhagen, A.; Cornely, O.A. Intensive care management of influenza-associated pulmonary aspergillosis. Clin. Microbiol. Infect. 2019, 25, 1501–1509. [Google Scholar] [CrossRef]
- Zou, P.; Wang, C.; Zheng, S.; Guo, F.; Yang, L.; Zhang, Y.; Liu, P.; Shen, Y.; Wang, Y.; Zhang, X.; et al. Invasive pulmonary aspergillosis in adults with avian influenza A (H7N9) pneumonia in China: A retrospective study. J. Infect. Dis. 2020, 221, S193–S197. [Google Scholar] [CrossRef]
- Hwang, D.M.; Chamberlain, D.W.; Poutanen, S.M.; Low, D.E.; Asa, S.L.; Butany, J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Pathol. 2005, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, Y.; Li, X.; Yang, L.; Zhang, W.; Kang, W. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N. Engl. J. Med. 2003, 349, 507–508. [Google Scholar] [CrossRef]
- Alanio, A.; Dellière, S.; Voicu, S.; Bretagne, S.; Mégarbane, B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J. Infect. 2020, 82, 84–123. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Noval, M.; Doub, J.B.; Heil, E. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia in a severely immunocompromised 25-year-old patient. Int. J. Infect. Dis. 2020, 99, 119–121. [Google Scholar] [CrossRef]
- Blaize, M.; Mayaux, J.; Luit, C.E.; Lampros, A.; Fekkar, A. COVID-19-related respiratory failure and lymphopenia do not seem associated with pneumocystosis. Am. J. Respir. Crit. Care Med. 2020, 202, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Sun, W.; Li, M.; Dong, L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin. Rheumatol. 2020, 39, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.; Snell, L.B.; Simons, R.; Douthwaite, S.T.; Lee, M.J. Coronavirus disease 2019 and Pneumocystis jirovecii pneumonia: A diagnostic dilemma in HIV. AIDS 2020, 34, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, M.A.; Alberici, F.; Bossini, N.; Scolari, F.; Pascucci, F.; Tomasoni, G.; Caruso, A. Pneumocystis jirevocii and SARS-CoV-2 co-infection: A common feature in transplant recipients? Vaccines 2020, 8, 544. [Google Scholar] [CrossRef]
- Guo, W.; Wang, M.; Ming, F.; Tang, W.; Liang, K. The diagnostic trap occurred in two COVID-19 cases combined Pneumocystis pneumonia in patient with AIDS. Res. Sq. 2020, 10, rs.3.rs-53350. [Google Scholar]
- Larzábal, F.J.; Vilela, A.; Brusca, S.; Saluzzi, I.; Ghergo, G.E.; Angiono, M.A. Diagnóstico simultáneo y evolución favorable de infección por Pneumocystis jirovecii, SARS-COV-2 y HIV avanzada. Medicina 2020, 80, 554–556. [Google Scholar] [PubMed]
- Mang, S.; Kaddu-Mulindwa, D.; Metz, C.; Becker, A.; Seiler, F.; Smola, S.; Maßmann, A.; Becker, S.L.; Papan, C.; Bals, R.; et al. Pneumocystis jirovecii Pneumonia and SARS-CoV-2 co-infection in newly diagnosed HIV-1 infection. Clin. Infect. Dis. 2020, 72, 1487–1489. [Google Scholar] [CrossRef]
- Menon, A.A.; Berg, D.D.; Brea, E.J.; Deutsch, A.J.; Kidia, K.K.; Thurber, E.G.; Polsky, S.B.; Yeh, T.; Duskin, J.A.; Holliday, A.M.; et al. A Case of COVID-19 and Pneumocystis jirovecii coinfection. Am. J. Respir. Crit. Care Med. 2020, 202, 136–138. [Google Scholar] [CrossRef]
- Mouren, D.; Goyard, C.; Catherinot, E.; Givel, C.; Chabrol, A.; Tcherakian, C.; Longchampt, E.; Vargaftig, J.; Farfour, E.; Legal, A.; et al. COVID-19 and Pneumocystis jirovecii pneumonia: Back to the basics. Respir. Med. Res. 2021, 79, 100814. [Google Scholar]
- Quintana-Ortega, C.; Remesal, A.; Ruiz de Valbuena, M.; de la Serna, O.; Laplaza-González, M.; Álvarez-Rojas, E.; Udaondo, C.; Alcobendas, R.; Murias, S. Fatal outcome of anti-MDA5 juvenile dermatomyositis in a paediatric COVID-19 patient: A case report. Mod. Rheumatol. Case Rep. 2021, 5, 101–107. [Google Scholar] [CrossRef]
- Rubiano, C.; Tompkins, K.; Sellers, S.A.; Bramson, B.; Eron, J.; Parr, J.B.; Schranz, A.J. Pneumocystis and severe acute respiratory syndrome coronavirus 2 coinfection: A case report and review of an emerging diagnostic dilemma. Open. Forum. Infect. Dis. 2020, 8, ofaa633. [Google Scholar] [CrossRef]
- Seitz, T.; Hoepler, W.; Weseslindtner, L.; Aberle, J.H.; Aberle, S.W.; Puchhammer-Stoeckl, E.; Baumgartner, S.; Traugott, M.; Karolyi, M.; Pawelka, E.; et al. Successful management of the first reported case in Austria of COVID-19 with ARDS. Infection 2020, 48, 647–651. [Google Scholar] [CrossRef]
- Riche, C.V.W.; Cassol, R.; Pasqualotto, A.C. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? J. Fungi 2020, 6, 286. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Wang, Z.; Chen, H.; Tian, L.; Liu, D. Nosocomial infection among patients with COVID-19: A retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect. Control Hosp. Epidemiol. 2020, 41, 982–983. [Google Scholar] [CrossRef]
- Lv, Z.; Cheng, S.; Le, J.; Huang, J.; Feng, L.; Zhang, B.; Li, Y. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: A retrospective cohort study. Microbes Infect. 2020, 22, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A latent threat to critically ill patients with COVID-19. Clin. Infect. Dis. 2020, ciaa1595. [Google Scholar] [CrossRef]
