Citronellal Exerts Its Antifungal Activity by Targeting Ergosterol Biosynthesis in Penicillium digitatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogens
2.2. Fruit
2.3. Chemicals
2.4. In Vivo Experiments of Citronellal
2.5. Additional Exogenous Ergosterol Assay
2.6. Plasma Membrane Integrity
2.7. Determination of Total Lanosterol and Ergosterol Contents
2.8. Determination of Sterols Composition by Gas Chromatography-Mass Spectrometry (GC-MS)
2.9. Real-Time Fluorescence Quantitative PCR (RTFQ-PCR) Analysis
2.10. Statistical Analysis
3. Results
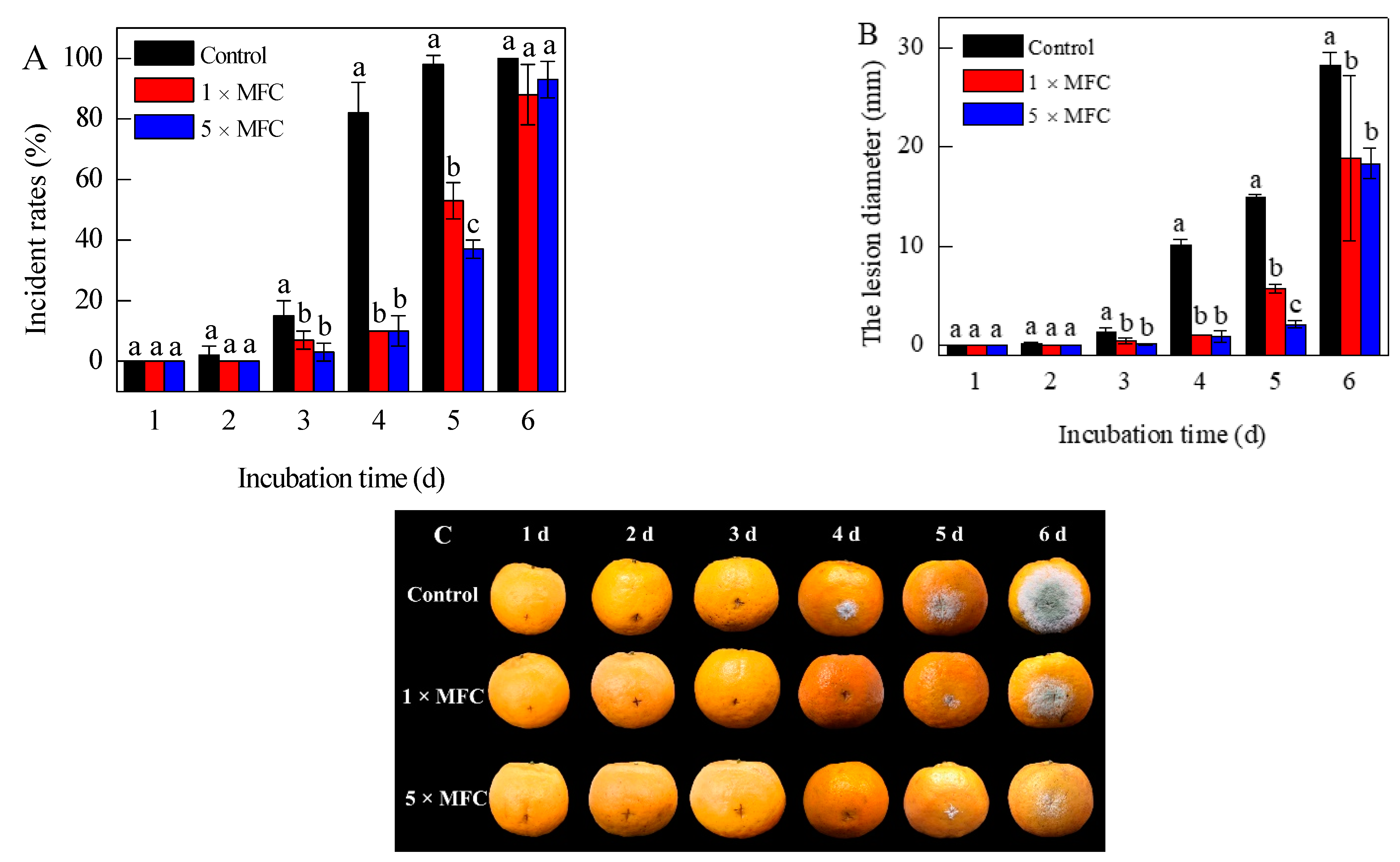
3.1. In Vivo Experiments of Citronellal
3.2. Additional Exogenous Ergosterol Assay
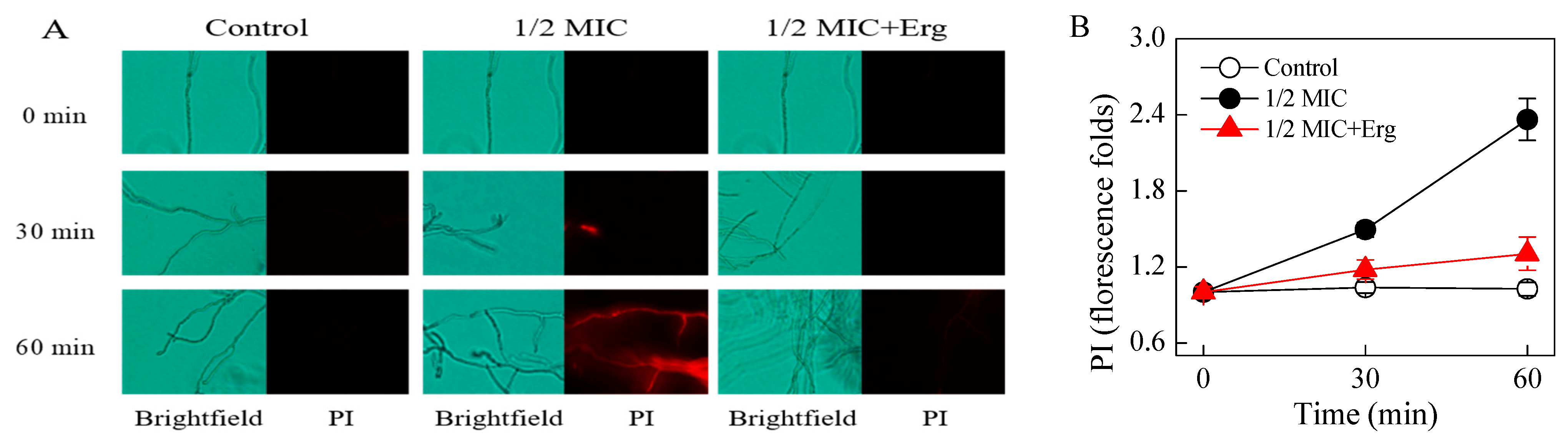
3.3. Effects of Citronellal on the Plasma Membrane Integrity
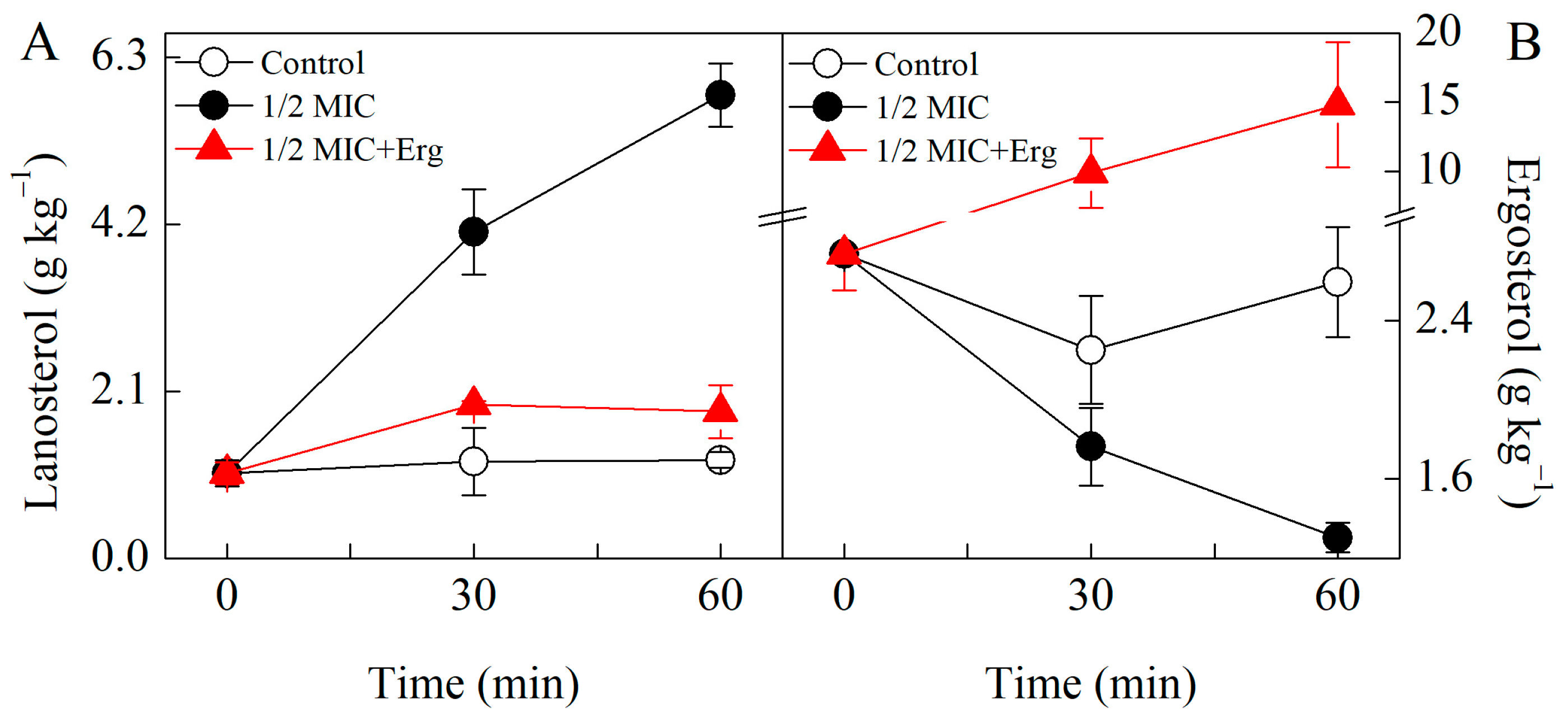
3.4. Lanosterol and Ergosterol Contents
3.5. Analysis of Sterols Composition by GC-MS
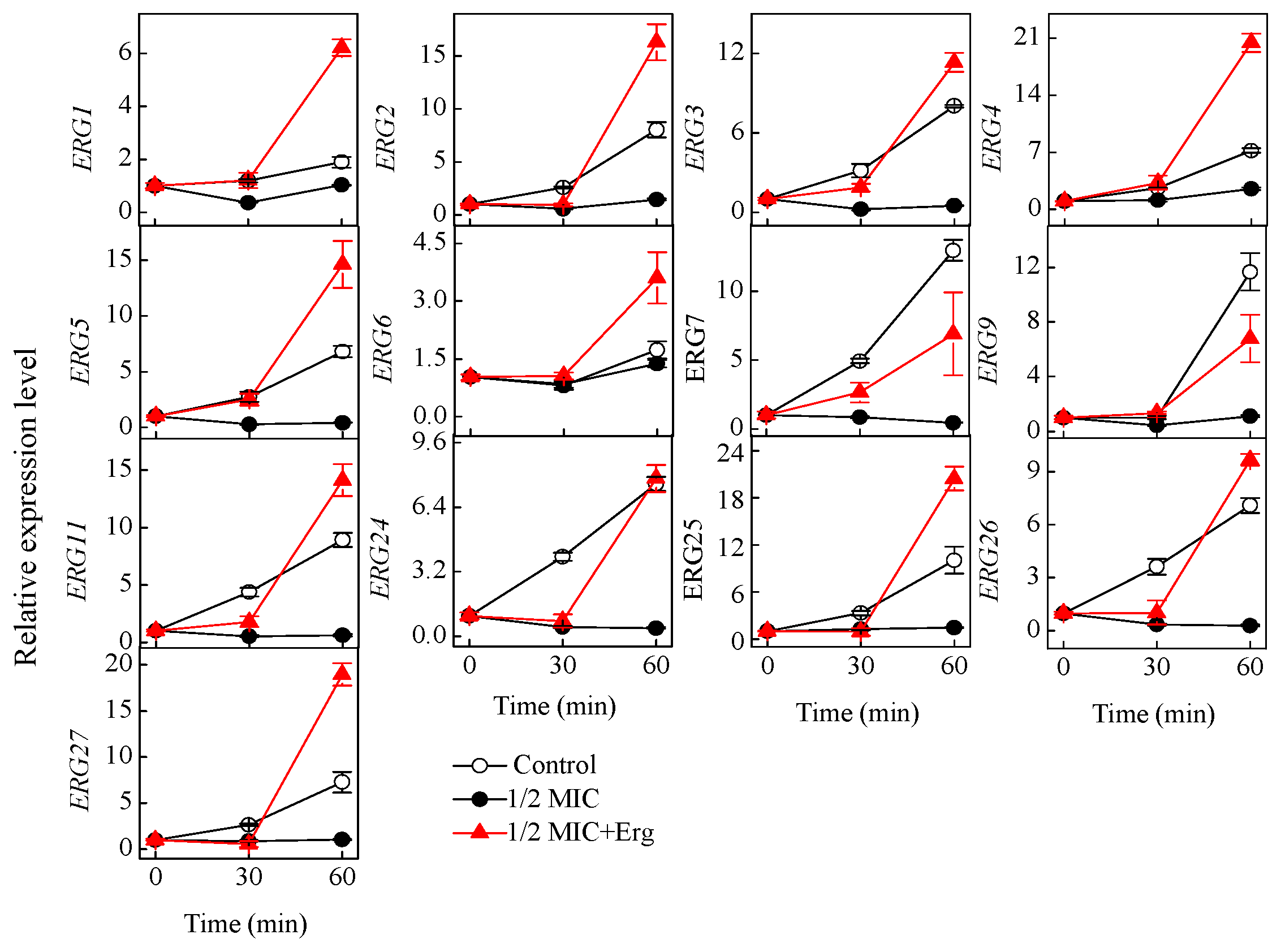
3.6. Effect of Citronellal on Gene Expression Levels in Ergosterol Biosynthesis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanetis, L.; Förster, H.; Adaskaveg, J.E. Comparative efficacy of the new postharvest fungicides azoxystrobin, fludioxonil, and pyrimethanil for managing citrus green mold. Plant Dis. 2007, 91, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.P.; Wang, J.Y.; Feng, D.; Ma, Z.H.; Li, H.Y. PdCYP51B, a new putative sterol 14a–demethylase gene of Penicillium digitatum involved in resistance to imazalil and other fungicides inhibiting ergosterol synthesis. Appl. Microbiol. Biotechnol. 2011, 91, 1107–1119. [Google Scholar] [CrossRef]
- Camele, I.; Elshafie, H.S.; Caputo, L.; De Feo, V. Anti-quorum sensing and antimicrobial effect of Mediterranean plant essential oils against phytopathogenic bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Grul’ova, D.; Zheljazkov, V.Z.; De Feo, V.; Camele, I. Biological investigations of essential oils extracted from three Juniperus species and evaluation of their antimicrobial, antioxidant and cytotoxic. J. Appl. Microbiol. 2020, 129, 1261–1271. [Google Scholar] [CrossRef]
