SCA Medium: A New Culture Medium for the Isolation of All Candida auris Clades
Abstract
1. Introduction
2. Materials and Methods
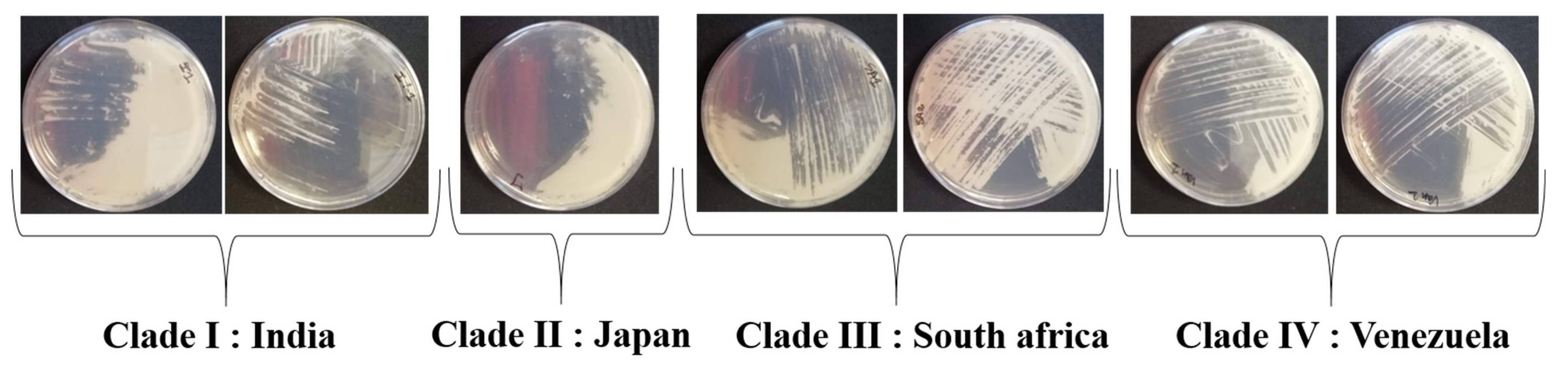
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Osei Sekyere, J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef]
- Chow, N.A.; De Groot, T.; Badali, H.; Abastabar, M.; Chiller, T.M.; Meis, J.F. Potential fifth clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 2019, 25, 1780–1781. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Browna, C.S. Candida auris: A review of the literature. Clin. Microbiol. Rev. 2018, 31, e00029-17. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-resistant Candida auris misidentified as Candida haemulonii: Characterization by matrix-assisted laser desorption ionization-time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by vitek 2, CLSI broth microdilution, and etest method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, M.; Miller, H.; Green, R.; Lee, R.; Durante, M.; Perkins, R.; Hewitt, C.; Simner, P.J.; Carroll, K.C.; Hayden, R.T.; et al. Can multidrug-resistant candida auris be reliably identified in clinical microbiology laboratories? J. Clin. Microbiol. 2017, 55, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Baron, S.A.; Yousfi, H.; Hadjadj, L.; Lalaoui, R.; Morand, S.; Rolain, J.M.; Bittar, F. Development and standardization of a specific real-time PCR assay for the rapid detection of Candida auris. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 1–5. [Google Scholar] [CrossRef]
- Lima, A.; Widen, R.; Vestal, G.; Uy, D.; Silbert, S. A TaqMan probe-based real-time PCR assay for the rapid identification of the emerging multidrug-resistant pathogen candida auris on the BD max system. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Leach, L.; Zhu, Y.; Chaturvedi, S. Development and Validation of a Real-Time PCR Assay for Rapid Detection of Candida auris from Surveillance Samples. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Spencer, J.E.; Lockhart, S.R.; Singleton, S.; Petway, D.J.; Bagarozzi, D.A.; Herzegh, O.T. A high-throughput and rapid method for accurate identification of emerging multidrug-resistant Candida auris. Mycoses 2019, 62, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, H.; Ranque, S.; Rolain, J.M.; Bittar, F. In vitro polymyxin activity against clinical multidrug-resistant fungi. Antimicrob. Resist. Infect. Control 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, H.; Cassagne, C.; Ranque, S.; Rolain, J.M.; Bittar, F. Repurposing of ribavirin as an adjunct therapy against invasive Candida strains in an in vitro study. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Lagier, J.C.; Khelaifia, S.; Alou, M.T.; Ndongo, S.; Dione, N.; Hugon, P.; Caputo, A.; Cadoret, F.; Traore, S.I.; Seck, E.H.; et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat. Microbiol. 2016, 1, 16203. [Google Scholar] [CrossRef]
- Bardet, L.; Le Page, S.; Leangapichart, T.; Rolain, J.M. LBJMR medium: A new polyvalent culture medium for isolating and selecting vancomycin and colistin-resistant bacteria. BMC Microbiol. 2017, 17, 220. [Google Scholar] [CrossRef]
- Jung, B.; Hoilat, G.J. MacConkey Medium; n: StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Calvo, B.; Melo, A.S.A.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New clonal strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef]
- L’Ollivier, C.; Cassagne, C.; Normand, A.C.; Bouchara, J.P.; Contet-Audonneau, N.; Hendrickx, M.; Fourquet, P.; Coulibaly, O.; Piarroux, R.; Ranque, S. A MALDI-TOF MS procedure for clinical dermatophyte species identification in the routine laboratory. Med. Mycol. 2013, 51, 713–720. [Google Scholar] [CrossRef]
- De Almeida, J.N.; Francisco, E.C.; Hagen, F.; Brandão, I.B.; Pereira, F.M.; Dias, P.H.P.; De Miranda Costa, M.M.; De Souza Jordão, R.T.; De Groot, T.; Colombo, A.L.; et al. Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. J. Fungi 2021, 7, 220. [Google Scholar] [CrossRef]
- Allaw, F.; Kara Zahreddine, N.; Ibrahim, A.; Tannous, J.; Taleb, H.; Bizri, A.R.; Dbaibo, G.; Kanj, S.S. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens 2021, 10, 157. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.W.; Dieleman, C.; Carbia, M.; Tap, R.M.; Hagen, F. Performance of two novel chromogenic media for the identification of multidrug-resistant candida auris compared with other commercially available formulations. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef]