- Ramadan, H.K.; Mahmoud, M.A.; Aburahma, M.Z.; Elkhawaga, A.A.; El-Mokhtar, M.A.; Sayed, I.M.; Hosni, A.; Hassany, S.M.; Medhat, M.A. Predictors of severity and co-infection resistance profile in COVID-19 patients: First report from upper Egypt. Infect. Drug Resist 2020, 13, 3409–3422. [Google Scholar] [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-resistant Candida auris infections in critically Ill coronavirus disease patients, India, April-July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef]
- Antinori, S.; Bonazzetti, C.; Gubertini, G.; Capetti, A.; Pagani, C.; Morena, V.; Rimoldi, S.; Galimberti, L.; Sarzi-Puttini, P.; Ridolfo, A.L. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: An increased risk for candidemia? Autoimmun. Rev. 2020, 19, 102564. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Buttrini, M.; Montecchini, S.; Piccolo, G.; Martinelli, M.; Dell’Anna, M.L.; Di Maio, A.; Arcangeletti, M.C.; Maccari, C.; De Conto, F.; et al. Detection of SARS-CoV-2 and other infectious agents in lower respiratory tract samples belonging to patients admitted to intensive care units of a tertiary-care hospital, located in an epidemic area, during the Italian lockdown. Microorganisms 2021, 9, 185. [Google Scholar] [CrossRef]
- Cataldo, M.A.; Tetaj, N.; Selleri, M.; Marchioni, L.; Capone, A.; Caraffa, E.; Caro, A.D.; Petrosillo, N.; INMICOVID-19 Co-Infection Group. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: An alarming “collateral effect”. J. Glob. Antimicrob. Resist. 2020, 23, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.R.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of carbapenem-resistant Gram-negatives and Candida auris during the COVID-19 pandemic in critically Ill patients: One step back in antimicrobial stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Torelli, R.; Vella, A.; Leone, P.M.; De Angelis, G.; De Carolis, E.; Ventura, G.; Sanguinetti, M.; Fantoni, M. Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: A fatal case report. J. Fungi 2020, 6, 163. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Germinario, B.N.; Ferrante, M.; Frangi, C.; Li Voti, R.; Muccini, C.; Ripa, M.; COVID-BioB Study Group. Candidemia in COVID-19 patients: Incidence and characteristics in a prospective cohort compared to historical non-COVID-19 controls. Clin. Infect. Dis. 2020, 30, ciaa1594. [Google Scholar] [CrossRef]
- Allaw, F.; Kara Zahreddine, N.; Ibrahim, A.; Tannous, J.; Taleb, H.; Bizri, A.R.; Dbaibo, G.; Kanj, S.S. First Candida auris outbreak during a COVID-19 pandemic in a tertiary-care center in Lebanon. Pathogens 2021, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Lozano, H.; Treviño-Rangel, R.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Mohsin, J.; Al-Huraizi, A.; Khamis, F. COVID-19 associated invasive candidiasis. J. Infect. 2021, 82, e45–e46. [Google Scholar] [CrossRef]
- Gorospe-Sarasúa, L.; Gallego-Rivera, J.I.; Muñoz-Molina, G.M.; Mirambeaux-Villalona, R.M.; Ajuria-Illarramendi, O.; González-García, A.; Barbolla-Díaz, I. Delayed Candida costochondritis and spondylitis in a post-COVID-19 patient previously treated with corticosteroids, antibiotics, and tocilizumab. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. 2020, ciaa1298. [Google Scholar] [CrossRef] [PubMed]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; de Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris outbreak in a COVID-19 specialty care unit-Florida, July-August 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef]
- Tafti, D.; Kluckman, M.; Dearborn, M.C.; Hunninghake, J.; Clayton, S. COVID-19 in patients with hematologic-oncologic risk factors: Complications in three patients. Cureus 2020, 12, e12064. [Google Scholar] [CrossRef]
- Bishburg, E.; Okoh, A.; Nagarakanti, S.R.; Lindner, M.; Migliore, C.; Patel, P. Fungemia in COVID-19 ICU patients, a single medical center experience. J. Med. Virol. 2020, 2810–2814. [Google Scholar] [CrossRef]
- Aesif, S.W.; Bribriesco, A.C.; Yadav, R.; Nugent, S.L.; Zubkus, D.; Tan, C.D.; Mehta, A.C.; Mukhopadhyay, S. Pulmonary pathology of COVID-19 following 8 weeks to 4 months of severe disease: A report of three cases, including one with bilateral lung transplantation. Am. J. Clin. Pathol. 2020, 155, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Macauley, P.; Epelbaum, O. Epidemiology and mycology of candidemia in non-oncological medical intensive care unit patients in a tertiary center in the united states: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses 2021. [Google Scholar] [CrossRef] [PubMed]
- Roman-Montes, C.M.; Martinez-Gamboa, A.; Diaz-Lomelí, P.; Cervantes-Sanchez, A.; Rangel-Cordero, A.; Sifuentes-Osornio, J.; Ponce-de-Leon, A.; Gonzalez-Lara, M.F. Accuracy of galactomannan testing on tracheal aspirates in COVID-19-associated pulmonary aspergillosis. Mycoses 2021, 64, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Platt, A.; Tornheim, J.A.; Zhang, S.X.; Datta, K.; Cardozo, C.; García-Vidal, C. Aspergillosis complicating severe coronavirus disease. Emerg. Infect. Dis. 2021, 27, 18–25. [Google Scholar] [CrossRef]
- Meijer, E.F.J.; Dofferhoand, A.S.M.; Meis, J.F.; Hoiting, O.; Buil, J.B. Azole-resistant COVID-19-associated pulmonary aspergillosis in an immunocompetent host: A case report. J. Fungi 2020, 6, 79. [Google Scholar] [CrossRef]