- Gru’ová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Goga’ová, Z.; Poráčová, J.; Camele, I.; De Feo, V. Thymol chemotype Origanum vulgare L. essential oil as a potential selective bio-based herbicide on monocot plant species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef]
- Kesdek, M.; Kordali, S.; Bozhuyuk, A.U.; Gudek, M. Larvicidal effect of Achillea biebersteinii Afan. (Asteraceae) essential oil against larvae of pine processionary moth, Thaumetopoea pityocampa (Denis & Schiffermüller, 1775) (Lepidoptera: Notodontidae). Turk. J. Agric. For. 2020, 44, 451–460. [Google Scholar] [CrossRef]
- Duan, X.F.; OuYang, Q.L.; Tao, N.G. Effect of applying cinnamaldehyde incorporated in wax on green mould decay in citrus fruits. J. Sci. Food Agric. 2017, 98, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F. The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chem. 2020, 309, 125520. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Shao, X.F.; Wei, Y.Y.; Jiang, S.; Xu, F.; Wang, H.F.; Gao, H.Y. Optimized preparation of tea tree oil complexation and their antifungal activity against Botrytis cinerea. Postharvest Biol. Technol. 2020, 162, 111114. [Google Scholar] [CrossRef]
- Kaur, H.; Bhardwaj, U.; Kaur, R. Cymbopogon nardus essential oil: A comprehensive review on its chemistry and bioactivity. J. Essent. Oil Res. 2021. [Google Scholar] [CrossRef]
- Rammanee, K.; Hongpattarakere, T. Effects of tropical citrus essential oils on growth, aflatoxin production, and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Bioprocess Technol. 2011, 4, 1050–1059. [Google Scholar] [CrossRef]
- Scora, K.M.; Scora, R.W. Effect of volatiles on mycelium growth of Penicillium digitatum, P. italicum, and P. ulaiense. J. Basic Microbiol. 1998, 38, 405–413. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Bara, A.; Salhi, N.; Terzi, A. In vitro evaluation of sub-lethal concentrations of plant-derived antifungal compounds on Fusaria growth and mycotoxin production. Molecules 2017, 22, 1271. [Google Scholar] [CrossRef]
