Neutrophil Cells Are Essential for The Efficacy of a Therapeutic Vaccine against Paracoccidioidomycosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fungus Strain
2.3. Intratracheal (i.t) Infection
2.4. Peptide Synthesis and Purification
2.5. Dioctadecyl-Dimethylammonium Bromide Formulation
2.6. Therapeutic Vaccine Schedule
2.7. Neutrophil Depletion
2.8. Lung Digestion and Flow Cytometry
2.9. Histopathology and Pulmonary Fungal Load
2.10. Cytokine Analysis
2.11. Statistical Analysis
3. Results
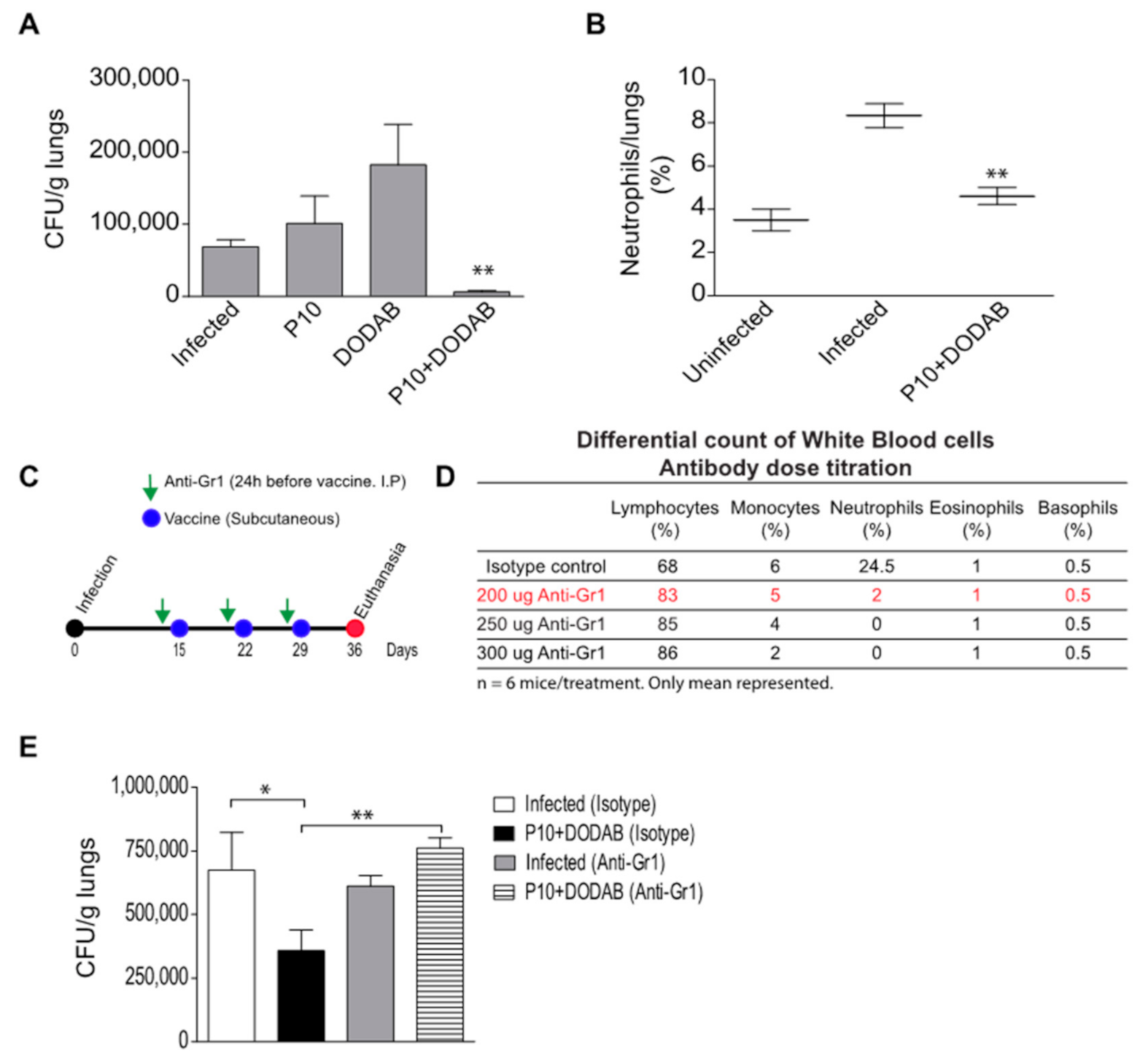
3.1. Pulmonary Fungal Load
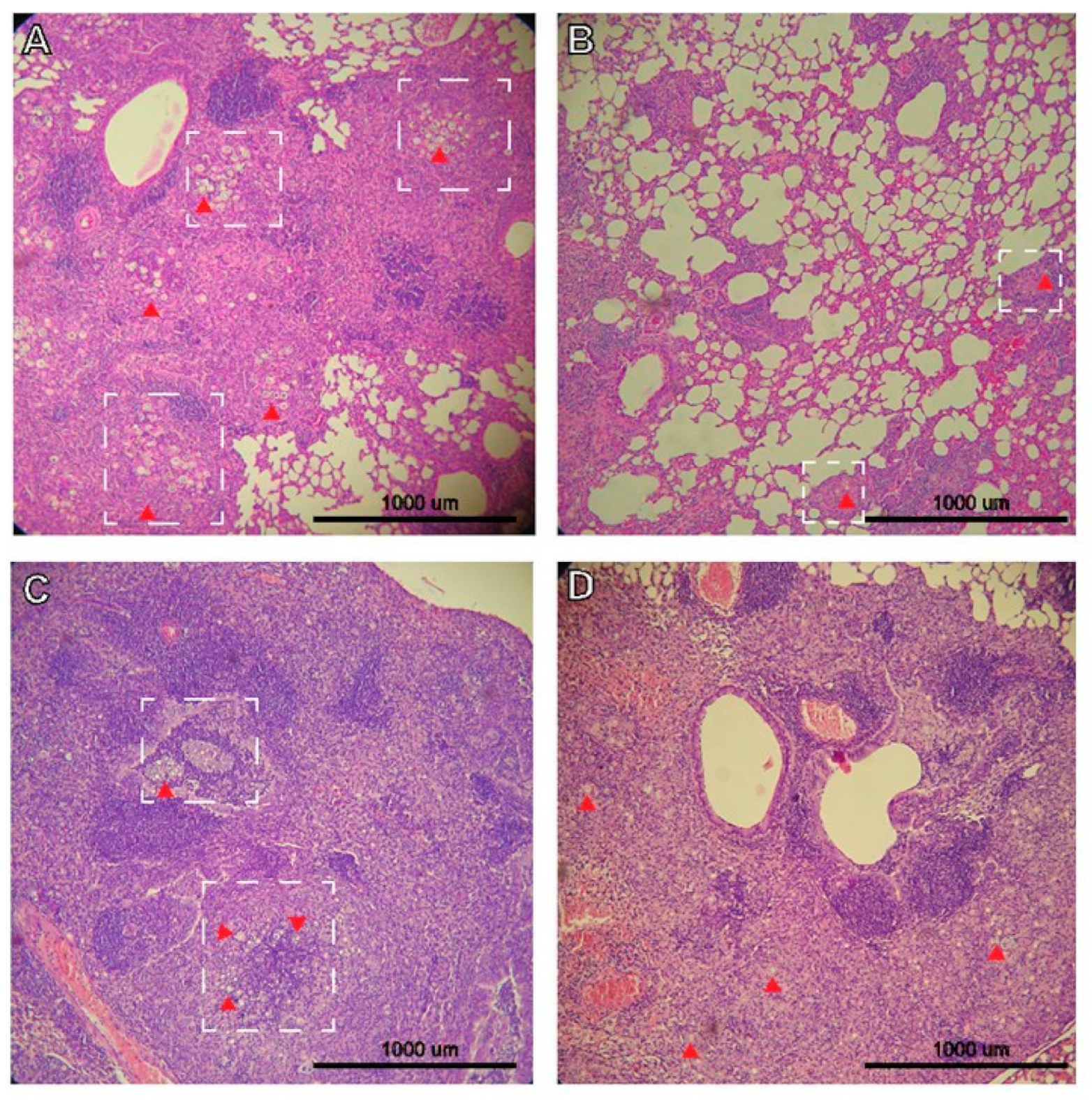
3.2. Histopathology
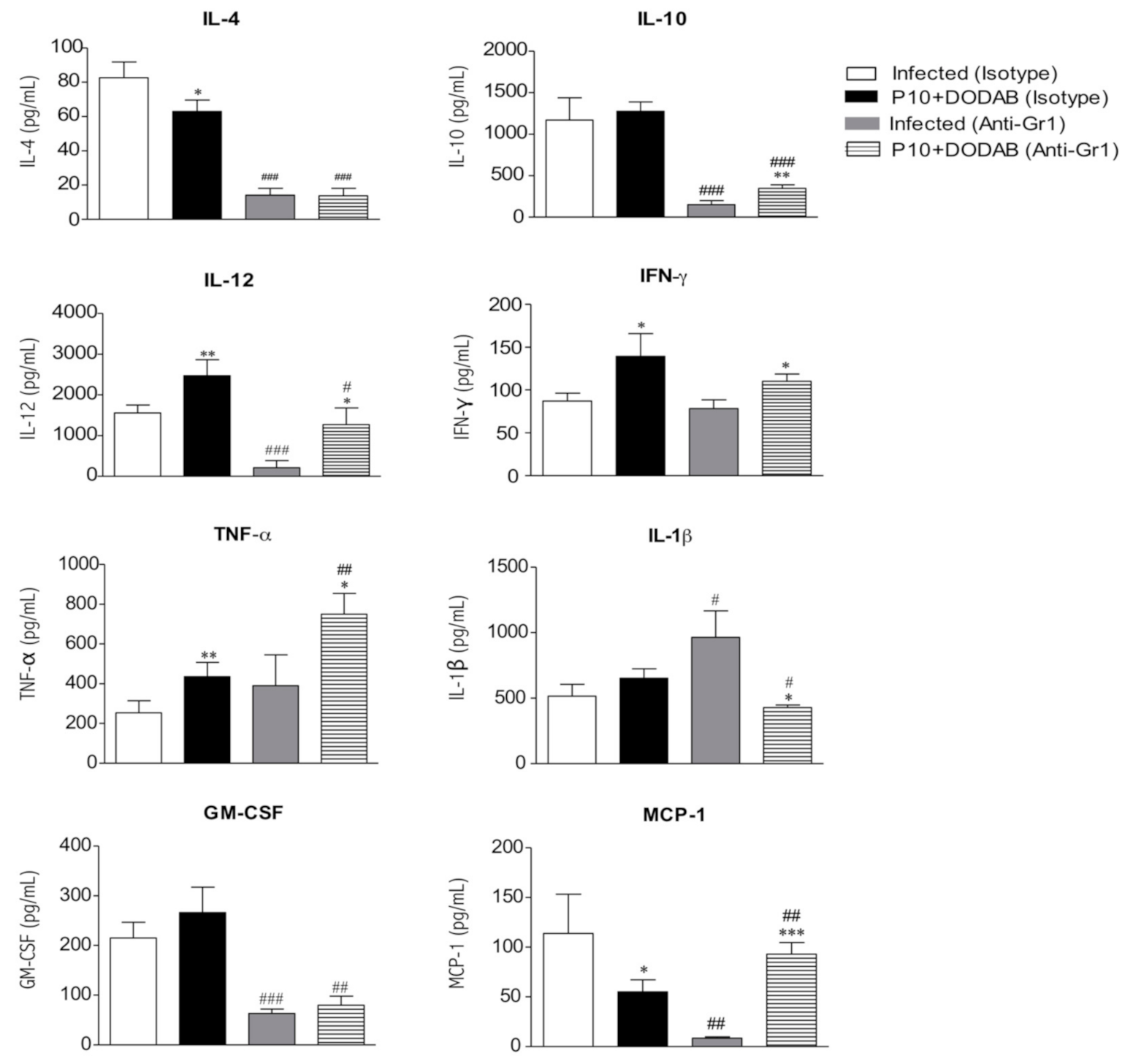
3.3. Cytokine Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez, R. Epidemiology of paracoccidioidomycosis. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 11–20. [Google Scholar] [CrossRef]
- De Melo Teixeira, M.; Theodoro, R.C.; De Oliveira, F.F.M.; Machado, G.C.; Hahn, R.C.; Bagagli, E.; San-Blas, G.; Felipe, M.S.S. Paracoccidioides lutzii sp. nov.: Biological and clinical implications. J. Music. Ther. 2015, 52, 19–28. [Google Scholar] [CrossRef]
- Hahn, R.C.; Rodrigues, A.M.; Fontes, C.J.F.; Nery, A.F.; Tadano, T.; De Pádua Queiroz, L.; De Camargo, Z.P. Case report: Fatal fungemia due to Paracoccidioides lutzii. Am. J. Trop. Med. Hyg. 2014, 91, 394–398. [Google Scholar] [CrossRef]
- Hrycyk, M.F.; Garcia Garces, H.; de Bosco, S.M.G.; de Oliveira, S.L.; Marques, S.A.; Bagagli, E. Ecology of Paracoccidioides brasiliensis, P. lutzii and related species: Infection in armadillos, soil occurrence and mycological aspects. Med. Mycol. 2018, 56, 950–962. [Google Scholar] [CrossRef]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; de Telles Filho, F.Q.; Mendes, R.P.; Colombo, A.L.; Moretti, M.L.; Kono, A.; Tresoldi, A.T.; Wanke, B.; Carvalho, C.R.; Benard, G.; et al. Consenso em paracoccidioidomicose. Rev. Soc. Bras. Med. Trop. 2006, 39, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.C.; Morato Conceição, Y.T.; Santos, N.L.; Ferreira, J.F.; Hamdan, J.S. Disseminated paracoccidioidomycosis: Correlation between clinical and in vitro resistance to ketoconazole and trimethoprim sulphamethoxazole. Mycoses 2003, 46, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Taborda, C.P.; Juliano, M.A.; Puccia, R.; Franco, M.; Travassos, L.R. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect. Immun. 1998, 66, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Braga, C.J.M.; Rittner, G.M.G.; Munoz Henao, J.E.; Teixeira, A.F.; Massis, L.M.; Sbrogio-Almeida, M.E.; Taborda, C.P.; Travassos, L.R.; Ferreira, L.C.S. Paracoccidioides brasiliensis Vaccine Formulations Based on the gp43-Derived P10 Sequence and the Salmonella enterica FliC Flagellin. Infect. Immun. 2009, 77, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.; Ferreira, K.S.; Almeida, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Prophylactic and Therapeutic Vaccination Using Dendritic Cells Primed with Peptide 10 Derived from the 43-Kilodalton Glycoprotein of Paracoccidioides brasiliensis. Clin. Vaccine Immunol. 2012, 19, 23–29. [Google Scholar] [CrossRef]
- Rittner, G.M.G.; Muñoz, J.E.; Marques, A.F.; Nosanchuk, J.D.; Taborda, C.P.; Travassos, L.R. Therapeutic DNA vaccine encoding peptide P10 against experimental paracoccidioidomycosis. PLoS Negl. Trop. Dis. 2012, 6, e1519. [Google Scholar] [CrossRef]
- De Amorim, J.; Magalhães, A.; Muñoz, J.E.; Rittner, G.M.G.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. DNA vaccine encoding peptide P10 against experimental paracoccidioidomycosis induces long-term protection in presence of regulatory T cells. Microbes Infect. 2013, 15, 181–191. [Google Scholar] [CrossRef]
- Marques, A.F.; da Silva, M.B.; Juliano, M.A.P.; Munhõz, J.E.; Travassos, L.R.; Taborda, C.P. Additive effect of P10 immunization and chemotherapy in anergic mice challenged intratracheally with virulent yeasts of Paracoccidioides brasiliensis. Microbes Infect. 2008, 10, 1251–1258. [Google Scholar] [CrossRef]
- Marques, A.F.; Da Silva, M.B.; Juliano, M.A.P.; Travassos, L.R.; Taborda, C.P. Peptide immunization as an adjuvant to chemotherapy in mice challenged intratracheally with virulent yeast cells of Paracoccidioides brasiliensis. Antimicrob. Agents Chemother. 2006, 50, 2814–2819. [Google Scholar] [CrossRef]