- Santana, M.F.; Pivoto, G.; Alexandre, M.A.A.; Baía-Da-silva, D.C.; da Silva Borboa, M.G.; Almedia Val, F.A.; Brito-Sousa, J.D.; Cardoso-Melo, G.; Monteiro, W.M.; Braga-Souza, J.V.; et al. Confirmed invasive pulmonary aspergillosis and COVID-19: The value of postmortem findings to support antemortem management. Rev. Soc. Bras. Med. Trop. 2020, 53, 1–4. [Google Scholar] [CrossRef]
- Meijer, E.F.J.; Dofferhoff, A.S.M.; Hoiting, O.; Meis, J.F. COVID-19-associated pulmonary aspergillosis: A prospective single-center dual case series. Mycoses 2021, 64, 457–464. [Google Scholar] [CrossRef]
- Sharma, A.; Hofmeyr, A.; Bansal, A.; Thakkar, D.; Lam, L.; Harrington, Z.; Bhonagiri, D. COVID-19 associated pulmonary aspergillosis (CAPA): An Australian case report. Med. Mycol. Case Rep. 2020, 31, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Skok, K.; Vander, K.; Setaffy, L.; Kessler, H.H.; Aberle, S.; Bargfrieder, U.; Trauner, M.; Lax, S.F. COVID-19 autopsies: Procedure, technical aspects and cause of fatal course. Experiences from a single-center. Pathol. Res. Pract. 2021, 217, 153305. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.; Farooqi, J.; Mahmood, S.F.; Jabeen, K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: An observational study from Pakistan. Mycoses 2020, 63, 766–770. [Google Scholar] [CrossRef]
- Wu, S.; Yang, S.; Chen, R.; Chen, H.; Xu, Y.; Lin, B. Dynamic Immune Response Profiles and Recovery of a COVID-19 Patient with Coinfection of Aspergillus fumigatus and Other Baseline Diseases: A Case Report. Omi. A. J. Integr. Biol. 2020, 24, 615–618. [Google Scholar] [CrossRef]
- Schein, F.; Munoz-Pons, H.; Mahinc, C.; Grange, R.; Cathebras, P.; Flori, P. Fatal aspergillosis complicating severe SARS-CoV-2 infection: A case report. J. Mycol. Med. 2020, 14, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.; Almaslamani, M.A.; Hashim, S.M.; Ibrahim, A.S.; Omrani, A.S. Fatal Coronavirus Disease 2019-associated Pulmonary Aspergillosis; A Report of Two Cases and Review of the Literature. IDCases 2020, 22, e00935. [Google Scholar] [CrossRef]
- Blaize, M.; Mayaux, J.; Nabet, C.; Nabet, C.; Lampros, A.; Marcelin, A.G.; Thellier, M.; Piarroux, R.; Demoule, A.; Fekkar, A. Fatal Invasive Aspergillosis and Coronavirus Disease in an Immunocompetent Patient. Emerg. Infect. Dis. 2020, 26, 1636–1637. [Google Scholar] [CrossRef]
- Nasri, E.; Shoaei, P.; Vakili, B.; Mirhendi, H.; Sadeghi, S.; Hajiahmadi, S.; Sadeghi, A.; Vaezi, A.; Badali, H.; Fakhim, H. Fatal Invasive Pulmonary Aspergillosis in COVID-19 Patient with Acute Myeloid Leukemia in Iran. Mycopathologia 2020, 185, 1077–1084. [Google Scholar]
- Fekkar, A.; Poignon, C.; Blaize, M.; Lampros, A. Fungal infection during COVID-19: Does Aspergillus mean secondary invasive aspergillosis? Am. J. Respir. Crit. Care Med. 2020, 202, 902–903. [Google Scholar] [CrossRef] [PubMed]
- Haglund, A.; Christensen, S.; Kristensen, L.; Gertsen, J.B.; Buus, L.; Lausch, K.R. Invasive pulmonary aspergillosis and hyperthermia in an immunocompetent patient with COVID-19. Med. Mycol. Case Rep. 2020, 31, 29–31. [Google Scholar] [CrossRef]
- Falces-Romero, I.; Ruiz-Bastián, M.; Díaz-Pollán, B.; Maseda, E.; García-Rodríguez, J. SARS-CoV-2 Working Group. Isolation of Aspergillus spp. in respiratory samples of patients with COVID-19 in a Spanish Tertiary Care Hospital. Mycoses 2020, 63, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Hassan, T.; Trzos-Grzybowska, M.; Thomas, J.; Quinn, A.; O’Sullivan, M.; Griffin, A.; Rogers, T.R.; Fe-Talento, A. Multi-triazole-resistant Aspergillus fumigatus and SARS-CoV-2 co-infection: A lethal combination. Med. Mycol. Case Rep. 2020, 31, 1–3. [Google Scholar] [CrossRef]
- Fekkar, A.; Lampros, A.; Mayaux, J.; Poignon, C.; Demeret, S.; Constantin, J.M.; Mercelin, A.-G.; Monsel, A.; Luyt, C.-E.; Blaize, M. Occurrence of Invasive Pulmonary Fungal Infections in Patients with Severe COVID-19 Admitted to the ICU. Am. J. Respir. Crit. Care Med. 2021, 203, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Segrelles-Calvo, G.; Araújo, G.R.S.; Llopis-Pastor, E.; Carrillo, J.; Hernández-Hernández, M.; Rey, L.; Rodríguez-Melean, N.; Escribano, I.; Antón, E.; Zamarro, C.; et al. Prevalence of opportunistic invasive aspergillosis in COVID-19 patients with severe pneumonia. Mycoses 2021, 64, 144–151. [Google Scholar] [CrossRef]
- Mitaka, H.; Perlman, D.C.; Javaid, W.; Salomon, N. Putative invasive pulmonary aspergillosis in critically ill patients with COVID-19: An observational study from New York City. Mycoses 2020, 63, 1368–1372. [Google Scholar] [CrossRef]
- Rothe, K.; Feihl, S.; Schneider, J.; Wallnöfer, F.; Wurst, M.; Lukas, M.; Treiber, M.; Lahmer, T.; Heim, M.; Dommasch, M.; et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: A retrospective cohort study in light of antibiotic stewardship. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Ghelfenstein-Ferreira, T.; Saade, A.; Alanio, A.; Bretagne, S.; Araujo de Castro, R.; Hamane, S.; Azoulay, E.; Bredin, S.; Delliére, S. Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU–A case report. Med. Mycol. Case Rep. 2020, 31, 15–18. [Google Scholar] [CrossRef]