- Wang, H.W.; Yang, Z.X.; Ying, G.Y.; Yang, M.H.; Nian, Y.J.; Wei, F.; Kong, W.J. Antifungal evaluation of plant essential oils and their major components against toxigenic fungi. Ind. Crop. Prod. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- Wu, Y.L.; OuYang, Q.L.; Tao, N.G. Plasma membrane damage contributes to antifungal activity of citronellal against Penicillium digitatum. J. Food Sci. Technol. 2016, 53, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- OuYang, Q.L.; Okwong, R.O.; Chen, Y.P.; Tao, N.G. Synergistic activity of cinnamaldehyde and citronellal against green mold in citrus fruit. Postharvest Biol. Technol. 2020, 162, 111095. [Google Scholar] [CrossRef]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fatima, Z.; Hameed, S. Citronellal-induced disruption of membrane homeostasis in Candida albicans and attenuation of its virulence attributes. Rev. Soc. Bras. Med. Trop. 2016, 49, 465–472. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.Z.; Wu, Z.; Li, N.; Chen, Y.L.; Qin, T.T.; Geng, H.; Xiong, L.; Liu, D.L. A novel sterol regulatory element–binding protein gene (sreA) identified in Penicillium digitatum is required for prochloraz resistance, full virulence and erg11 (cyp51) regulation. PLoS ONE 2015, 10, e0117115. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Mancini, E.; Camele, I.; De Martino, L.; De Feo, V. In vivo antifungal activity of two essential oils from Mediterranean plants against postharvest brown rot disease of peach fruit. Ind. Crop. Prod. 2015, 66, 11–15. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Mancini, E.; Sakr, S.; De Martino, L.; Mattia, C.A.; De Feo, V.; Camele, I. Antifungal activity of some constituents of Origanum vulgare L. essential oil against postharvest disease of peach fruit. J. Med. Food 2015, 18, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. 