The Pacific Tree-Parasitic Fungus Cyclocybe parasitica Exhibits Monokaryotic Fruiting, Showing Phenotypes Known from Bracket Fungi and from Cyclocybe aegerita
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Culture Maintenance, and Nuclear State Verification
2.2. Fruiting Setup for C. parasitica ICMP 11668 and Its Progeny Monokaryons
2.3. Cross Sections
2.4. Mating of Selected C. parasitica Monokaryons
3. Results
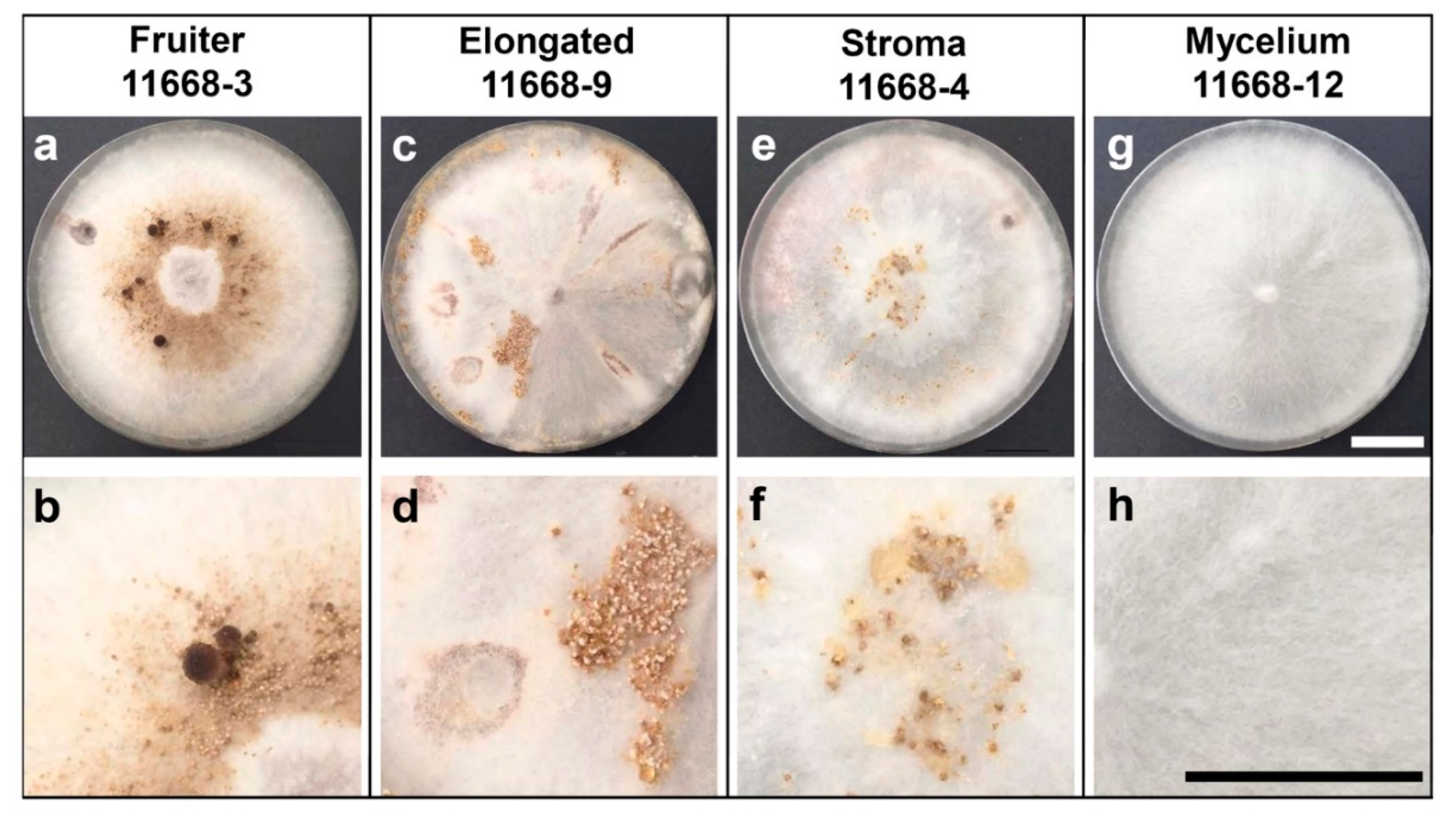
3.1. Fruiting Phenotypes of C. parasitica ICMP 11668 and Its Monokaryotic Progeny
3.2. Mating Compatibility of Fruiting-Competent Monokaryons
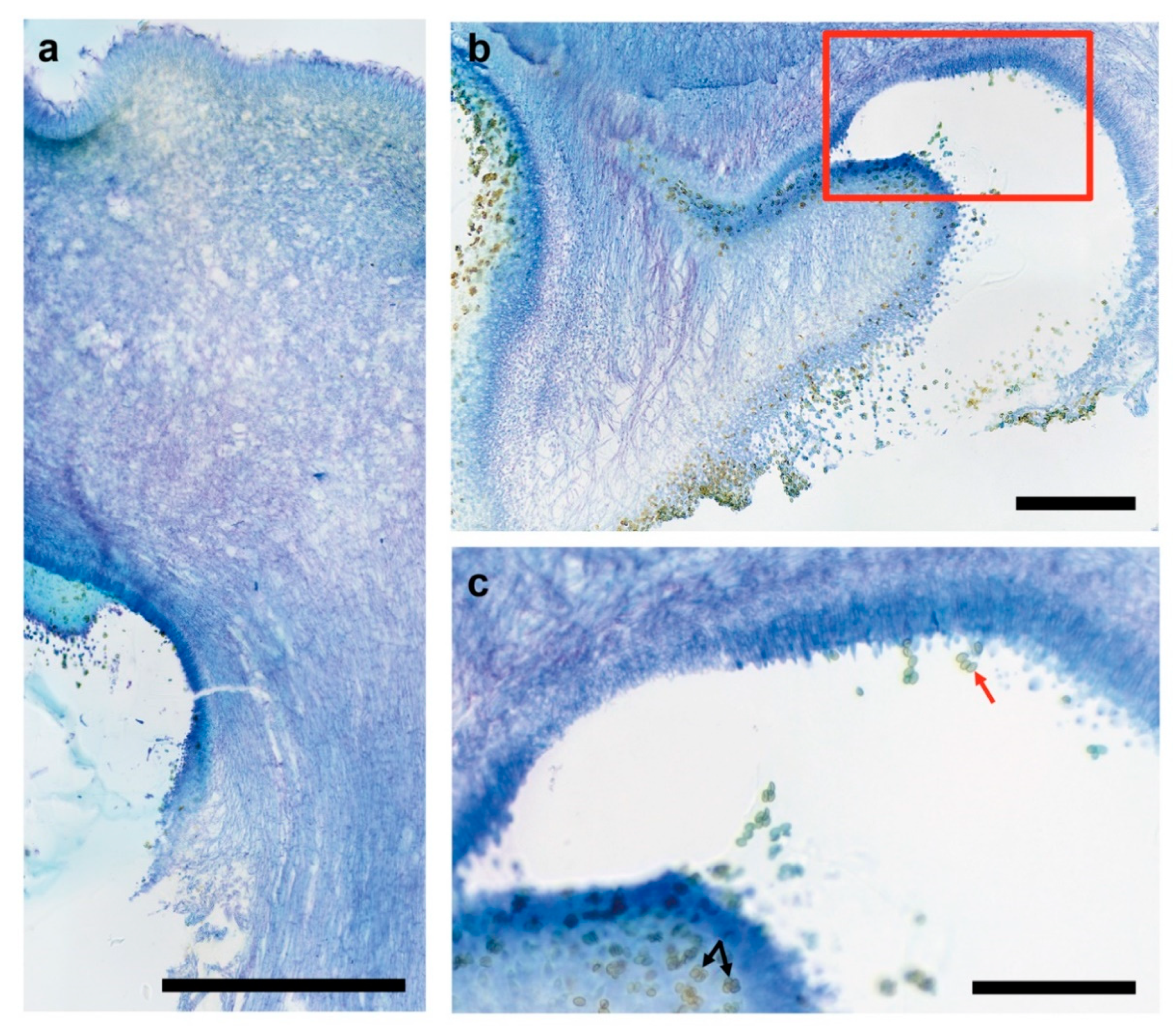