- Das, S.; Singh, S.; Tawde, Y.; Chakrabarti, A.; Rudramurthy, S.M.; Kaur, H.; Shankarnarayan, S.A.; Ghosh, A. A selective medium for isolation and detection of Candida auris, an emerging pathogen. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Fraser, M.; Johnson, E.M. CHROMagarTM Candida Plus: A novel chromogenic agar that permits the rapid identification of Candida auris. Med. Mycol. 2021, 59, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.G.; Hakim, S.T.; Kazmi, S.U. Use of CHROMagar Candida for the presumptive identification of Candida species directly from clinical specimens in resource-limited settings. Libyan J. Med. 2010, 5, 1–6. [Google Scholar] [CrossRef]
| Type | Strain/Sample | Nb | Source | Origin | Identification | qPCR C. auris (Ibrahim et al., 2021) |
|---|---|---|---|---|---|---|
| Gram-positive bacteria | Bacillus cereus | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested |
| Corynebacterium amycolatum | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Corynebacterium jeikeium | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Corynebacterium propinquum | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Corynebacterium striatum | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Enterococcus faecalis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Enterococcus faecium | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Micrococcus luteus | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus aureus | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus capitis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus cohnii | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus epidermidis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus haemolyticus | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus lugdunensis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus pasteuri | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus saprophyticus | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus simulans | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus warneri | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Streptococcus agalactiae | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Streptococcus dysgalactiae | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Streptococcus equinus | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Streptococcus mitis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Streptococcus pneumoniae | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Staphylococcus hominis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Streptococcus salivarius | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Subtotal | 25 | |||||
| Gram-negative bacteria | Achromobacter xylosoxidans | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested |
| Acinetobacter baumannii | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Bacteroides fragilis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Citrobacter braakii | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Citrobacter freundii | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Citrobacter koseri | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Enterobacter aerogenes | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Enterobacter asburiae | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Enterobacter cloacae | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Enterobacter kobeii | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Escherichia coli | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Haemophilus influenzae | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Haemophilus parainfluenzae | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Hafnia alvei | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Klebsiella oxytoca | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Klebsiella pneumoniae | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Moraxella catarrhalis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Morganella morganii | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Pasteurella multocida | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Proteus mirabilis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Proteus vulgaris | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Providencia stuartii | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Pseudomonas aeruginosa | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Raoultella ornithinolytica | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Stenotrophomonas maltophilis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Subtotal | 25 | |||||