2011, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.J.; Xing, F.G.; Selvaraj, J.N.; Wang, Y.; Zhao, Y.J.; Zhou, L.; Liu, X.; Liu, Y. Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin A production. PLoS ONE 2014, 9, e108285. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Ahmad, I.; Cameotra, S.S. Phenyl aldehyde and propanoids exert multiple sites of action towards cell membrane and cell wall targeting ergosterol in Candida albicans. AMB Express 2013, 3, 54. [Google Scholar] [CrossRef]
- OuYang, Q.L.; Tao, N.G.; Jing, G.X. Transcriptional profiling analysis of Penicillium digitatum, the causal agent of citrus green mold, unravels an inhibited ergosterol biosynthesis pathway in response to citral. BMC Genom. 2016, 17, 599. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.Z.; Zeng, H.; Zhang, P.; Tessema, A.; Peng, X. Efficacy and possible mechanisms of perillaldehyde in control of Aspergillus niger, causing grape decay. Int. J. Food Microbiol. 2015, 202, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.G.; Jia, L.; Zhou, H.E. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, R.O.; Teixeira, A.P.C.; Oliveira, W.A.; Lima, E.D.; Lima, I.O. Investigation of the antifungal activity of carvacrol against strains of Cryptococcus neoformans. Pharma. Biol. 2016, 54, 2591–2596. [Google Scholar] [CrossRef]
- OuYang, Q.L.; Tao, N.G.; Zhang, M.L. A damaged oxidative phosphorylation mechanism is involved in the antifungal activity of citral against Penicillium digitatum. Front. Microbiol. 2018, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Staudacher, V.; Krauss, J.; Giera, M.; Bracher, F.A. Convenient cellular assay for the identification of the molecular target of ergosterol biosynthesis inhibitors and quantification of their effects on total ergosterol biosynthesis. Steroids 2013, 78, 483–493. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real–Time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2013, 3, 439. