Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications
Abstract
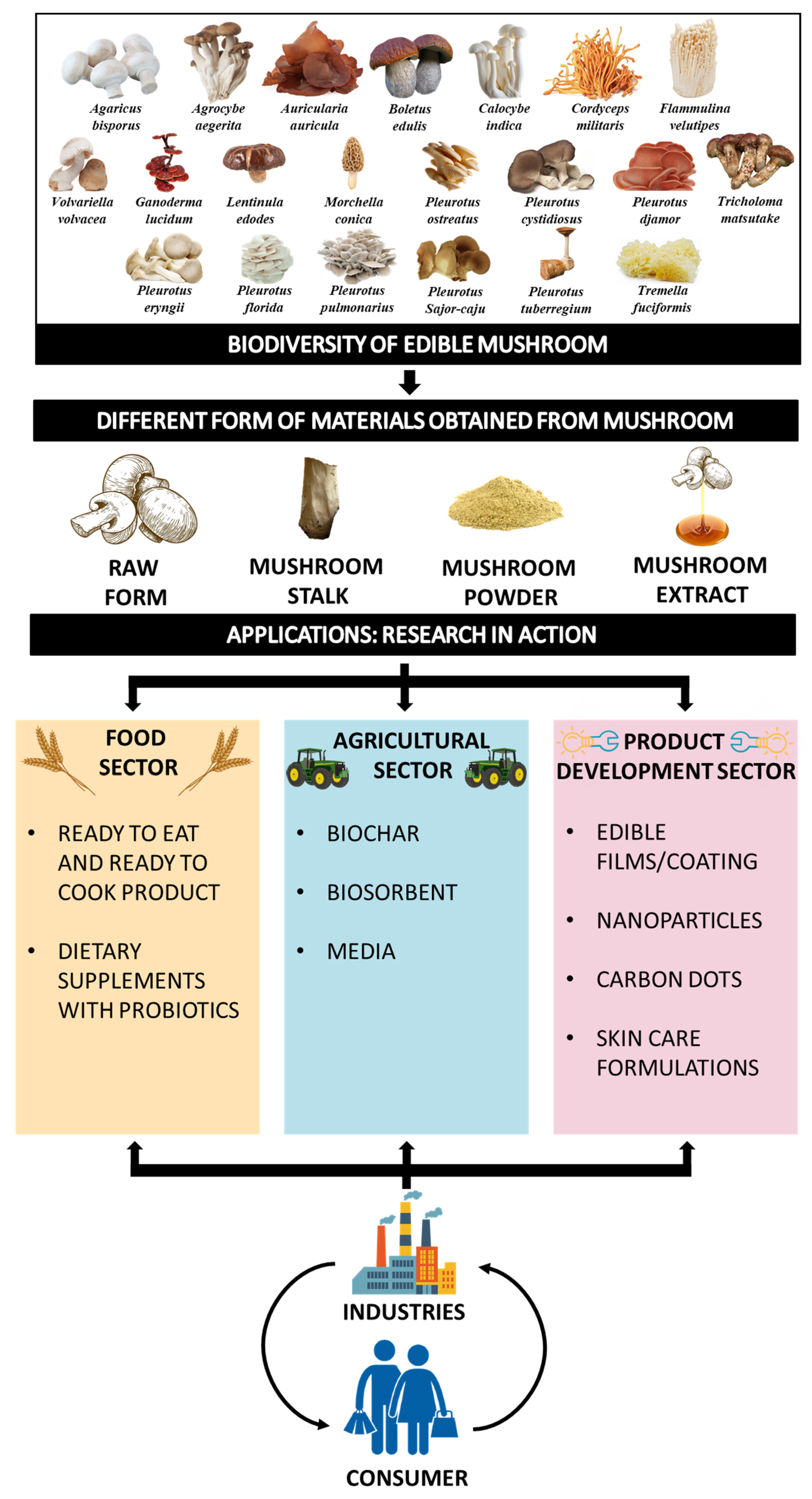
1. Introduction
2. Edible Mushrooms Fortified in Ready-to-Eat and Ready-to-Cook Foods
3. Edible Mushrooms Based Films/Coatings
4. Mushrooms as a Source of Prebiotics for Food Supplementation
5. Edible Mushrooms Based Media
6. Edible Mushrooms Derived Biosorbents
7. Edible Mushrooms Derived Biochar
8. Edible Mushrooms Derived Nanoparticles (NPs)
9. Edible Mushrooms Derived Carbon Dots
10. Edible Mushrooms Based Skin Care Formulations
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Chang, S.T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ergönül, P.G.; Akata, I.; Kalyoncu, F.; Ergönül, B. Fatty acid compositions of six wild edible mushroom species. Sci. World J. 2013, 2013, 163964. [Google Scholar] [CrossRef]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D’Arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef]
- Longvah, T.; Deosthale, Y.G. Composition and nutritional studies on edible wild mushroom from Northeast India. Food Chem. 1998, 63, 331–334. [Google Scholar] [CrossRef]
- Maga, J.A. Mushroom flavor. J. Agric. Food Chem. 1981, 29, 1–4. [Google Scholar] [CrossRef]
- Mattila, P.; Konko, K.; Euvola, M.; Pihlava, J.; Astola, J.; Vahteristo, L. Contents of vitamins, mineral elements and some phenolic compound in cultivated mushrooms. J. Agric. Food Chem. 2001, 42, 2449–2453. [Google Scholar] [CrossRef]
- Clifford, A.J.; Heid, M.K.; Peerson, J.M.; Bills, N.D. Bioavailability of food folates and evaluation of food matrix effects with a rat bioassay. J. Nutr. 1991, 121, 445–453. [Google Scholar] [CrossRef]
- Bano, Z.; Rajarathnam, S. Pleurotus mushrooms. Part II. Chemical composition, nutritional value, post-harvest physiology, preservation, and role as human food. Crit. Rev. Food Sci. Nutr. 1988, 27, 87–158. [Google Scholar] [CrossRef]
- Ribeiroa, B.; Pinhoa, P.G.; Andradea, P.B.; Baptistab, P.; Valentao, P. Fatty acid composition of wild edible mushrooms species: A comparative study. Microchem. J. 2009, 93, 29–35. [Google Scholar] [CrossRef]
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; Ferreira, I.C.F.R.; Pintado, M. Valorization of mushroom by-products as a source of value-added compounds and potential applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef]
- Lim, S.-H.; Lee, Y.-H.; Kang, H.-W. Efficient recovery of lignocellulolytic enzymes of spent mushroom compost from oyster mushrooms, Pleurotus spp., and potential use in dye decolorization. Mycobiology 2013, 41, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Soliman, A.; Abbas, M.; Ahmed, S. Preparation, canning and evaluation process of vegetable mixture diets (ready-to-eat) supplemented with mushroom. Suez Canal Univ. J. Food Sci. 2017, 4, 19–28. [Google Scholar] [CrossRef][Green Version]
- Salehi, F. Characterization of different mushrooms powder and its application in bakery products: A review. Int. J. Food Prop. 2019, 22, 1375–1385. [Google Scholar] [CrossRef]
- Ng, S.H.; Robert, S.D.; Ahmad, W.A.N.W.; Ishak, W.R.W. Incorporation of dietary fiber-rich oyster mushroom (Pleurotus sajor-caju) powder improves postprandial glycaemic response by interfering with starch granule structure and starch digestibility of biscuit. Food Chem. 2017, 227, 358–368. [Google Scholar] [CrossRef]
- Rathore, H.; Sehwag, S.; Prasad, S.; Sharma, S. Technological, nutritional, functional and sensorial attributes of the cookies fortified with Calocybe indica mushroom. J. Food Meas. Charact. 2019, 13, 976–987. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; Brennan, M.; Guan, W.; Liu, J.; Wang, M.; Brennan, C. In vitro gastric digestion antioxidant and cellular radical scavenging activities of wheat-shiitake noodles. Food Chem. 2020, 330, 127214. [Google Scholar] [CrossRef]
- Prodhan, U.K.; Linkon, K.M.M.R.; Al-Amin, M.F.; Alam, M.J. Development and quality evaluation of mushroom (Pleurotus sajor-caju) enriched biscuits. Emir. J. Food Agric. 2015, 27, 542–547. [Google Scholar] [CrossRef]
- Ren, A.; Pan, S.; Li, W.; Chen, G.; Duan, X. Effect of various pretreatments on quality attributes of vacuum-fried shiitake mushroom chips. J. Food Qual. 2018, 2018, 4510126. [Google Scholar] [CrossRef]
- Farzana, T.; Mohajan, S. Effect of incorporation of soy flour to wheat flour on nutritional and sensory quality of biscuits fortified with mushroom. Food Sci. Nutr. 2015, 3, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Ray, A.B. Development and shelf-life evaluation of tomato-mushroom mixed ketchup. J. Food Sci. Technol. 2016, 53, 2236–2243. [Google Scholar] [CrossRef]
- Khan, M.U.; Qazi, I.M.; Ahmed, I.; Ullah, S.; Khan, A.; Jamal, S. Development and quality evaluation of banana mushroom blended jam. Pak. J. Sci. Ind. Res. Ser. B 2017, 60, 11–18. [Google Scholar]
- Rachappa, P.; Sudharma, D.C.; Chauhan, O.P.; Patki, P.E.; Nagaraj, R.; Naik, S.; Naik, R. Development and evaluation of white button mushroom based snacks. J. Food Process. Technol. 2020, 11, 824. [Google Scholar]
- Mohajan, S.; Orchy, T.N.; Farzana, T. Effect of incorporation of soy flour on functional, nutritional, and sensory properties of mushroom–moringa-supplemented healthy soup. Food Sci. Nutr. 2018, 6, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.A.; Derbyshire, E.; Tiwari, B.K.; Brennan, C.S. Enrichment of extruded snack products with coproducts from chestnut mushroom (Agrocybe aegerita) production: Interactions between dietary fiber, physicochemical characteristics, and glycemic load. J. Agric. Food Chem. 2012, 60, 4396–4401. [Google Scholar] [CrossRef]