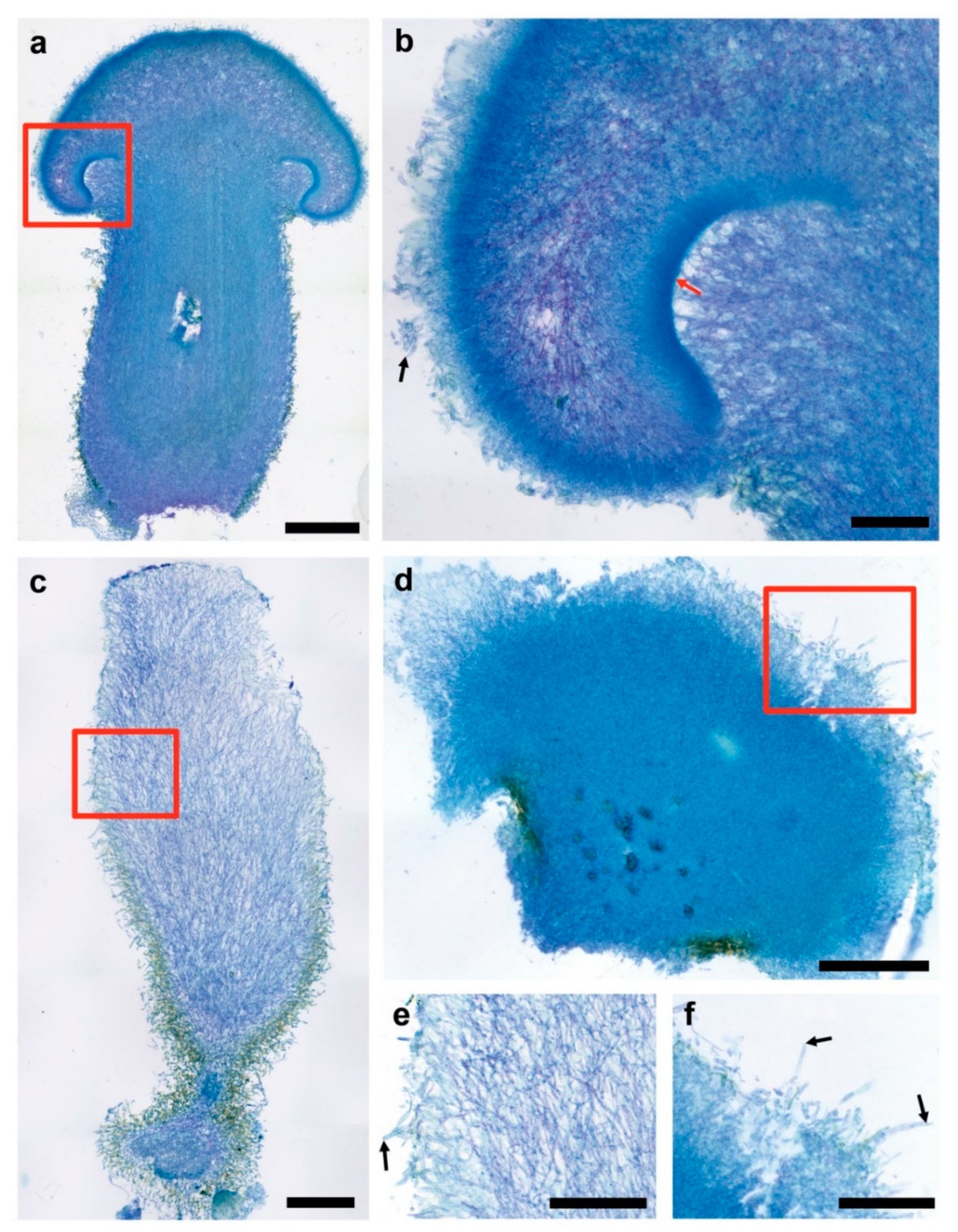
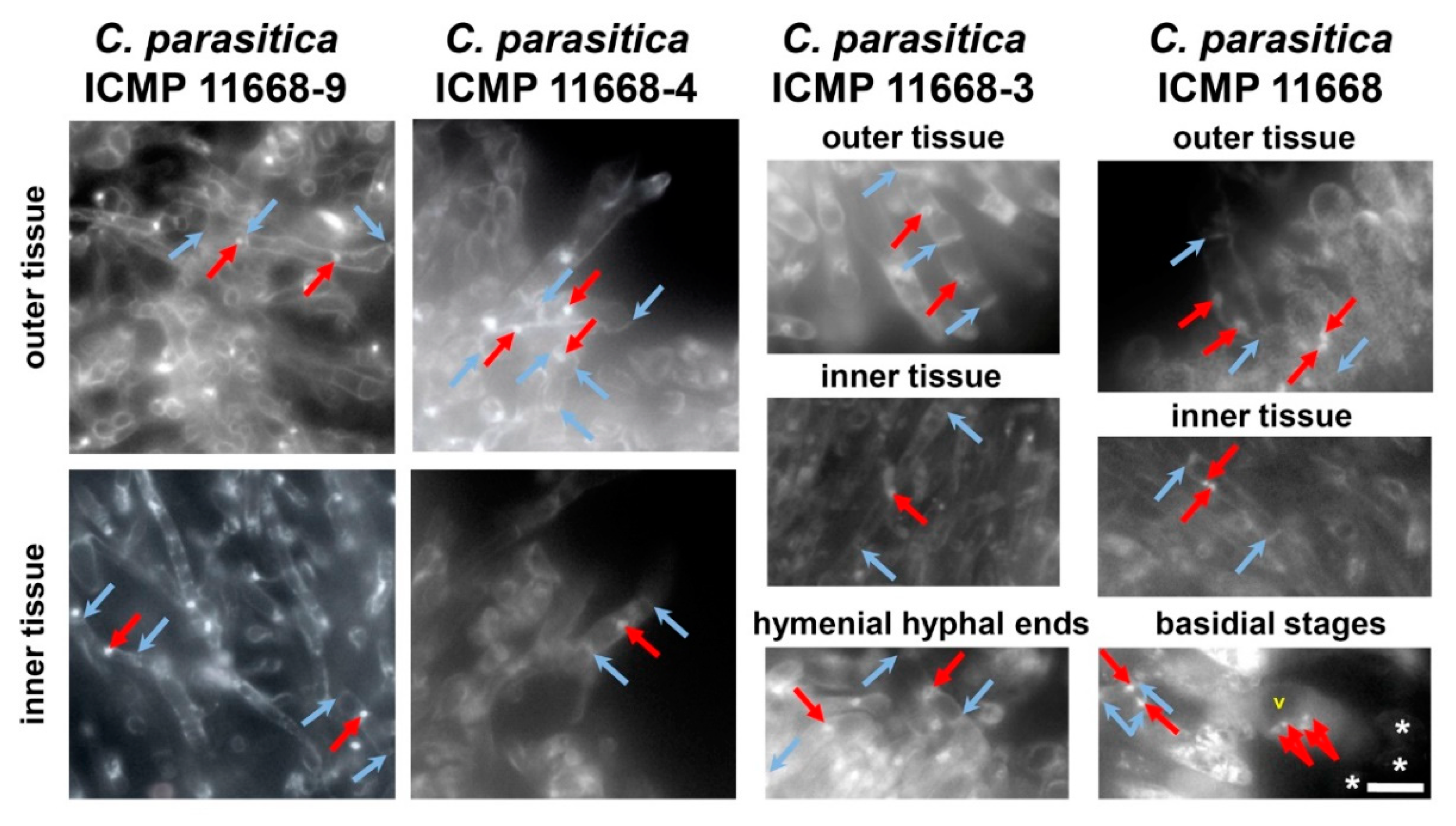
3.3. Fruiting Structure Anatomy in C. parasitica ICMP 11668 and Its Progeny
4. Discussion
4.1. Dikaryotic and Monokaryotic Basidiome Morphogenesis in C. parasitica
4.2. Open Questions: Nature of Monokaryotic Fruiting s.str. and Its Potential Occurence in Wild Populations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevenson, G. A parasitic member of the Bolbitiaceae—Agrocybe parasitica sp. nov. N. Z. J. For. Sci. 1982, 27, 130–133. [Google Scholar]
- Mitchell, A.; Savage, G.P. Agrocybe parasitica: The mushroom of the future? Proc. Nutr. Soc. N. Z. 1990, 115, 175–178. [Google Scholar]