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Cramer, R.A. Regulation of sterol biosynthesis in the human fungal pathogen Aspergillus fumigatus: Opportunities for therapeutic development. Front. Microbiol. 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Tsai, H.F.; Mandal, A.; Walker, B.A.; Noble, J.A.; Fukuda, Y.; Bennett, J.E. Sterol uptake and sterol biosynthesis act coordinately to mediate antifungal resistance in Candida glabrata under azole and hypoxic stress. Mol. Med. Rep. 2018, 17, 6585–6597. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.D.; Zhu, Y.B.; Du, G.C.; Zhou, J.W.; Chen, J. Exogenous ergosterol protects Saccharomyces cerevisiae from D-limonene stress. J. Appl. Microbiol. 2013, 114, 482–491. [Google Scholar] [CrossRef]
- Abe, F.; Hiraki, T. Mechanistic role of ergosterol in membrane rigidity and cycloheximide resistance in Saccharomyces cerevisiae. BBA Biomembr. 2009, 1788, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Esquivel, B.D.; White, T.C. Overexpression or deletion of ergosterol biosynthesis genes alters doubling time, response to stress agents, and drug susceptibility in Saccharomyces cerevisiae. mBio 2018, 9, e01291-18. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Tong, Z.C.; Zhang, X.; Wang, Y.Y.; Fang, W.G.; Li, L.; Luo, Z.S. Unveiling the mechanisms for the plant volatile organic compound linalool to control gray mold on strawberry fruits. J. Agric. Food Chem. 2019, 67, 9265–9276. [Google Scholar] [CrossRef]
- Ottilie, S.; Goldgof, G.M.; Calvet, C.M.; Jennings, G.K.; Lamonte, G.; Schenken, J.; Vigil, E.; McCall, L.; Lopes, E.S.C.; Gunawan, F.; et al. Rapid chagas disease drug target discovery using directed evolution in drug-sensitive yeast. ACS Chem. Biol. 2017, 12, 422–434. [Google Scholar] [CrossRef]
- Vale-Silva, L.A.; Coste, A.T.; Ischer, J.E.; Parker, J.E.; Kelly, E.; Pinto, E.; Sanglard, D. Azole resistance by loss of function of the sterol Δ5,6- desaturase gene (ERG3) in Candida albicans does not necessarily decrease virulence. Antimicrob. Agents Chemother. 2012, 56, 1960–1968. [Google Scholar] [CrossRef]