- Bello, M.; Oluwamukomi, M.O.; Enujiugha, V.N. Nutrient composition and sensory properties of biscuit from mushroom-wheat composite flours. Arch. Curr. Res. Int. 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Khider, M.; Seoudi, O.; Abdelaliem, Y.F. Functional processed cheese spreads with high nutritional value as supplemented with fresh and dried mushrooms. Int. J. Nutr Food Sci. 2017, 6, 45–52. [Google Scholar] [CrossRef]
- Cornelia, M.; Chandra, J. Utilization of white oyster mushroom powder (Pleurotus ostreatus (Jacq.) P. Kumm.) in the making of biscuit as emergency food product. Eurasia J. Biosci. 2019, 13, 1859–1866. [Google Scholar]
- Shalaby, S.M.; Mohamed, A.G.; Farahat, E.S. Preparation of functional and nutritional spreadable processed cheese fortified with vegetables and mushrooms. Int. J. Curr Res. 2018, 10, 74075–74082. [Google Scholar]
- Rosli, W.I.W.; Solihah, M.A. Nutritional composition and sensory properties of oyster mushroom-based patties packed with biodegradable packaging. Sains Malays. 2014, 43, 65–71. [Google Scholar]
- Chauhan, N.; Vaidya, D.; Gupta, A.; Pandit, A. Fortification of pasta with white button mushroom: Functional and rheological properties. Int. J. Food Ferment. Technol. 2017, 7, 87–96. [Google Scholar] [CrossRef]
- Chaudhari, P.D.N.; Wandhekar, S.S.; Shaikh, A.A.; Devkatte, A.N. Preparation and characterization of cookies prepared from wheat flour fortified with mushroom (Pleurotussajor-caju) and spiced with cardamom. Int. J. Res. Anal. Rev. 2018, 5, 386–389. [Google Scholar]
- Lu, X.; Brennan, M.A.; Serventi, L.; Liu, J.; Guan, W.; Brennan, C.S. Addition of mushroom powder to pasta enhances the antioxidant content and modulates the predictive glycaemic response of pasta. Food Chem. 2018, 264, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.H.; Heo, J.Y.; Lee, C.; Lo, Y.M.; Moon, B. Quality and sensory characterization of white jelly mushroom (Tremella fuciformis) as a meat substitute in pork patty formulation. J. Food Process. Preserv. 2014, 38, 2014–2019. [Google Scholar] [CrossRef]
- Arora, B.; Kamal, S.; Sharma, V.P. Nutritional and quality characteristics of instant noodles supplemented with oyster mushroom (P. ostreatus). J. Food Process Preserv. 2018, 42, e13521. [Google Scholar] [CrossRef]
- Patinho, I.; Saldaña, E.; Selani, M.M.; de Camargo, A.C.; Merlo, T.C.; Menegali, B.S.; Contreras-Castillo, C.J. Use of Agaricus bisporus mushroom in beef burgers: Antioxidant, flavor enhancer and fat replacing potential. Food Prod. Process Nutr. 2019, 1, 1–15. [Google Scholar] [CrossRef]
- Srivastava, A.; Attri, B.; Verma, S. Development and evaluation of instant soup premix using oyster mushroom powder. Mushroom Res. 2019, 28, 65–69. [Google Scholar] [CrossRef]
- Salehi, F.; Kashaninejad, M.; Asadi, F.; Najafi, A. Improvement of quality attributes of sponge cake using infrared dried button mushroom. J. Food Sci. Technol. 2016, 53, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, F.L.; Akinwande, B.A.; Ade-Omowaye, B.I.O. Physicochemical properties of novel cookies produced from orange-fleshed sweet potato cookies enriched with sclerotium of edible mushroom (Pleurotus tuber-regium). J. Saudi Soc. Agric. Sci. 2020, 19, 174–178. [Google Scholar]
- Parvin, R.; Farzana, T.; Mohajan, S.; Rahman, H.; Rahman, S.S. Quality improvement of noodles with mushroom fortified and its comparison with local branded noodles. NFS J. 2020, 20, 37–42. [Google Scholar] [CrossRef]
- Jeong, C.H.; Shim, K.H. Quality characteristics of sponge cakes with addition of Pleurotus eryngii mushroom powders. J. Korean Soc. Food Sci. Nutr. 2004, 33, 716–722. [Google Scholar]
- Lu, X.; Brennan, M.A.; Serventi, L.; Mason, S.; Brennan, C.S. How the inclusion of mushroom powder can affect the physicochemical characteristics of pasta. Int. J. Food Sci. Technol. 2016, 51, 2433–2439. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Kumar, D. Fruit and vegetable peels: Utilization of high value horticultural waste in novel industrial applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou, B.-S.; Punotai, K.; Olson, D.; Williams, T.; Wood, D.; Rodov, V.; Poverenov, E.; McHugh, T. Layer-by-layer alginate and fungal chitosan based edible coatings applied to fruit bars. J. Food Sci. 2018, 83, 1880–1887. [Google Scholar] [CrossRef]
- Du, H.; Hu, Q.; Yang, W.; Pei, F.; Kimatu, B.M.; Ma, N.; Zhao, L. Development, physiochemical characterization and forming mechanism of Flammulina velutipes polysaccharide-based edible films. Carbohydr. Polym. 2016, 152, 214–221. [Google Scholar] [CrossRef]
- Poverenov, E.; Arnon-Rips, H.; Zaitsev, Y.; Bar, V.; Danay, O.; Horev, B.; Rodov, V. Potential of chitosan from mushroom waste to enhance quality and storability of fresh-cut melons. Food Chem. 2018, 268, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, W.; Zhao, K.; Ma, Y.; Cheng, S.; Zhou, J.; Wu, Z. Producing a novel edible film from mushrooms (L. edodes and F. velutipes) by-products with a two-stage treatment namely grinding and bleaching. J. Food Eng. 2020, 275, 109862. [Google Scholar] [CrossRef]
- Olufunmilola, O.M.; Shian, A.J.; Dooshima, I.B. Effects of plasticizer concentration and mushroom (Pleurotus pulmonarius) flour inclusion on the sensory, mechanical and barrier properties of cassava starch based edible films. Eur. J. Food Sci. Technol. 2019, 7, 47–62. [Google Scholar]
- Asad, F.; Anwar, H.; Yassine, H.M.; Ullah, M.I.; Kamran, Z.; Sohail, M.U. White button mushroom, Agaricus bisporus (Agaricomycetes), and a probiotics mixture supplementation correct dyslipidemia without influencing the colon microbiome profile in hypercholesterolemic rats. Int. J. Med. Mushrooms 2020, 22, 235–244. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Van Doan, H.; Doolgindachbaporn, S.; Suksri, A. Effects of Eryngii mushroom (Pleurotus eryngii) and Lactobacillus plantarum on growth performance, immunity and disease resistance of Pangasius catfish (Pangasius bocourti, Sauvage 1880). Fish Physiol. Biochem. 2016, 42, 1427–1440. [Google Scholar] [CrossRef]