- Sasoni, N.; Rodriguez-Müller, M.; Posse, G.; González, J.; Leonardelli, F.; Garcia-Effron, G. SARS-CoV-2 and Aspergillus section Fumigati coinfection in an immunocompetent patient treated with corticosteroids. Rev. Iberoam. Micol. 2021, 28, S1130–S1406. [Google Scholar]
- Patti, R.K.; Dalsania, N.R.; Somal, N.; Sinha, A.; Mehta, S.; Ghitan, M.; Seneviratne, C.; Kupfer, Y. Subacute Aspergillosis “Fungal Balls” Complicating COVID-19. J. Investig. Med. High Impact. Case Rep. 2020, 8, 3. [Google Scholar] [CrossRef]
- Fernandez, N.B.; Caceres, D.H.; Bee, K.D.; Irrazabal, C.; Delgado, G.; Farias, L.; Chiller, T.M.; Verweij, P.E.; Stecher, D. Ventilator-associated pneumonia involving Aspergillus flavus in a patient with coronavirus disease 2019 (COVID-19) from Argentina. Med. Mycol. Case Rep. 2020, 31, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, H.; Fernández-Ruiz, M.; Gutiérrez, E.; Sevillano, Á.; Caravaca-Fontán, F.; Morales, E.; López-Medrano, F.; Aguado, J.M.; Praga, M.; Andrés, A. Invasive pulmonary aspergillosis associated with COVID-19 in a kidney transplant recipient. Transpl. Infect. Dis. 2020, 23, e13501. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Valerio, M.; Álvarez-Uría, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 2021, 64, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Rogers, T.R.; Talento, A.F. COVID-19 associated invasive pulmonary aspergillosis: Diagnostic and therapeutic challenges. J. Fungi 2020, 6, 115. [Google Scholar] [CrossRef]
- Borman, A.M.; Palmer, M.D.; Fraser, M.; Patterson, Z.; Mann, C.; Oliver, D.; Linton, C.J.; Gough, M.; Brown, P.; Dzietczyk, A.; et al. COVID-19-associated invasive aspergillosis: Data from the UK national mycology reference laboratory. J. Clin. Microbiol. 2021, 59, e02136-20. [Google Scholar]
- Yusuf, E.; Vonk, A.; van den Akker, J.P.C.; Bode, L.; Sips, G.J.; Rijnders, B.J.A.; de Steenwinkel, J.; Verkaik, N.J.; Vogel, M.; van der Eerden, M.; et al. Frequency of Positive Aspergillus Tests in COVID-19 Patients in Comparison to Other Patients with Pulmonary Infections Admitted to the ICU. J. Clin. Microbiol. 2020, 2, 278–320. [Google Scholar]
- Werthman-ehrenreich, A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. Am. J. Emerg. Med. 2021, 42, e5–e264. [Google Scholar] [CrossRef]
- Mehta, S.; Pandey, A. Rhino-Orbital Mucormycosis Associated with COVID-19. Cureus 2020, 12, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Do Monte, E.S.; Dos Santos, M.E.L.; Ribeiro, I.B.; De Oliveira Luz, G.; Baba, E.R.; Hirsch, B.S.; Pereora Funari, M.; De Moura, E.G.H. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: A case report. Clin. Endosc. 2020, 53, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R. Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia 2021, 1–10. [Google Scholar] [CrossRef]
- Placik, D.A.; Taylor, W.L.; Wnuk, N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol. Case Rep. 2020, 15, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Waizel-Haiat, S.; Guerrero-Paz, J.A.; Sanchez-Hurtado, L.; Calleja-Alarcon, S.; Romero-Gutierrez, L. A Case of Fatal Rhino-Orbital Mucormycosis Associated with New Onset Diabetic Ketoacidosis and COVID-19. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Pasero, D.; Sanna, S.; Liperi, C.; Piredda, D.; Branca, G.P.; Casadio, L.; Simeo, R.; Buselli, A.; Rizzo, R.; Bussu, F.; et al. A challenging complication following SARS-CoV-2 infection: A case of pulmonary mucormycosis. Infection 2020, 1–6. [Google Scholar] [CrossRef]
- Zurl, C.; Hoenigl, M.; Schulz, E.; Hatzl, S.; Gorkiewicz, G.; Krause, R.; Eller, P.; Prattes, J. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically Ill COVID-19 patient with underlying hematological malignancy. J. Fungi 2021, 7, 88. [Google Scholar] [CrossRef]
- Mrittika, S.; Summeet, L.; Tatyarao, P.L.; Ragini, P.; Santosh, G.H. Mucor in a Viral Land: A Tale of Two Pathogens. Indian J. Opththalmol. 2021, 69, 244–252. [Google Scholar] [CrossRef]
- Chang, C.C.; Senining, R.; Kim, J.; Goyal, R. An Acute Pulmonary Coccidioidomycosis Coinfection in a Patient Presenting with Multifocal Pneumonia with COVID-19. J. Investig. Med. High Impact Case Rep. 2020, 8, 4–6. [Google Scholar] [CrossRef]
- Shah, A.S.; Heidari, A.; Civelli, V.F.; Sharma, R.; Clark, C.S.; Munoz, A.D.; Ragland, A.S.; Johnson, R.H. The Coincidence of 2 Epidemics, Coccidioidomycosis and SARS-CoV-2: A Case Report. J. Investig. Med. High Impact Case Rep. 2020, 8. [Google Scholar] [CrossRef]
- Basso, R.P.; Poester, V.R.; Benelli, J.L.; Stevens, D.A.; Zogbi, H.E.; da Vasconcellos, I.C.S.; Pasqualotto, A.C.; Xavier, M.O. COVID-19-Associated Histoplasmosis in an AIDS Patient. Mycopathologia 2020, 1. [Google Scholar] [CrossRef]
- Bertolini, M.; Mutti, M.F.; Barletta, J.A.E.; Falak, A.; Cuatz, D.; Sisto, A.; Ragusa, M.A.; Fernandez Claros, N.O.; Rolón, M.J. COVID-19 Associated with AIDS-Related Disseminated Histoplasmosis: A Case Report. Int. J. STD AIDS 2020, 31, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.A.; Marin, E.; Caceres, D.H.; Romero, M.; Depardo, R.; Priarone, M.M.; Rey, L.; Vázquez, M.; Verweij, P.E.; Chiller, T.M.; et al. Coronavirus Disease 2019 (Covid-19) in a Patient with Disseminated Histoplasmosis and HIV—A Case Report from Argentina and Literature Review. J. Fungi 2020, 6, 275. [Google Scholar] [CrossRef] [PubMed]