| Genes | Primer (5′-3′) | Length (bp) |
|---|---|---|
| ERG9 | TCTTTGTTGAGGCCGGGTTT ACTTGGGGCGTTTCAACAGA | 51 |
| ERG1 | ATCCCCGATAACCTGCCTCT CCCTTGACGCCTCCATTCTT | 52 |
| ERG7 | GCGCTGGCGATTGGTCGATG CAGGCCCAGTTTCCGGGCTCC | 219 |
| ERG11 | GCGGAATCAAGAGGGACGAT GCCCTAGCACACACTTCAGA | 150 |
| ERG24 | AGAGCTTCACAGTTCCAGCC CGATGCCTCGCTGACAAATG | 155 |
| ERG25 | ATCGAAAGCTTCCTACGGGG GCGCATCAATAGGCTGAGGA | 80 |
| ERG26 | ACCAGACCCCCTGCATCTAT TTGGGATCCGTGCTCTAGGA | 162 |
| ERG27 | TTTCGATCTGCTGCCGTCTT GCGCGCTTCGAGTTGTAAAT | 91 |
| ERG6 | CGCGTGATGCCGCCTTCAAC TGAGCCTTGCGGGCCTCACG | 184 |
| ERG3 | CAGGCCATGGCCGCAATGCC GGTGCAGGCCACGGTGGATCC | 190 |
| ERG5 | TCTCGCCATTGGCGGATGCG GGCCAACAATGGCGCCCTTG | 240 |
| ERG4 | GCTGGAACCGCTACTTCCTT AGACAAACAGGTAGGCGACG | 51 |
| ERG2 | ACATCTTCGACCCGGAACAC TTGGGACCGACTTTCTGCTC | 121 |
| actin183 | TGCGCTGAACCGAACTGCCG TCGGGAGCCTCGAAGCGCTC | 183 |
| Treatments | Inhibitory Rate (%) | |||
|---|---|---|---|---|
| 1 d | 2 d | 3 d | 4 d | |
| 1/4 MIC | 40.48 ± 4.12 c | 19.42 ± 3.36 d | 16.67 ± 4.12 e | 14.57 ± 1.99 f |
| 1/4 MIC+50 mg/LErg | 42.86 ± 7.14 c | 23.30 ± 3.67 d | 16.67 ± 2.38 f | 21.19 ± 1.15 e |
| 1/4 MIC+150 mg/LErg | 40.48 ± 4.12 c | 5.82 ± 3.36 f | 3.17 ± 2.75 g | 5.30 ± 3.03 g |
| 1/4 MIC+250 mg/LErg | 50.00 ± 7.14 c | 16.50 ± 3.36 e | 19.84 ± 5.99 e | 15.23 ± 5.00 f |
| 1/2 MIC | 66.67 ± 8.25 b | 76.70 ± 2.91 c | 68.25 ± 5.99 c | 59.60 ± 9.18 c |
| 1/2 MIC+50 mg/LErg | 66.67 ± 8.25 b | 80.58 ± 6.06 b | 65.08 ± 4.96 c | 57.62 ± 8.96 c |
| 1/2 MIC+150 mg/LErg | 52.38 ± 4.12 bc | 76.70 ± 2.91 c | 50.79 ± 7.27 d | 42.38 ± 7.16 d |
| 1/2 MIC+250 mg/LErg | 52.38 ± 8.25 b | 82.52 ± 5.04 b | 74.60 ± 7.65 c | 65.56 ± 11.30 c |
| MIC | 100.00 ± 0.00a | 100.00 ± 0.00a | 95.24 ± 0.00 b | 90.73 ± 1.15 b |
| MIC+50 mg/LErg | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 95.24 ± 0.00 b | 85.43 ± 14.91 b |