- Daneshmand, A.; Sadeghi, G.H.; Karimi, A.; Vaziry, A. Effect of oyster mushroom (Pleurotus ostreatus) with and without probiotic on growth performance and some blood parameters of male broilers. Anim. Feed Sci. Techol. 2011, 170, 91–96. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of probiotic. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Faraki, A.; Noori, N.; Gandomi, H.; Banuree, S.A.H.; Rahmani, F. Effect of Auricularia auricula aqueous extract on survival of Lactobacillus acidophilus La-5 and Bifidobacterium bifidum Bb-12 and on sensorial and functional properties of synbiotic yogurt. Food Sci. Nutr. 2020, 8, 1254–1263. [Google Scholar] [CrossRef]
- Roy, D.; Fahim, A. The effect of different level of mushroom (Agaricus bisporus) and probiotics (Saccharomyces cerevisiae) on sensory evaluation of broiler meat. J. Entomol. Zool. Stud. 2019, 7, 347–349. [Google Scholar]
- Oyetayo, V.O.; Oyetayo, F.L. Hematological parameters of rats fed mushroom, Pleurotus sajor-caju diets and orogastrically dosed with probiotic Lactobacillus fermentum Ovl. Int. J. Probiotics Prebiotics 2007, 2, 39–42. [Google Scholar]
- Van Doan, H.; Hoseinifar, S.H.; Dawood, M.A.; Chitmanat, C.; Tayyamath, K. Effects of Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum on mucosal, serum immunology and growth performance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 70, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.L.; Isikhuemhen, O.S.; Ibrahim, S.A. Performance assessment of broiler chickens given mushroom extract alone or in combination with probiotics. Poult Sci. 2007, 86, 1856–1860. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; Vandenberghe, L.P.S. Overview of applied solid-state fermentation in Bazil. Biochem. Eng. J. 2008, 13, 205–218. [Google Scholar] [CrossRef]
- Zhang, R.H.; Li, X.J.; Fadel, J.G. Oyster mushroom cultivation with rice and wheat straw. Bioresource Technol. 2002, 82, 277–284. [Google Scholar] [CrossRef]
- Sanchez, J.E.; Royse, D.J. Scytalidium thermophilum- colonized grain, corncobs and chopped wheat straw substrates for the production of Agaricus bisporus. Bioresour. Technol. 2009, 100, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Reid, B.J.; Fermor, T.R. Impact of composting strategies on the treatment of soils contaminated with organic pollutants. Environ. Pollut. 2001, 112, 269–283. [Google Scholar] [CrossRef]
- Fidanza, M.A.; Sam fond, D.L.; Beyen, D.M.; Aurentz, D.J. Analysis of fresh mushroom compost. Hort. Technol. 2010, 20, 449–453. [Google Scholar] [CrossRef]
- Rajput, R.; Prasad, G.; Chopra, A.K. Scenario of solid waste management in present Indian context. Casp. J. Environ. Sci. 2009, 7, 45–53. [Google Scholar]
- Run-Hua, Z.; Zeng-Qiang, D.; Zhi-Guo, L. Use of spent mushroom substrate as growing media for tomato and cucumber seedlings. Pedosphere 2012, 22, 333–342. [Google Scholar]
- Medina, E.; Paredes, C.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Spent mushroom substrates as component of growing media for germination and growth of horticultural plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.V.; Wang, C.H. Use of spent mushroom substrate and manure compost for honeydew melon seedlings. J. Plant. Growth Regul. 2015, 34, 417–424. [Google Scholar]
- Wu, S.; Lan, Y.; Huang, D.; Peng, Y.; Huang, Z.; Gelbič, I.; Carballar-Lejarazu, R.; Guan, X.; Zhang, L.; Zou, S. Use of spent mushroom substrate for production of Bacillus thuringiensis by solid-state fermentation. J. Econ. Entomol. 2014, 107, 137–143. [Google Scholar] [CrossRef]
- Rajavat, A.S.; Rai, S.; Pandiyan, K.; Kushwaha, P.; Choudhary, P.; Kumar, M.; Chakdar, H.; Singh, A.; Karthikeyan, N.; Bagul, S.Y.; et al. Sustainable use of the spent mushroom substrate of Pleurotus florida for production of lignocellulolytic enzymes. J. Basic Microbiol. 2020, 60, 173–184. [Google Scholar] [CrossRef]
- Qiao, J.J.; Zhang, Y.F.; Sun, L.F.; Liu, W.W.; Zhu, H.J.; Zhang, Z. Production of spent mushroom substrate hydrolysates useful for cultivation of Lactococcus lactis by dilute sulfuric acid, cellulase and xylanase treatment. Bioresour. Technol. 2011, 102, 8046–8051. [Google Scholar] [CrossRef]
- Singh, G.; Tiwari, A.; Rathore, H.; Prasad, S.; Hariprasad, P.; Sharma, S. Valorization of paddy straw using de-oiled cakes for P. ostreatus cultivation and utilization of spent mushroom substrate for biopesticide development. Waste Biomass Valroi. 2021, 12, 333–346. [Google Scholar] [CrossRef]
- Sendi, H.; Mohamed, M.T.M.; Anwar, M.P.; Saud, H.M. Spent mushroom waste as a media replacement for peat moss in kai-lan (Brassica oleracea var. Alboglabra) production. Sci. World J. 2013, 2013, 258562. [Google Scholar] [CrossRef]
- Liu, C.J.; Duan, Y.L.; Jin, R.Z.; Han, Y.Y.; Hao, J.H.; Fan, S.X. Spent mushroom substrates as component of growing media for lettuce seedlings. In Proceedings of the 4th International Conference on Agricultural and Biological Sciences, Hangzhou, China, 26–29 June 2018; Volume 185, p. 012016. [Google Scholar]
- Antimanon, S.; Chamkhuy, W.; Sutthiwattanakul, S.; Laoteng, K. Efficient production of arachidonic acid of Mortierella sp. by solid-state fermentation using combinatorial medium with spent mushroom substrate. Chem. Pap. 2018, 72, 2899–2908. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Samoraj, M.; Chojnacka, K. Solid-state solubilization of bones by B. megaterium in spent mushroom substrate as a medium for a phosphate enriched substrate. J. Chem. Technol. Biotechnol. 2017, 92, 1397–1405. [Google Scholar] [CrossRef]