- Pino-Tamayo, P.A.; Puerta-Arias, J.D.; Lopera, D.; Urán-Jiménez, M.E.; González, Á. Depletion of Neutrophils Exacerbates the Early Inflammatory Immune Response in Lungs of Mice Infected with Paracoccidioides brasiliensis. Mediators Inflamm 2016, 2016, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Arango, J.C.J.C.; González, Á. Depletion of neutrophils promotes the resolution of pulmonary inflammation and fibrosis in Mice infected with paracoccidioides brasiliensis. PLoS ONE 2016, 11, e0163985. [Google Scholar] [CrossRef] [PubMed]
- Lincopan, N.; Espíndola, N.M.; Vaz, A.J.; da Costa, M.H.B.; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Novel immunoadjuvants based on cationic lipid: Preparation, characterization and activity in vivo. Vaccine 2009, 27, 5760–5771. [Google Scholar] [CrossRef]
- Di Pilato, M.; Esteban, M. Neutrophil and vaccine. Cell Cycle 2015, 14, 1615–1616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jee, J.; Bonnegarde-Bernard, A.; Duverger, A.; Iwakura, Y.; Cormet-Boyaka, E.; Martin, T.L.; Steiner, H.E.; Bachman, R.C.; Boyaka, P.N. Neutrophils negatively regulate induction of mucosal IgA responses after sublingual immunization. Mucosal Immunol. 2015, 8, 735–745. [Google Scholar] [CrossRef][Green Version]
- Stephen, J.; Scales, H.E.; Benson, R.A.; Erben, D.; Garside, P.; Brewer, J.M. Neutrophil swarming and extracellular trap formation play a significant role in Alum adjuvant activity. NPJ Vaccines 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Trentini, M.M.; de Oliveira, F.M.; Kipnis, A.; Junqueira-Kipnis, A.P. The role of neutrophils in the induction of specific Th1 and Th17 during Vaccination against tuberculosis. Front. Microbiol. 2016, 7, 898. [Google Scholar] [CrossRef]