- Mrittika, S.; Santosh, G.H.; Namrata, S.; Mahipal, S.S. COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian J. Opththalmol. 2021, 69, 488–509. [Google Scholar] [CrossRef]
- Arenas-Jiménez, J.J.; Plasencia-Martínez, J.M.; García-Garrigós, E. When pneumonia is not COVID-19 Cuando la neumonía no es COVID-19]. Radiología 2021, 63, 180–192. [Google Scholar] [CrossRef]
- Pfaller, M.A. Molecular approaches to diagnosing and managing infectious diseases: Practicality and costs. Emerg. Infect. Dis. 2001, 7, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M. Invasive Fungal Disease Complicating Coronavirus Disease 2019: When It Rains, it pours. Clin. Infect. Dis. 2020, ciaa1342, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; van de Veerdonk, F.L.; Jenks, J.D.; Koehler, P.; Krause, R.; Cornely, O.A.; Perlín, D.S.; Lass-Flörl, C.; Hoenigl, M.; et al. COVID-19 associated pulmonary aspergillosis (CAPA)—From immunology to treatment. J. Fungi 2020, 6, 91. [Google Scholar] [CrossRef] [PubMed]
| Number of Cases/Sex/Age (Years)/Country | Risk Factors | Diagnostic Method | Clinical Management | Fatality Rate (%) | Reference |
|---|---|---|---|---|---|
| 10/8 male and 2 female/average between 46–68/France | Prolonged corticosteroid treatment in 3 patients | RT-qPCR; detection of β-D-glucan in serum | Cotrimoxazole in 4 patients, 6 patients were not treated | 30.0 | [20] |
| 1/male/25/USA | HIV, CD4 = 32 cells/mm3 | Detection of antigen in respiratory secretions by bronchoscopy | Trimethoprim-sulfamethoxazole, prednisone | 0.0 | [21] |
| 2/female/78 and the other was not reported/France | Lymphocytopenia | qPCR in BAL | Specific anti-Pneumocystis treatment | 100.0 | [22] |
| 1/female/72/China | 30-year-old rheumatoid arthritis and glucocorticoid treatment | High troughput sequencing analysis | Caspofungin acetate, glucocorticoids | 0.0 | [23] |
| 1/male/55/UK | HIV, CD4 422 cells/μL | PCR multiplex | Cotrimoxazole and prednisolone | 0.0 | [24] |
| 1/male/65/Italy | Kidney transplant, immunosuppressive treatment | qPCR | Trimethoprim-sulfamethoxazole | 100.0 | [25] |
| 2/ND/ND/China | HIV | ND | Clindamycin for one patient and trimethoprim-sulfamethoxazole for the other | 0.0 | [26] |
| 1/female/46/Argentina | HIV, absolute CD4+ count 67 cells/μL | Grocott stain of a sputum sample | Ceftriaxone, azithromycin, trimethoprim-sulfamethoxazole, prednisone, and fluconazole | 0.0 | [27] |
| 1/male/52/Germany | HIV, CD4+ 12 cells/μL viral load of 360,000 HIV-1 RNA copies/mL | Pneumocystis detection in BAL by a non-specified method, high LDH | Trimethoprim-sulfamethoxazol, prednisone | 0.0 | [28] |
| 1/female/83/USA | Lymphocytopenia | qPCR of tracheal aspirate | Trimethoprim-sulfamethoxazole | 0.0 | [29] |
| 1/male/65/France | Chemotherapy for chronic lymphocytic leukemia, lymphopenia | qPCR in BAL | Trimethoprim-sulfamethoxazole | 0.0 | [30] |
| 1/female/11/Spain | anti-MDA5 JDM | RT-PCR | Trimethoprim-sulfamethoxazole | 100.0 | [31] |
| 1/male/36/USA | HIV, absolute CD4 cell count was <10 cells/µL | DFA and PCR | Trimethoprim-sulfamethoxazole and prednisone | 100.0 | [32] |
| Number of Cases/Sex/Age (Years)/Country | Etiological Agent | Risk Factors | Diagnostic Method | Clinical Management | Fatality Rate (%) | Reference |
|---|---|---|---|---|---|---|
| 1/male/72/Austria | C. glabrata | Mechanical ventilation, central venous catheter, and hospitalization time | Culture | Caspofungin | 0.0 | [33] |
| 11/7 male and 4 female/average 59/Brazil | C. albicans, C. glabrata and C. tropicalis | DM, central venous catheter, antibiotics treatment, and HIV | Culture | Fluconazole, anidulafungin, voriconazole, and amphotericin B deoxycholate | 72.7 | [34] |
| 41/21 male and 20 female/average 62/Brazil | C. albicans, C. tropicalis, C. parapsilosis and C. glabrata | DM, mechanical ventilation, central venous catheter, surgery, hospitalization time, and hypotensive | Culture | Anidulafungin and fluconazole | 61.0 | [35] |
| 60/32 male and 28 female/average 51/China | Candida spp. | ND | Real-time PCR | ND | ND | [36] |
| 4/ND/ND/China | C. albicans, and C. glabrata | DM, mechanical ventilation, septic shock, acute respiratory and acute renal injury, and glucocorticoid treatment | Culture | ND | ND | [37] |
| 2/ND/ND/China | C. albicans | DM, central venous catheter, peripherally inserted central catheter, glucocorticoid treatment, antibiotics treatment, and hematological disease | ND | ND | ND | [38] |
| 6/3 male and 3 female/average 62/China | C. albicans, C. parapsilosis C. lusitaniae and C. tropicalis | DM, bacterial co-infection, higher white blood cell, and neutrophil counts, and higher levels of D-dimer, IL-6, IL-10, reactive protein-c, and procalcitonin | Culture and MALDI-TOF | ND | ND | [39] |
| 20/13 male and 7 female/average 63/Colombia | C. auris, C. albicans, C. tropicalis, C. parapsilosis C. orthopsilosis, C. glabrata | Mechanical ventilation, invasive hemodynamic support, prolonged stay in the ICU, DM, cancer, antibiotics treatment, and steroids | Culture and MALDI-TOF | Fluconazole, caspofungin, and voriconazole | 60.0 | [40] |