- Park, N.; Yun, Y.-S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess. Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Abdi, O.; Kazemi, M. A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater. Environ. Sci. 2015, 6, 1386–1399. [Google Scholar]
- Menaga, D.; Rajakumar, S.; Ayyasamy, P.M. Spent mushroom substrate: A crucial biosorbent for the removal of ferrous iron from groundwater. SN Appl. Sci. 2021, 3, 32. [Google Scholar] [CrossRef]
- Akar, S.T.; Gorgulu, A.; Kaynak, Z.; Anilan, B.; Akar, T. Biosorption of reactive blue 49 dye under batch and continuous mode using a mixed biosorbent of macro-fungus Agaricus bisporus and Thuja orientalis cones. Chem. Eng. J. 2009, 148, 26–34. [Google Scholar] [CrossRef]
- Eliescu, A.; Georgescu, A.A.; Nicolescu, C.M.; Bumbac, M.; Cioateră, N.; Mureșeanu, M.; Buruleanu, L.C. Biosorption of Pb(II) from aqueous solution using mushroom (Pleurotus ostreatus) biomass and spent mushroom substrate. Anal. Lett. 2020, 53, 2292–2319. [Google Scholar] [CrossRef]
- Tay, C.C.; Liew, H.H.; Yin, C.Y.; Abdul-Talib, S.; Surif, S.; Suhaimi, A.B.; Yong, S.K. Biosorption of cadmium ions using Pleurotus ostreatus: Growth kinetics, isotherm study and biosorption mechanism. Korean J. Chem. Eng. 2011, 28, 825–830. [Google Scholar] [CrossRef]
- Yildirim, A.; Acay, H. Biosorption studies of mushrooms for two typical dyes. JOTCSA 2020, 7, 295–306. [Google Scholar] [CrossRef][Green Version]
- Qu, J.; Zang, T.; Gu, H.; Li, K.; Hu, Y.; Ren, G.; Xu, X.; Jin, Y. Biosorption of copper ions from aqueous solution by Flammulina velutipes spent substrate. BioResources 2015, 10, 8058–8075. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Zheng, H.; Luan, X.; Li, H.; Wang, Y.; Du, Q.; Sui, K.; Li, H.; Xia, Y. Adsorption of Congo red with hydrothermal treated shiitake mushroom. Mater. Res. Express. 2020, 7, 015103. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Tu, H.; Li, F.; Li, X.; Yang, J.; Liao, J.; Yang, Y.; Liu, N.; Sun, Q. Characteristics of uranium biosorption from aqueous solutions on fungus Pleurotus ostreatus. Environ. Sci. Pollut. Res. 2016, 23, 24846–24856. [Google Scholar] [CrossRef]
- Mahmood, T.; Khan, A.; Naeem, A.; Hamayun, M.; Muska, M.; Farooq, M.; Hussain, F. Adsorption of Ni(II) ions from aqueous solution onto a fungus Pleurotus ostreatus. Desalin. Water Treat. 2016, 57, 7209–7218. [Google Scholar] [CrossRef]
- Amin, F.; Talpur, F.N.; Balouch, A.; Afridi, H.I.; Khaskheli, A.A. Efficient entrapping of toxic Pb(II) ions from aqueous system on a fixed-bed column of fungal biosorbent. Geol. Ecol. Landsc. 2018, 2, 39–44. [Google Scholar] [CrossRef][Green Version]
- Wu, J.; Zhang, T.; Chen, C.; Feng, L.; Su, X.; Zhou, L.; Chen, Y.; Xia, A.; Wang, X. Spent substrate of Ganodorma lucidumas a new bio-adsorbent for adsorption of three typical dyes. Bioresour. Technol. 2018, 266, 134–138. [Google Scholar] [CrossRef]
- Amin, F.; Talpur, F.N.; Balouch, A.; Afridi, H.I.; Surhio, M.L. Statistical methodology for biosorption of nitrate (NO3−) ions from aqueous solution by Pleurotus eryngii fungal biomass. Model. Earth Syst. Environ. 2017, 3, 1101–1112. [Google Scholar] [CrossRef]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface change along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Bruun, E.W.; Hauggaard-Nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenerg. 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Zhang, H.; Voroney, R.; Price, G. Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biol. Biochem. 2015, 83, 19–28. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Pandit, B.H.; Martinsen, V.; Cornelissen, G.; Conte, P.; Kammann, C.I. Fourfold increase in pumpkin yield in response to low-dosage root zone application of urine-enhanced biochar to a fertile tropical soil. Agriculture 2015, 5, 723–741. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.W.; Conte, P.; Joseph, S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Ynag, G.; Zhang, X.; Qi, H.; Ma, J.; Xiao, Y.; et al. Adsorption characteristics of Pb(II) using biochar derived from spent mushroom substrate. Sci Rep. 2019, 9, 15999. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, H.; Cheng, H.; Yan, Y.; Chang, M.; Cao, Y.; Huang, F.; Zhang, G.; Yan, M. Spent Ganoderma lucidum substrate derived biochar as a new bio-adsorbent for Pb2+/Cd2+ removal in water. Chemosphere 2020, 241, 125121. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, N.; Luo, Y.; Zhang, H.; Su, L.; Oh, K.; Cheng, H. Efficient removal of Cu(II), Zn(II), and Cd(II) from aqueous solutions by a mineral-rich biochar derived from a spent mushroom (Agaricus bisporus) substrate. Materials 2020, 14, 35. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, G.; He, Y.; Chen, C.; Liu, X.; Li, G.; Gu, Y.; Zhao, Y. Mixed heavy metals removal from wastewater by discarded mushroom-stick biochar: Adsorption properties and mechanisms. Environ. Sci. Process. Impacts 2019, 21, 584–592. [Google Scholar] [CrossRef]
- Sewu, D.D.; Jung, H.; Kim, S.S.; Lee, D.S.; Woo, S.H. Decolorization of cationic and anionic dye-laden wastewater by steam-activated biochar produced at an industrial-scale from spent mushroom substrate. Bioresour. Technol. 2019, 277, 77–86. [Google Scholar] [CrossRef]
- Chen, G.J.; Peng, C.Y.; Fang, J.Y.; Dong, Y.Y.; Zhu, X.H.; Cai, H.M. Biosorption of fluoride from drinking water using spent mushroom compost biochar coated with aluminum hydroxide. Desalin Water Treat. 2016, 57, 12385–12395. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Sharma, A.; Tejwan, N.; Bhardwaj, S.; Bhardwaj, P.; Nepovimova, E.; Shami, A.; Kalia, A.; Kumar, A.; Abd-Elsalam, K.A.; et al. Pleurotus macro fungi-assisted nanoparticle synthesis and its potential applications: A review. J. Fungi 2020, 6, 351. [Google Scholar] [CrossRef] [PubMed]
- Owaid, M.N.; Ibraheem, I.J. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 2017, 9, 5–23. [Google Scholar] [CrossRef]