- Silva, E.B.; Goodyear, A.; Sutherland, M.D.; Podnecky, N.L.; Gonzalez-Juarrero, M.; Schweizer, H.P.; Dow, S.W. Correlates of immune protection following cutaneous immunization with an attenuated Burkholderia pseudomallei vaccine. Infect. Immun. 2013, 81, 4626–4634. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Luft, V.D.; Amorim, J.; Magalhães, A.; Thomaz, L.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Immunization with P10 Peptide Increases Specific Immunity and Protects Immunosuppressed BALB/c Mice Infected with Virulent Yeasts of Paracoccidioides brasiliensis. Mycopathologia 2014, 178, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Leliefeld, P.H.C.; Koenderman, L.; Pillay, J. How neutrophils shape adaptive immune responses. Front. Immunol. 2015, 6, 471. [Google Scholar] [CrossRef]
- Mócsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef]
- Abadie, V.; Badell, E.; Douillard, P.; Ensergueix, D.; Leenen, P.J.M.; Tanguy, M.; Fiette, L.; Saeland, S.; Gicquel, B.; Winter, N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 2005, 106, 1843–1850. [Google Scholar] [CrossRef]
- Hampton, H.R.; Bailey, J.; Tomura, M.; Brink, R.; Chtanova, T. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat. Commun. 2015, 6, 7139. [Google Scholar] [CrossRef] [PubMed]
- Neeland, M.R.; Shi, W.; Collignon, C.; Taubenheim, N.; Meeusen, E.N.T.; Didierlaurent, A.M.; de Veer, M.J. The Lymphatic Immune Response Induced by the Adjuvant AS01: A Comparison of Intramuscular and Subcutaneous Immunization Routes. J. Immunol. 2016, 197, 2704–2714. [Google Scholar] [CrossRef]
- Pina, A.; Hila, P.; Saldiva, N.; Elena, L.; Restrepo, C.; Calich, V.L.G. Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts. J. Leukoc. Biol. 2006, 79, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357. [Google Scholar] [CrossRef]
- Andersson, H.; Andersson, B.; Eklund, D.; Ngoh, E.; Persson, A.; Svensson, K.; Lerm, M.; Blomgran, R.; Stendahl, O. Apoptotic Neutrophils Augment the Inflammatory Response to Mycobacterium tuberculosis Infection in Human Macrophages. PLoS ONE 2014, 9, e101514. [Google Scholar] [CrossRef] [PubMed]