| MIC+150 mg/LErg | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 95.24 ± 0.00 b | 93.38 ± 1.15 b |
| MIC+250 mg/LErg | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 95.24 ± 0.00 b | 93.38 ± 1.15 b |
| MFC | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 2 MIC+50 mg/LErg | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 2 MIC+150 mg/LErg | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 2 MIC+250 mg/LErg | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| Substances | Control | 1/2 MIC | 1/2 MIC + Erg | ||||
|---|---|---|---|---|---|---|---|
| Time (min) | |||||||
| 0 | 30 | 60 | 30 | 60 | 30 | 60 | |
| squalene | + | + | + | + | + | + | + |
| ergosterol | + | + | + | + | + | + | + |
| ergosta-5,7,22,24(28)-tetraenol | − | + | − | − | − | − | − |
| ergosta-7,22-dienol | − | − | + | + | + | + | − |
| lanosterol | + | + | + | + | + | + | + |
| eburicol | − | − | − | + | + | - | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
OuYang, Q.; Liu, Y.; Oketch, O.R.; Zhang, M.; Shao, X.; Tao, N. Citronellal Exerts Its Antifungal Activity by Targeting Ergosterol Biosynthesis in Penicillium digitatum. J. Fungi 2021, 7, 432. https://doi.org/10.3390/jof7060432
OuYang Q, Liu Y, Oketch OR, Zhang M, Shao X, Tao N. Citronellal Exerts Its Antifungal Activity by Targeting Ergosterol Biosynthesis in Penicillium digitatum. Journal of Fungi. 2021; 7(6):432. https://doi.org/10.3390/jof7060432
Chicago/Turabian StyleOuYang, Qiuli, Yangmei Liu, Okwong Reymick Oketch, Miaoling Zhang, Xingfeng Shao, and Nengguo Tao. 2021. "Citronellal Exerts Its Antifungal Activity by Targeting Ergosterol Biosynthesis in Penicillium digitatum" Journal of Fungi 7, no. 6: 432. https://doi.org/10.3390/jof7060432
APA StyleOuYang, Q., Liu, Y., Oketch, O. R., Zhang, M., Shao, X., & Tao, N. (2021). Citronellal Exerts Its Antifungal Activity by Targeting Ergosterol Biosynthesis in Penicillium digitatum. Journal of Fungi, 7(6), 432. https://doi.org/10.3390/jof7060432