- Sriramulu, M.; Shanmugam, S.; Ponnusamy, V.K. Agaricus bisporus mediated biosynthesis of copper nanoparticles and its biological effects: An in-vitro study. Colloid Interface Sci. Commun. 2020, 35, 100254. [Google Scholar] [CrossRef]
- Madhanraj, R.; Eyini, M.; Balaji, P. Antioxidant assay of gold and silver nanoparticles from edible Basidiomycetes mushroom fungi. Free Radic. Antioxid. 2017, 7, 137–142. [Google Scholar] [CrossRef]
- Bhat, R.; Sharanabasava, V.G.; Deshpande, R.; Shetti, U.; Sanjeev, G.; Venkataraman, A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus florida and its anti-cancer evaluation. J. Photochem. B Biol. 2013, 125, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Abd Ellah, N.H.; Bohara, R.A.; Rehan, I.F.; Marraiki, N.; Batiha, G.E.S.; Hetta, H.F.; Singh, M.P. Pleurotus sajor-caju-mediated synthesis of silver and gold nanoparticles active against colon cancer cell lines: A new era of Herbonanoceutics. Molecules 2020, 25, 3091. [Google Scholar] [CrossRef]
- Zeng, D.; Zhao, J.; Luk, K.H.; Cheung, S.T.; Wong, K.H.; Chen, T. Potentiation of in vivo anti-cancer efficacy of selenium nanoparticles by mushroom polysaccharides surface decoration. J. Agric. Food Chem. 2019, 67, 2865–2876. [Google Scholar] [CrossRef]
- Ismail, A.F.M.; Ahmed, M.M.; Salem, A.A.M. Biosynthesis of silver nanoparticles using mushroom extracts: Induction of apoptosis in HepG2 and MCF-7 cells via caspases stimulation and regulation of BAX and Bcl-2 gene expressions. J. Pharm. Biomed. Sci. 2015, 5, 1–9. [Google Scholar]
- Aygün, A.; Özdemir, S.; Gülcan, M.; Cellat, K. Synthesis and Characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J. Pharm. Biomed. Anal. 2020, 178, 112970. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.J.P.; Murugan, M.; Jeyaraj, M.; Rathinam, N.K.; Sangiliyandi, G. Synthesis of silver nanoparticles using pine mushroom extract: A potential antimicrobial agent against E. coli and B. subtilis. J. Ind. Eng. Chem. 2014, 20, 2325–2331. [Google Scholar] [CrossRef]
- Mirunalini, S.; Arulmozhi, V.; Deepalakshmi, K.; Krishnaveni, M. Intracellular biosynthesis and antibacterial activity of silver nanoparticles using edible mushrooms. Not. Sci. Biol. 2012, 4, 55–61. [Google Scholar] [CrossRef]
- Manimaran, K.; Murugesan, S.; Ragavendran, C.; Balasubramani, G.; Natarajan, D.; Ganesan, A.; Seedevi, P. Biosynthesis of TiO2 nanoparticles using edible mushroom (Pleurotus djamor) extract: Mosquito larvicidal, histopathological, antibacterial and anti-cancer effect. J. Clust Sci. 2020, 1–12. [Google Scholar] [CrossRef]
- Manimaran, K.; Balasubramani, G.; Ragavendran, C.; Natarajan, D.; Murugesan, S. Biological applications of synthesized ZnO nanoparticles using Pleurotus djamor against mosquito larvicidal, histopathology, antibacterial, antioxidant and anti-cancer effect. J. Clust. Sci. 2020, 1–13. [Google Scholar] [CrossRef]
- Gurunathan, S.; Raman, J.; Malek, S.N.A.; John, P.A.; Vikineswary, S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: A potential cytotoxic agent against breast cancer cells. Int J. Nanomed. 2013, 8, 4399–4413. [Google Scholar]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.Z.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-derived carbon dots for toxic metal ion detection and as antibacterial and anti-cancer agents. ACS Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Pacquiao, M.R.; de Luna, M.D.G.; Thongsai, N.; Kladsomboon, S.; Paoprasert, P. Highly fluorescent carbon dots from enokitake mushroom as multi-faceted optical nanomaterials for Cr6+ and VOC detection and imaging applications. Appl. Surf. Sci. 2018, 453, 192–203. [Google Scholar] [CrossRef]
- Zulfajri, M.; Rasool, A.; Huang, G.G. A fluorescent sensor from oyster mushroom-carbon dots for sensing nitroarenes in aqueous solutions. New J. Chem. 2020, 44, 10525–10535. [Google Scholar] [CrossRef]
- Zulfajri, M.; Liu, K.C.; Pu, Y.H.; Rasool, A.; Dayalan, S.; Huang, G.G. Utilization of carbon dots derived from Volvariella volvacea mushroom for a highly sensitive detection of Fe3+ and Pb2+ ions in aqueous solutions. Chemosensors 2020, 8, 47. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Wang, Y.; Wang, S.; Miao, H.; Yang, L. Carbon dots derived from fungus for sensing hyaluronic acid and hyaluronidase. Sens. Actuators B Chem. 2017, 251, 503–508. [Google Scholar] [CrossRef]
- Millikan, L.E. Cosmetology, cosmetics, cosmeceuticals: Definitions and regulations. Clin. Dermatol. 2001, 19, 371–374. [Google Scholar] [CrossRef]
- Antignac, E.; Nohynek, G.J.; Re, T.; Clouzeau, J.; Toutain, H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem. Toxicol. 2011, 49, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Bahkali, A.H.; Moslem, M.A. Fungi-an unusual source for cosmetics. Fungal Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- Camassola, M. Mushrooms-the incredible factory for enzymes and metabolites productions. Ferment. Technol. 2013, 2. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Barreiro, M.F.; González-Paramás, A.M.; Ferreira, I.C.F.R. Development of mushroom-based cosmeceutical formulations with anti-inflammatory, anti-tyrosinase, antioxidant, and antibacterial properties. Molecules 2016, 21, 1372. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Ferreira, I.C.F.R. The potential of Ganoderma lucidum extracts as bioactive ingredients in topical formulations, beyond its nutritional benefits. Food Chem. Toxicol. 2017, 108, 139–147. [Google Scholar] [CrossRef]
- Gupta, N.; Dubey, A.; Prasad, P.; Roy, M. Formulation and evaluation of herbal fairness cream comprising hydroalcoholic extracts of Pleurotus ostreatus, Glycyrrhiza glabra and Camellia sinensis. UK J. Pharm. Biosci. 2015, 3, 41. [Google Scholar] [CrossRef]