| Yeast | Candida albicans | 73 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested |
| Candida glabrata | 8 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida krusei | 4 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida parapsilosis | 6 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida lusitaniae | 3 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida tropicalis | 6 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida zelanoides | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida lipolytica | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida inconspicua | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida intermedia | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida guilliermondii | 3 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida bracarensis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida utilis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida bovina | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida dubliniensis | 2 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida norvegensis | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida kefyr | 2 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida beverwijkiae | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Cryptococcus diffluens | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Cryptococcus uniguttulatus | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Cryptococcus neoformans | 2 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Saccharomyces cerevisiae | 2 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Rhodotorula mucilaginosa | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Yarrowia lipolitica | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida haemulonii | 1 | Clinical | Netherlands | MALDI-TOF-MS | Not tested | |
| Candida duobushaemulonii | 1 | Clinical | Netherlands | MALDI-TOF-MS | Not tested | |
| Trichosporon asahii | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Kodamaea ohmeri | 1 | Clinical | Marseille, France | MALDI-TOF-MS | Not tested | |
| Candida auris Clade I | 2 | Clinical | Netherlands | MALDI-TOF-MS | Positive | |
| Candida auris Clade II | 1 | Clinical | DSMZ collection | MALDI-TOF-MS | Positive | |
| Candida auris Clade III | 2 | Clinical | Netherlands | MALDI-TOF-MS | Positive | |
| Candida auris Clade IV | 2 | Clinical | Netherlands | MALDI-TOF-MS | Positive | |
| Subtotal | 135 | |||||
| Human samples | Stool samples | 200 | Clinical | Marseille, France | Negative | |
| Total | 385 |
| Crystal Violet | Bile Salts | C. auris Clade I | C. auris Clade II | C. auris Clade III | C. auris Clade VI | C. tropicalis |
|---|---|---|---|---|---|---|
| 0 mg/L | 0 g/L | ++ | ++ | ++ | ++ | ++ |
| 0 mg/L | 0.5 g/L | ++ | ++ | ++ | ++ | ++ |
| 0 mg/L | 1 g/L | ++ | ++ | ++ | ++ | ++ |
| 0 mg/L | 1.5 g/L | ++ | + | ++ | ++ | + |
| 0.5 mg/L | 0 g/L | ++ | ++ | ++ | ++ | - |
| 0.5 mg/L | 0.5 g/L | ++ | ++ | ++ | ++ | - |
| 0.5 mg/L | 1 g/L | ++ | + | ++ | ++ | - |
| 0.5 mg/L | 1.5 g/L | ++ | + | ++ | ++ | - |
| Pancreatic Digest of Casein | Peptic Digest of Animal Tissue | NaCl | Mannitol | Crystal Violet | Agar | pH | Chloramphenicol | Gentamicin | |
|---|---|---|---|---|---|---|---|---|---|
| Welsh et al. broth | 5 g | 5 g | 100 g | 20 g | - | - | 5.6 | 50 mg/L | 50 mg/L |
| SCA medium | 5 g | 5 g | 100 g | 20 g | 0.5 mg | 15 g | 7 | 50 mg/L | 50 mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.; Peyclit, L.; Abdallah, R.; Khelaifia, S.; Chamieh, A.; Rolain, J.-M.; Bittar, F. SCA Medium: A New Culture Medium for the Isolation of All Candida auris Clades. J. Fungi 2021, 7, 433. https://doi.org/10.3390/jof7060433
Ibrahim A, Peyclit L, Abdallah R, Khelaifia S, Chamieh A, Rolain J-M, Bittar F. SCA Medium: A New Culture Medium for the Isolation of All Candida auris Clades. Journal of Fungi. 2021; 7(6):433. https://doi.org/10.3390/jof7060433
Chicago/Turabian StyleIbrahim, Ahmad, Lucie Peyclit, Rim Abdallah, Saber Khelaifia, Amanda Chamieh, Jean-Marc Rolain, and Fadi Bittar. 2021. "SCA Medium: A New Culture Medium for the Isolation of All Candida auris Clades" Journal of Fungi 7, no. 6: 433. https://doi.org/10.3390/jof7060433
APA StyleIbrahim, A., Peyclit, L., Abdallah, R., Khelaifia, S., Chamieh, A., Rolain, J.-M., & Bittar, F. (2021). SCA Medium: A New Culture Medium for the Isolation of All Candida auris Clades. Journal of Fungi, 7(6), 433. https://doi.org/10.3390/jof7060433