| 5/ND/ND/Egypt | C. albicans and C. glabrata | Antibiotics treatment, anticoagulants, mechanical ventilation, oxygen therapy, ARDS, and renal injury | Culture | ND | ND | [41] |
| 15/11 male and 4 female/average 63/India | C. auris, C albicans, C. tropicalis, and C. krusei | Mechanical ventilation, prolonged hospitalization time, DM, central lines and urinary catheters, and asthma | Culture, MALDI-TOF, and sequencing | Amphotericin B and micafungin | 53.3 | [42] |
| 53/female 30 and male 23/average 63.1/Iran | C. albicans, C. glabrata, C. dubliniensis, C. parapsilosis, C. tropicalis, and C. krusei | Broad-spectrum antibiotics treatment, corticosteroid treatment, mechanical ventilation, and ICU stay period | PCR and sequencing | Fluconazole, nystatin, and caspofungin | ND | [43] |
| 3/male/average 67.6/Italy | C. albicans, C. parapsilosis and C. tropicalis | Central venous catheter, parental nutrition, antibiotics treatment, and steroids treatment | Culture | Caspofungin, and fluconazole | 0.0 | [44] |
| 36/ND/ND/Italy | C. albicans, C. lusitaniae, C. glabrata, C. parapsilosis, and C. inconspicua | ND | Culture and MALDI-TOF | ND | ND | [45] |
| 7/ND/ND/Italy | C. albicans, C. inconspicua and C. parapsilosis | ICU stay period, mechanical ventilation, central venous catheter, antibiotics treatment, and corticosteroids treatment | Culture | Echinocandins | ND | [46] |
| 6/ND/ND/Italy | C. auris | ICU length of stay, broad-spectrum antibiotics treatment, and asthma | Culture and MALDI-TOF, sequencing | Echinocandins | 50.0 | [47] |
| 1/male/79/Italy | C. glabrata | Mechanical ventilation, antibiotics treatment, DM, and surgery | Culture and MALDI-TOF | Caspofungin | 100.0 | [48] |
| 3/ND/ND/Italy | C. albicans, C. parapsilosis and C. tropicalis | Antibiotics treatment, HIV, cancer, DM, anti-inflammatory treatment, and hospital length of stay | Culture | ND | ND | [49] |
| 21/16 male and 5 female/average 71/Italy | C. albicans and non -albicans, Candida spp. | Cancer, HIV, antibiotics treatment, parental nutrition, corticosteroid treatment, DM, ICU length of stay, central venous catheter, and surgery | Culture | ND | 57.1 | [50] |
| 14/8 male and 6 female/average 72/Lebanon | C. auris | Cancer, ICU length of stay, mechanical ventilation, urinary catheter, central venous catheter, broad-spectrum antibiotics treatment, and steroids treatment | Culture and MALDI-TOF | Caspofungin and anidulafungin | 35.7 | [51] |
| 12/10 male and 2 female/average 55/Mexico | C. auris and C. glabrata | Mechanical ventilation, peripherally inserted central lines, urinary catheter, asthma, steroids treatment, and prolonged ICU stay | Culture-MALDI-TOF, and sequencing | Isavuconazole, anidulafungin, caspofungin, amphotericin B, and voriconazole | 83.3 | [52] |
| 5/male/average 59/Oman | C. albicans, C. glabrata and C tropicalis | Mechanical ventilation, ICU prolonged length of stay, broad-spectrum antibiotics treatment, and central line catheter | Culture and MALDI-TOF | Amphotericin B, caspofungin, and voriconazole | 60.0 | [53] |
| 1/male/53/Spain | C. albicans | Mechanical ventilation, corticosteroid treatment, broad-spectrum antibiotics treatment, and central venous catheter | Culture | Fluconazole | 0.0 | [54] |
| 3/ND/ND/UK | C. albicans | Central line, mechanical ventilation, immunomodulatory therapy, and broad-spectrum antibiotics treatment | Culture and MALDI-TOF | ND | ND | [55] |
| 17/Ratio male:female was 2:1/aveage 58/UK | C. albicans and C. parapsilosis | Cancer, corticosteroid treatment, ventilation support, asthma, DM, and central venous catheter | Culture and MALDI-TOF | Liposomal amphotericin B, fluconazole, caspofungin, and voriconazole | 38.5 | [56] |
| 35/21 male and 14 female/average 69/USA | C. auris | Central venous catheter, mechanical ventilator, urinary catheter, DM, cancer, nasogastric and gastric tube | Culture | ND | ND | [57] |
| 1/male/54/USA | C. albicans | Cancer, and mechanical ventilation | Culture | ND | ND | [58] |
| 8/4 male and 4 female/average 63/USA | C. albicans, C. glabrata, C. parapsilosis and C. tropicalis | ICU length of stay, mechanical ventilation, and central venous catheter | Culture and MALDI-TOF | Caspofungin and fluconazole | 38.0 | [59] |
| 1/male/46/USA | C. albicans | Mechanical ventilation, cancer, and surgery | Culture | ND | 33.0 | [60] |
| 12/9 male and 3 female/average 62/USA | C. albicans, C. parapsilosis, C. glabrata, C. tropicalis, and C. dublinensis | Mechanical ventilation, central venous catheter, ICU stay, and broad-spectrum antibiotics treatment | Culture and MALDI-TOF | ND | 75.0 | [61] |
| Number of Cases/Sex/Age (Years)/Country | Etiological Agent | Risk Factors | Diagnostic Method | Clinical Management | Fatality Rate (%) | Reference |
|---|---|---|---|---|---|---|
| 1/female/72/China | A. fumigatus | Leflunomide for rheumatoid arthritis, Methylprednisolone, Tocilizumab, and glucocorticoid treatment | High-performance sequencing analysis | Caspofungin acetate | 0.0 | [23] |
| 5/male:female ratio 2.2:1/Average 57/United Kingdom | A. fumigatus | Solid neoplasm | AspICU algorithm, BAL, culture, PCR, BDG, GM | Voriconazole caspofungin liposomal amphotericin B | 53.0 | [56] |
| 14/ND/average 50.35/Mexico | Aspergillus spp., A. fumigatus, A. flavus, A. niger | Obesity, DM, hypertension, active smoker and HIV | Culture, MALDI-TOF, sGM | Voriconazole, anidulafungin | 57.0 | [62] |