- Hapsari, R.; Elya, B.; Amin, J. Formulation and evaluation of antioxidant and tyrosinase inhibitory effect from gel containing the 70% ethanolic Pleurotus ostreatus extract. Int. J. Med. Arom. Plants 2012, 2, 135–140. [Google Scholar]
- Lourith, N.; Pungprom, S.; Kanlayavattanakul, M. Formulation and efficacy evaluation of the safe and efficient moisturizing snow mushroom hand sanitizer. J. Cosmet. Dermatol. 2021, 20, 554–560. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Fernandes, I.P.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Ferreira, I.C.F.R. Mushroom-based cosmeceutical ingredients: Microencapsulation and in vitro release profile. Ind. Crops Prod. 2018, 124, 44–52. [Google Scholar] [CrossRef]
| Edible Mushroom Common Name | Scientific Name | Food Product | Beneficial Effects | Reference |
|---|---|---|---|---|
| Milky white | Calocybe indica | Cookies | Increase in protein, fiber, minerals and β-glucan, phenolic, flavonoids and antioxidants; decrease in starch, reduction in glycemic index | [17] |
| Oyster | Pleurotus sajor-caju | Biscuits | Increase in concentration of protein, dietary fiber, ash and reduction in carbohydrate | [19] |
| Shiitake | Lentinula edodes | Chips | Improvement in quality attributes (color, sensory evaluation) | [20] |
| Oyster | Pleurotus ostreatus | Biscuits | Enhancement of nutritional quality | [21] |
| White button | Agaricus bisporus | Ketchup | Increase in ash content, crude fiber, protein, total soluble solids, and reducing sugars; decrease in total sugars | [22] |
| Oyster | Pleurotus ostreatus | Jam | Increase in total soluble solids, percent acidity and reducing sugar, decrease in pH and non-reducing sugar | [23] |
| White button | Agaricus bisporus | Mushroom tikki and stuffed mushroom | Increase in protein, dietary fiber, antioxidant and phenolic components | [24] |
| Oyster | Pleurotus ostreatus | Soup | Increase in nutritional value | [25] |
| Chestnut | Agrocybe aegerita | Snacks | Manipulation of glycemic response of individuals | [26] |
| Oyster | Pleurotus sajur-caju | Biscuit | Increase in the mineral content | [27] |
| Oyster | Pleurotus ostreatus | Vegetable mixture diets | Highly acceptable, nutritious, delicious, ready-to-eat diet | [14] |
| Oyster | Pleurotus ostreatus | Processed cheese spreads | High moisture, ash and protein content, total viable counts and spore former bacteria was lower in processed cheese supplemented with mushrooms | [28] |
| Oyster | Pleurotus ostreatus | Biscuit | Higher moisture, protein, ash content, higher hardness, darker and redder in color | [29] |
| Oyster | Pleurotus ostreatus | Spreadable processed cheese | Increase in total solids, protein, fibers and carbohydrates | [30] |
| Oyster | Pleurotus sajor-caju | chicken patty | Reduction in fat content, no change in protein and β-glucan | [31] |
| White button | Agaricus bisporus | Pasta | Improved antioxidant activity, increase moisture content, carbohydrates, decreased crude fiber, crude protein, and fat | [32] |
| Oyster | Pleurotus sajor-caju | Cookies | High protein content, low-fat content, high fiber, minerals and vitamin content | [33] |
| White button | Agaricus bisporus | Pasta | Decrease in the extent of starch degradation, increase in total phenolic content and antioxidant capacities | [34] |
| White jelly | Tremella fuciformis | Patty | Oil holding capacity of mushroom has a positive effect on cooking yield of patty as well as senses | [35] |
| Oyster | Pleurotus ostreatus | Instant noodles | Increase in protein and fiber content | [36] |
| White button | Agaricus bisporus | Beef burgers | Reduction in the fat content of beef burgers | [37] |
| Oyster | Pleurotus ostreatus | Instant soup premix | Rich in protein, crude fiber, minerals and low in fat, carbohydrate and energy value | [38] |
| White button | Agaricus bisporus | Sponge cake | Increase in apparent viscosity, volume, springiness and cohesiveness values | [39] |
| Oyster | Pleurotus sajor-caju | Biscuit | Reduction in starch pasting viscosities, starch gelatinization enthalpy value, increases in protein, crude fiber and mineral content | [16] |
| Shiitake | Lentinula edodes | Noodles | Improvement in nutritional profile and reduction in the glycemic index of foods | [18] |
| King tuber | Pleurotus tuber-regium | Cookies | Higher protein, ash, crude fiber, water-soluble vitamins and minerals | [40] |
| Oyster | Pleurotus ostreatus | Noodles | Lower level of carbohydrate, fat, and sodium | [41] |
| King trumpet | Pleurotus eryngii | Sponge cake | Increase in ash and proteincontent | [42] |
| White button, Shitake, Porcini | Agaricus bisporus, Lentinula edodes, Boletus edulis | Pasta | High firmness and tensile strength | [43] |
| Edible Mushrooms Common Name | Scientific Name | Product Used | Compounds | Key Findings | References |
|---|---|---|---|---|---|
| White button | Agaricus bisporus | Fruit bars | Chitosan | Increased antioxidant capacity, ascorbic acid content, fungal growth prevention and firmness | [45] |
| White button | Agaricus bisporus | Fresh-cut melons | Chitosan | Enhance fruit firmness, inhibit off-flavors and reduce the microbial counts (up to 4 log CFU g−1). | [47] |
| Velvet shank | Flammulina velutipes | ND | Polysaccharide | High tensile strength, barrier property to water vapor and oxygen | [46] |
| Shiitake, Velvet shank | Lentinula edodes, Flammulina velutipes | ND | Insoluble dietary fibers | Highest tensile strength and an effective barrier to water vapor | [48] |
| Indian oyster | Pleurotus pulmonarius | ND | Flour | Significant barrier properties and mechanical strength | [49] |
| Edible Mushrooms Common Name | Scientific Name | Probiotic Used | Form of Mushroom Used | Applications | References |