- Marwick, J.A.; Mills, R.; Kay, O.; Michail, K.; Stephen, J.; Rossi, A.G.; Dransfield, I.; Hirani, N. Neutrophils induce macrophage anti-inflammatory reprogramming by suppressing NF-κB activation. Cell Death Dis. 2018, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.J.; Mamoni, R.L.; Musatti, C.C.; Papaiordanou, P.M.O.; Blotta, M.H.S.L. Cytokines and lymphocyte proliferation in juvenile and adult forms of paracoccidioidomycosis: Comparison with infected and non-infected controls. Microbes Infect. 2002, 4, 139–144. [Google Scholar] [CrossRef]
- De Oliveira, L.L.; Coltri, K.C.; Barros Cardoso, C.R.; Roque-Barreira, M.C.; Panunto-Castelo, A. T helper 1-inducing adjuvant protects against experimental paracoccidioidomycosis. PLoS Negl. Trop. Dis. 2008, 2, e183. [Google Scholar] [CrossRef]
- Travassos, L.R.; Taborda, C.P. New advances in the development of a vaccine against paracoccidioidomycosis. Front. Microbiol. 2012, 3, 212. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, O.; Muñoz, J.E.; Lincopan, N.; Teixeira, A.F.; Ferreira, L.C.S.; Travassos, L.R.; Taborda, C.P. The role of adjuvants in therapeutic protection against paracoccidioidomycosis after immunization with the P10 peptide. Front. Microbiol. 2012, 3, 154. [Google Scholar] [CrossRef]
- Holanda, R.A.; Muñoz, J.E.; Dias, L.S.; Silva, L.B.R.; Santos, J.R.A.; Pagliari, S.; Vieira, É.L.M.; Paixão, T.A.; Taborda, C.P.; Santos, D.A.; et al. Recombinant vaccines of a CD4+T-cell epitope promote efficient control of Paracoccidioides brasiliensis burden by restraining primary organ infection. PLoS Negl. Trop. Dis. 2017, 11, e0005927. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, M.; Ma, D. TNF-α, a good or bad factor in hematological diseases? Stem. Cell Investig. 2010, 1. [Google Scholar] [CrossRef]
- Rodrigues, D.R.; Dias-Melicio, L.A.; Calvi, S.A.; Peraçoli, M.T.S.; Soares, A.M.V.C. Paracoccidioides brasiliensis killing by IFN-γ, TNF-α and GM-CSF activated human neutrophils: Role for oxygen metabolites. Med. Mycol. 2007, 45, 27–33. [Google Scholar] [CrossRef]