| 20/ND/elderly/USA and Spain | A. fumigatus | Severe immunosuppression due to hematological neoplasm or transplants, hypertensionlung disease, steroid therapy | BAL, culture, BDG | Voriconazole, posaconazole, liposomal mphotericin B | 100.0 | [63] |
| 1/female/74/Netherlands | A. fumigatus | Hospitalization in the ICU | Culture, GM, BDG | Voriconazole, liposomal amphotericin B, caspofungin | 100.0 | [64] |
| 1/male/71/Brazil | A. penicillioides | Hypertension DM, chronic kidney disease | Histopathology, GM, Confirmation by nucleotide sequencing | Post-mortem diagnosis | 100.0 | [65] |
| 13/11 male and 2 female/average 54 to 78/Netherlands | A. fumigatus | Immunosuppression, ICU, VMI, prolonged use of corticosteroids treatment | BDG, GM, Fungal PCR targeting the Cyp51A gene | Voriconazole, caspofungin, liposomal amphotericin B | 40.0 to 50.0 | [66] |
| 1/female/66/Australia | A. section Fumigati | Hypertension, smoking history, osteopenia, Facklamia hominis blood culture and Escherichia coli urine culture on admission | Non-bronchoscopic endotracheal aspirate with Gram staining | Voriconazole | 0.0 | [67] |
| 1/male/ND/Austria | Aspergillus spp. | Chronic degenerative disease, neoplasia, immunosuppression | Autopsy | Post-mortem diagnosis | 100.0 | [68] |
| 5/3 male and 2 female/average 69/Pakistan | A. fumigatus, A. flavus, A. niger | DM, high blood pressure | Culture, IgM, BDG | Voriconazole, liposomal amphotericin B | 40.0 | [69] |
| 1/male/46/China | A. fumigatus | DM, stage 2 hypertension | Culture, MALDI-TOF | Voriconazole | 0.0 | [70] |
| 1/female/87France | Aspergillus spp. | ND | GM, ELISA, Western blot, PCR | Voriconazole | 100.0 | [71] |
| 1/female/58/Qatar | A. niger, A. terreus | Diabetic nephropathy, hypertension, hyperlipidemia, chronic hepatitis B infection, elderly patient | Culture | Anidulafungin, liposomal amphotericin B, voriconazole | 100.0 | [72] |
| 1/male/74/France | A. fumigatus | Asymptomatic myelodysplastic syndrome (hypereosinophilia, with CD8+ T-cell lymphocytosis), Hashimoto’s thyroiditis, and hypertension | Culture, PCR, BDG, GM | No antifungal treatment was initiated due to the rapid and fatal course in the patient | 100.0 | [73] |
| 1/female/42/Iran | Aspergillus spp. | Acute myeloid leukemia, DM | GM, ELISA for Aspergillus | Liposomal amphotericin B | 100.0 | [74] |
| 2/1 male and 1 female/66 and 38 respectively/France | Aspergillus spp. A. niger | DM, obesity, hypertension, rheumatoid arthritis in methotrexate treatment | GM, PCR, BDG, Culture | ND | 0.0 | [75] |
| 1/male/52/Denmark | A. fumigatus sensu stricto | DM, obesity, percutaneous coronary intervention | GM, MALDI-TOF | Voriconazole | 0.0 | [76] |
| 10/8 male and 2 female/average between 51 and 76/Spain | A. fumigatus, A. nidulans | Hematological neoplasms, immunosuppression, DM, obesity, ICU COPD, Age > 65 | Culture, MALDI-TOF, GM ELISA, AspICU algorithm | Corticosteroids, voriconazole, caspofungin, amphotericin B | 70.0 | [77] |
| 1/male/66/Ireland | A. fumigatus with TR34/L98H mutation in Cyp51A gene | DM, hypertension, hyperlipidemia, obesity, grounds maintenance worker | BDG, GM | Liposomal amphotericin B | 100.0 | [78] |
| 6/ND/average 55/France | A. fumigatus | Overweight, hypertension, DM, active smokers, COPD, Immunodepression | Culture, GMN, BDG, PCR | Voriconazole, caspofungin | 57.2 | [79] |
| 7/5 male and 2 female/average 59.6 ± 15.21/Spain | A. fumigatus, A. flavus, A. niger | DM, obesity, sleep apnea, hypertension | PCR ITS1-5.8S-ITS2, GM | Itraconazole, liposomal amphotericin B | 86.0 | [80] |
| 4/male/average 79/USA | A. fumigatus | COPD | AspICU algorithm, EORTC/MSG, culture, sGM | Voriconazole | 100.0 | [81] |
| 9/ND/average 63.5/Germany | A. fumigatus | Hypertension | Microbiological follow-up tests | Echinocandins, voriconazole, fluconazole, addition of liposomal amphotericin B | 13.0 | [82] |
| 1/male/56/France | A. fumigatus with TR34/L98H mutation in Cyp51A gene | DM, hypertension, hyperlipidemia, obesity | Tracheal aspirate, Cx + PCREUCAST, Pan-azole-resistance, autopsy was not performed | No antifungal treatment was given because patient died before results were obtained | 100.0 | [83] |
| 1/male/73/Argentina | A. section Fumigati | Pulmonary embolism, and thrombophlebitis | GM, Pan-fungal nested PCR of 18S-rDNA | Voriconazole, liposomal amphotericin B, fluconazole | 0.0 | [84] |
| 1/male/73/USA | Aspergillus spp., A. flavus | Hypertension | TAC, GM, culture | Voriconazole | 0.0 | [85] |
| 1/male/85/Argentina | A. flavus | Hypertension | MALDI-TOF | Anidulafungin, voriconazole | 100.0 | [86] |
| 1/female/55/Spain | A. fumigatus | Hypertension active smoker, liver hemangiomas, kidney transplant recipient | Sputum, culture, GM, BDG | Isavuconazole | 0.0 | [87] |
| 8/6 male and 2 female/52 and 74 respectively/Spain | A. fumigatus, A. terreus, A. awamori, A. citrinoterreus, A. lentulus | Hypertension, obesity, asthma, kidney transplant recipient | Culture, sGM, PCR | Voriconazole, isavuconazole, liposomal amphotericin B | 100.0 | [88] |
| 1/male/80/France | A. flavus | Removed thyroid cancer | Tracheal aspirate culture | Voriconazole, isavuconazole | 100.0 | [89] |