|---|---|---|---|---|---|
| White button | Agaricus bisporus | Probiotics mixture (Protexin 6 × 107CFU gm−1) | Powder | Lowered total cholesterol, LDL cholesterol, triglyceride concentrations, oxidative stress and dyslipidemia in hypercholesterolemic rats | [50] |
| Wood ear/Jew’s ear | Auricularia auricula | Lactobacillus acidophilus La-5, Bifidobacterium bifidum Bb-12 | Extract | Enhancement in the survival rate of probiotics toabout 0.43 and 0.51 log CFU g−1; improved probiotic protection and functional properties of symbiotic yogurt | [55] |
| White button | Agaricus bisporus | Saccharomyces cerevisiae | Powder | Improvement in the meat quality with the incorporation of mushroom and probiotics in the broiler diet | [56] |
| Oyster | Pleurotus sajor-caju | Lactobacillus fermentum OVL | Powder | Increase in neutrophil count in rats, decrease in lymphocyte count | [57] |
| Oyster | Pleurotus ostreatus | PrimaLac (Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidium, Enterococcus faecium) | Powder | Decrease in abdominal fat on the carcass, increase in HDL concentration in plasma | [53] |
| Caterpillar | Cordyceps militaris | Lactobacillus plantarum | Spent mushroom substrate | Increase in the specific growth rate, weight gain, final weight in fish fed supplemented diets | [58] |
| Shiitake | Lentinus edodes | 1.0 ×108 CFU g−1(Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifdium, Enterococcus faecium) | Extract | No weight gain in broiler chickens | [59] |
| King oyster | Pleurotus eryngii | Lactobacillus plantarum | Powder | Growth stimulation, immunity and disease resistance | [52] |
| Edible Mushrooms Common Name | Scientific Name | Media Composition | Purpose/Utilization | References |
|---|---|---|---|---|
| Velvet shank | Flammulina velutipes | Spent mushroom substrate, perlite, and vermiculite | Growing media for tomato and cucumber seedlings | [66] |
| White button, Oyster | Agaricus bisporus, Pleurotus ostreatus | Spent mushroom substrate, and Sphagnum peat | Growing media for tomato, courgette and pepper | [67] |
| Velvet shank | Flammulina velutipes | Spent mushroom substrate, and chicken manure compost | Growing media for honeydew melon | [68] |
| Velvet shank | Flammulina velutipes | Spent mushroom substrate, calcium carbonate, wheat bran, and yeast extract and inorganic salts | Production media for Bacillus thuringiensis | [69] |
| Oyster | Pleurotusf lorida | Spent mushroom substrate | Production media for lignocellulolytic enzymes | [70] |
| Oyster | Pleurotus ostreatus | Spent mushroom substrate | Production media for Lactococcus lactis | [71] |
| Oyster | Pleurotus ostreatus | Spent mushroom substrate, paddy straw, and soybean cake | Biopesticide (Trichoderma asperellum) development | [72] |
| ND | ND | Spent mushroom substrate and peat moss | Growing media for Chinese kale | [73] |
| ND | ND | Spent mushroom substrate, perlite, and vermiculite | Growing media for lettuce seedlings | [74] |
| ND | ND | Spent mushroom substrate, polished rice, full-fat soybean, and rice bran | Production media for arachidonic acid by Mortierella sp. | [75] |
| ND | ND | Spent mushroom substrate, and poultry cooked bones | Production media for solubilizationphosphate by Bacillus megaterium | [76] |
| Edible Mushrooms Common Name | Scientific Name | Drying Temperature/Time | Applications | References |
|---|---|---|---|---|
| Oyster | Pleurotus florida | RT/24 h | Showed 100% removal of Fe2+ from the water sample | [79] |
| White button | Agaricus bisporus | 80 °C/24 h | Successfully biosorbed Reactive Blue 49 dye (1.85 × 10−4 mol g−1) from water | [80] |
| Oyster | Pleurotus ostreatus | 40 °C/24 h | Showed greater adsorption against Pb2+(85.91 mg g−1) in water | [81] |
| Oyster | Pleurotus ostreatus | 60 °C/24 h | Biosorbed 3.8 mg g−1 of Cd2+ | [82] |
| Oyster, Black morels | Pleurotus ostreatus, Morchella conica | RT/4 days | Adsorbed methylene blue (82.81 and 38.47 mg g−1) and for malachite green (64.13 and 39.28 mg g−1) | [83] |
| Velvet shank | Flammulina velutipes | 60 °C/24 h | Maximum removal capacity against copper ions was 15.56 mg g−1 | [84] |
| Shiitake | Lentinula edodes | Freeze-dried/24 h | Maximum absorption against Congo red was 217.86 mg g−1 | [85] |
| Oyster | Pleurotus ostreatus | 78 °C/48 h | Showed maximum biosorption against uranium ion (19.95 mg g−1) | [86] |
| Oyster | Pleurotus ostreatus | 80 °C/ND | Showed maximum biosorption against Ni2+ (20.71 mg g−1) | [87] |
| King trumpet | Pleurotus eryngii | 60 °C/24 h | Showed maximum biosorption against Pb2+ (3.30 mg g−1) | [88] |
| Lingzhi | Ganoderma lucidum | 60 °C/72 h | Maximum biosorption against malachite green (40.65 mg g−1), safranine T (33.00 mg g−1), and methylene (22.37 mg g−1) | [89] |
| King trumpet | Pleurotus eryngii | 60 °C/24 h | Removed 88.38% of NO3− | [90] |
| Edible Mushrooms Common Name | Scientific Name | Process and Conditions Required for Biochar Formation | Applications | References |
|---|---|---|---|---|
| Oyster, Shiitake | Pleurotus ostreatus, Lentinula edodes | Pyrolysis at 700 °C for 2 h | Adsorbed 326mg g−1 and 398mg g−1 of lead Pb(II) from the water | [99] |
| Lingzhi | Ganoderma lucidum | Pyrolysis at 650 °C for 2 h | Showed maximal adsorption against Pb2+ (262.76 mg g−1) and Cd2+ (75.82 mg g−1) | [100] |
| White button | Agaricus bisporus | Pyrolysis at 750 °C for 3 h | Showed maximal adsorption against Cu2+(65.2 mg g−1), Cd2+(76.3 mg g−1), and Zn2+(44.4 mg g−1) in water | [101] |
| ND | ND | Pyrolysis at 300 °C for 90 min | Showed maximal adsorption against Pb2+ (21.0 mg g−1), Cu2+(18.8 mg g−1), Cd2+(11.2 mg g−1) and Ni2+(9.8 mg g−1) in water | [102] |
| ND | ND | Pyrolysis at 450 °C for 4 h | Showed maximal adsorption against crystal violet (1057mg g−1) in wastewater | [103] |