- Bernardino, S.; Pina, A.; Felonato, M.; Costa, T.A.; Frank de Araújo, E.; Feriotti, C.; Bazan, S.B.; Keller, A.C.; Leite, K.R.M.; Calich, V.L.G. TNF-α and CD8+ T Cells Mediate the Beneficial Effects of Nitric Oxide Synthase-2 Deficiency in Pulmonary Paracoccidioidomycosis. PLoS Negl. Trop. Dis. 2013, 7, e2325. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.A.; Bazan, S.B.; Feriotti, C.; Araújo, E.F.; Bassi, Ê.J.; Loures, F.V.; Calich, V.L.G. In Pulmonary Paracoccidioidomycosis IL-10 Deficiency Leads to Increased Immunity and Regressive Infection without Enhancing Tissue Pathology. PLoS Negl. Trop. Dis. 2013, 7, e2512. [Google Scholar] [CrossRef]
- Bazzoni, F.; Tamassia, N.; Rossato, M.; Cassatella, M.A. Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: Lessons from neutrophils. Eur. J. Immunol. 2010, 40, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Daley, J.M.; Thomay, A.A.; Connolly, M.D.; Reichner, J.S.; Albina, J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008, 83, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Wojtasiak, M.; Pickett, D.L.; Tate, M.D.; Londrigan, S.L.; Bedoui, S.; Brooks, A.G.; Reading, P.C. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J. Gen. Virol. 2010, 91, 2158–2166. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, L.d.S.; Silva, L.B.R.; Nosanchuk, J.D.; Taborda, C.P. Neutrophil Cells Are Essential for The Efficacy of a Therapeutic Vaccine against Paracoccidioidomycosis. J. Fungi 2021, 7, 416. https://doi.org/10.3390/jof7060416
Dias LdS, Silva LBR, Nosanchuk JD, Taborda CP. Neutrophil Cells Are Essential for The Efficacy of a Therapeutic Vaccine against Paracoccidioidomycosis. Journal of Fungi. 2021; 7(6):416. https://doi.org/10.3390/jof7060416
Chicago/Turabian StyleDias, Lucas dos Santos, Leandro B. R. Silva, Joshua D. Nosanchuk, and Carlos Pelleschi Taborda. 2021. "Neutrophil Cells Are Essential for The Efficacy of a Therapeutic Vaccine against Paracoccidioidomycosis" Journal of Fungi 7, no. 6: 416. https://doi.org/10.3390/jof7060416
APA StyleDias, L. d. S., Silva, L. B. R., Nosanchuk, J. D., & Taborda, C. P. (2021). Neutrophil Cells Are Essential for The Efficacy of a Therapeutic Vaccine against Paracoccidioidomycosis. Journal of Fungi, 7(6), 416. https://doi.org/10.3390/jof7060416