| 30/24 male and 6 female/between 38 and 86 respectively/Germany, France, Netherlands, Belgium, Italy, Austria | A. fumigatus, Aspergillus spp. A. flavus | Obesity, DM, hypertension, chronic kidney disease, hyperlipidemia | Culture + GM, sGM, PCR | Voriconazole, isavuconazole, caspofungin, liposomal amphotericin B | 50.0 | [90] |
| 6/ND/ND/United Kingdom | A. fumigatus | COVID-19 requiring hospitalization in the ICU | Culture, Microscopy, GM, BDG, PCR | ND | ND | [91] |
| 10/ND/average 62/Netherlands | Aspergillus spp. | GM, PCR, Culture | ND | ND | [92] |
| Number of Cases/Sex/Age (Years)/Country | Etiological Agent | Risk Factors | Diagnostic Method | Clinical Management | Fatality Rate (%) | Reference |
|---|---|---|---|---|---|---|
| 1/male/60/India | Unidentified | DM, glucocorticoid treatment, and broad-spectrum antibiotics treatment | Clinical and suggestive MRI | Amphotericin B | 0.0 | [94] |
| 1/male/86/Brazil | Unidentified | Glucocorticoid treatment and broad-spectrum antibiotics treatment | Histopathology, Gastric ulcer biopsy | ND | 0.0 | [95] |
| 1/female/33/USA | Unidentified | Uncontrolled DM | Clinical and suggestive MRI | Amphotericin B and sinonasal debridement | 0.0 | [93] |
| 1/male/55/India | R. microsporus | DM, glucocorticoid treatment, broad-spectrum antibiotics treatment, systemic high blood pressure, end-stage kidney disease, ischemic cardiomyopathy | Sputum sample culture | Liposomal amphotericin B, upper right lobectomy | 100.0 | [96] |
| 1/male/49/USA | Rhizopus spp. | Glucocorticoid treatment, broad-spectrum antibiotics treatment | Histopathology, Right upper lobe biopsy | Amphotericin B | 0.0 | [97] |
| 1/male/24/Mexico | Lichteimia (Absidia) spp. | Uncontrolled DM, diabetic ketoacidosis | Culture | Amphotericin B | 0.0 | [98] |
| 1/male/66/Italy | Rhizopus spp. | Broad-spectrum antibiotics treatment | Bronchial aspirate culture | Liposomal amphotericin B, isavuconazole, thoracocentesis | 0.0 | [99] |
| 1/male/53/Austria | R. microsporus | Neoplasia, glucocorticoids treatment | dPCR and sequencing, complete microscopic autopsy of lung tissue | ND | 0.0 | [100] |
| 6/male/average 60.5/India | 2-Unidentified and 4-Mucor spp. | DM, diabetic ketoacidosis, glucocorticoid treatment, and uncontrolled DM | Culture and histopathology | FESS and amphotericin B | 100.0 | [101] |
| Number of Cases/Sex/Age (Years)/Country | Etiological Agent | Risk Factors | Diagnostic Method | Clinical Management | Fatality Rate (%) | Reference |
|---|---|---|---|---|---|---|
| 1/female/48/USA | C. immitis | Heart failure, lived in an endemic area: Bakersfield, California | IgM and IgG by immunodiffusion assay with complement-fixation titers of 1:2 | Fluconazole | 0.0 | [102] |
| 1/male/48/USA | C. immitis | Uncontrolled DM, lives in endemic area: California | Positive serology for Coccidioides spp. with complement-fixation titers of 1:32 | ND | 0.0 | [103] |
| 1/female/43/Brazil | H. capsulatum | HIV infection with TCD4+ lymphocyte count of 113 cells/mm3, cocaine use, lived in an endemic area: Rio Grande, Brazil | Gomori-Grocott staining of expectoration, H. capsulatum urinary antigen | Itraconazole | 0.0 | [104] |
| 1/male/43/Argentina | H. capsulatum | HIV infection with TCD4+ lymphocyte count of 16.3 cells/mm3,lived in an endemic area: Buenos Aires, Argentina | Giemsa staining, Blood culture and skin biopsy culture | Amphotericin B deoxycholate, itraconazole | 0.0 | [105] |
| 1/female/36/Argentina | H. capsulatum | HIV infection with TCD4+ lymphocyte count of 3 cells/mm3, drug use: marijuana and cocaine, lived in endemic area: Buenos Aires, Argentina | Wright and Giemsa staining of expectoration, Histoplasma serum and urinary antigen | Amphotericin B deoxycholate, itraconazole | 0.0 | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frías-De-León, M.G.; Pinto-Almazán, R.; Hernández-Castro, R.; García-Salazar, E.; Meza-Meneses, P.; Rodríguez-Cerdeira, C.; Arenas, R.; Conde-Cuevas, E.; Acosta-Altamirano, G.; Martínez-Herrera, E. Epidemiology of Systemic Mycoses in the COVID-19 Pandemic. J. Fungi 2021, 7, 556. https://doi.org/10.3390/jof7070556
Frías-De-León MG, Pinto-Almazán R, Hernández-Castro R, García-Salazar E, Meza-Meneses P, Rodríguez-Cerdeira C, Arenas R, Conde-Cuevas E, Acosta-Altamirano G, Martínez-Herrera E. Epidemiology of Systemic Mycoses in the COVID-19 Pandemic. Journal of Fungi. 2021; 7(7):556. https://doi.org/10.3390/jof7070556
Chicago/Turabian StyleFrías-De-León, María Guadalupe, Rodolfo Pinto-Almazán, Rigoberto Hernández-Castro, Eduardo García-Salazar, Patricia Meza-Meneses, Carmen Rodríguez-Cerdeira, Roberto Arenas, Esther Conde-Cuevas, Gustavo Acosta-Altamirano, and Erick Martínez-Herrera. 2021. "Epidemiology of Systemic Mycoses in the COVID-19 Pandemic" Journal of Fungi 7, no. 7: 556. https://doi.org/10.3390/jof7070556
APA StyleFrías-De-León, M. G., Pinto-Almazán, R., Hernández-Castro, R., García-Salazar, E., Meza-Meneses, P., Rodríguez-Cerdeira, C., Arenas, R., Conde-Cuevas, E., Acosta-Altamirano, G., & Martínez-Herrera, E. (2021). Epidemiology of Systemic Mycoses in the COVID-19 Pandemic. Journal of Fungi, 7(7), 556. https://doi.org/10.3390/jof7070556