| ND | ND | Pyrolysis at 500 °C for 2 h | Showed maximal adsorption against fluoride (36.5 mg g−1) in water | [104] |
| Edible Mushrooms Common Name | Scientific Name | Types of Nanoparticles Synthesized | Reaction Temperature/Time | Morphology | Size | Applications | References |
|---|---|---|---|---|---|---|---|
| White button | Agaricus bisporus | Copper | RT/24 h | Spherical | 2–10 nm | Antibacterial activity against Enterobacter aerogens; Antioxidant activity using DPPH, and ABTS; Anti-cancer activity against cancer cell lines SW620 (colon cancer) | [107] |
| Brown oyster | Pleurotus cystidiosus | Gold | 29 °C/24 h | ND | ND | Antioxidant activity using DPPH, and ABTS | [108] |
| Oyster | Pleurotus florida | Gold | 70 °C/1.5 h | Spherical | 2–14 nm | Anti-cancer activity against cancer cell lines A-549 (Human lung carcinoma), K-562 (Human chronic myelogenous leukemia bone marrow), HeLa (Human cervix) and MDA-MB (Human adenocarcinoma mammary gland) | [109] |
| Oyster | Pleurotus ostreatus | Gold | 29 °C/24 h | Spherical | 22.9 nm | Antioxidant activity using DPPH, and ABTS | [108] |
| Oyster | Pleurotus sajor-caju | Gold | RT/12 h | Spherical | 16–18 nm | Anti-cancer activity against cancer cell lines HCT-116 (colon cancer) | [110] |
| King tuber | Pleurotus tuber-regium | Selenium | RT/24 h | Spherical | 91–102 nm | Anti-cancer activity against gastric adenocarcinoma AGS | [111] |
| Oyster | Pleurotus ostreatus | Silver | 25 °C/48 h | Spherical | 17.5 nm | Anti-cancer activity against cancer cell lines HepG2 (human liver) and MCF-7 (breast) | [112] |
| Lingzhi | Ganoderma lucidum | Silver | ND/ND | Spherical | 15–22 nm | Antioxidant activity using DPPH; Antibacterial activity against Staphylococcus aureus, Enterococcus hirae, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa, Legionella pneumophila subsp. Pneumophila; and antifungal activity against Candida albicans | [113] |
| Matsutake | Tricholoma matsutake | Silver | RT/30 min | Spherical | 10–70 nm | Antibacterial activity against Bacillus cereus, Escherichia coli | [114] |
| Milky white, Oyster, White button, Lingzhi | Calocybe indica, Pleurotus ostreatus, Agaricu sbisporus, Ganoderma lucidum | Silver | RT/12 h | Spherical | 80–100 nm | Antibacterial activity against Staphylococcus aureus | [115] |
| Pink oyster | Pleurotus djamor | Titanium oxide | RT/20 min | Spherical | 31 nm | Antibacterial activity against Corynebacterium diphtheria, Pseudomonas fluorescens, and Staphylococcus aureus; Anti-cancer activity against cancer cell lines A-549 (Human lung carcinoma); larval toxicity against Aedes aegypti, Culex quinquefasciatus | [116] |
| Pink oyster | Pleurotus djamor | Zinc oxide | RT/24 h | Sphere | 74.36 nm | Antioxidant activity using DPPH, ABTS, and H2O2; larval toxicity against Aedes aegypti, Culex quinquefasciatus; Antibacterial activity against Corynebacterium diphtheria, Pseudomonas fluorescens, and Staphylococcus aureus | [117] |
| Edible Mushrooms Common Name | Scientific Name | Production Conditions | Applications | References |
|---|---|---|---|---|
| Oyster | Pleurotus sp. | Hydrothermal/120 °C/4 h | Selective sensitivity for Pb2+; Antibacterial activity against Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa; Anti-cancer activity against breast cancer cells (MDA-MB-231) | [119] |
| Velvet shank | Flammulina velutipes | Hydrothermal/250 °C/4 h | Sensed Cr6+ with a limit of detection 0.73 µM and volatile organic compounds | [120] |
| Oyster | Pleurotu ssp. | Hydrothermal/200 °C/25 h | Sensed nitroarenes in water samples | [121] |
| Paddy straw | Volvariella volvacea | Hydrothermal/200 °C/25 h | Sensed Pb2 with limit of detection 12 nM and for Fe3+ 16 nM | [122] |
| ND | ND | Hydrothermal/200 °C/6 h | Sensed hyaluronic acid and hyaluronidase | [123] |
| Edible Mushrooms Common Name | Scientific Name | Product Base | Applications | References |
|---|---|---|---|---|
| White button, Oyster, Shiitake | Agaricus bisporus, Pleurotus ostreatus, Lentinula edodes | Cream | Anti-inflammatory; anti-tyrosinase; antioxidant and antibacterial activity | [128] |
| Lingzhi | Ganoderma lucidum | Cream | Anti-tyrosinase; antioxidant and antibacterial activity | [129] |
| Oyster | Pleurotus ostreatus | Cream | Skin fairness | [130] |
| Oyster | Pleurotus ostreatus | Gel | Anti-tyrosinase; antioxidant activity | [131] |
| Snow | Tremella fuciformis | Gel | Hand sanitizer | [132] |
| White button, Oyster | Agaricus bisporus, Pleurotus ostreatus | Cream | Anti-tyrosinase; antioxidant and antibacterial activity | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Cruz-Martins, N.; Dhanjal, D.S.; Singh, R.; Chopra, C.; Verma, R.; Abd-Elsalam, K.A.; et al. Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications. J. Fungi 2021, 7, 427. https://doi.org/10.3390/jof7060427
Kumar H, Bhardwaj K, Sharma R, Nepovimova E, Cruz-Martins N, Dhanjal DS, Singh R, Chopra C, Verma R, Abd-Elsalam KA, et al. Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications. Journal of Fungi. 2021; 7(6):427. https://doi.org/10.3390/jof7060427
Chicago/Turabian StyleKumar, Harsh, Kanchan Bhardwaj, Ruchi Sharma, Eugenie Nepovimova, Natália Cruz-Martins, Daljeet Singh Dhanjal, Reena Singh, Chirag Chopra, Rachna Verma, Kamel A. Abd-Elsalam, and et al. 2021. "Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications" Journal of Fungi 7, no. 6: 427. https://doi.org/10.3390/jof7060427
APA StyleKumar, H., Bhardwaj, K., Sharma, R., Nepovimova, E., Cruz-Martins, N., Dhanjal, D. S., Singh, R., Chopra, C., Verma, R., Abd-Elsalam, K. A., Tapwal, A., Musilek, K., Kumar, D., & Kuča, K. (2021). Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications. Journal of Fungi, 7(6), 427. https://doi.org/10.3390/jof7060427
