- Hood, I.A. An Introduction to Fungi on Wood in Queensland; University of New England: Armidale, Australia, 2003; 388p. [Google Scholar]
- Fuhrer, B. A Field Guide to Australian Fungi; Bloomings Books Pty Ltd.: Melbourne, Australia, 2005; 360p. [Google Scholar]
- iNaturalist.org. Available online: https://doi.org/10.15468/ab3s5xaccessedviaGBIF.org (accessed on 26 April 2021).
- Watling, R. Observations on the Bolbitaceae—30. Some Brazillian Taxa. Bol. Soc. Argent. Bot. 1992, 28, 77–103. [Google Scholar]
- Frings, R.A.; Maciá-Vicente, J.G.; Buße, S.; Čmoková, A.; Kellner, H.; Hofrichter, M.; Hennicke, F. Multilocus phylogeny- and fruiting feature-assisted delimitation of European Cyclocybe aegerita from a new Asian species complex and related species. Mycol. Prog. 2020, 19, 1001–1016. [Google Scholar] [CrossRef]
- Watling, R.; Taylor, G.M. Observations on the Bolbitiaceae: 27. Preliminary account of the Bolbitiaceae of New Zealand. Bibl. Mycol. 1987, 117, 1–61. [Google Scholar]
- Singer, R. Naucoria Fries and affinitive genera in the USSR. Acta Inst. Bat. Komarov. Acad. Sci. USSR 1950, 2, 402–498. [Google Scholar]
- Watling, R.; Gregory, N.M. Census catalogue of world members of the Bolbitiaceae. Bibl. Mycol. 1981, 82, 1–224. [Google Scholar]
- Nauta, M.M. Agrocybe Fay. In Flora Agaricina Neerlandica; Noordeloos, M.E., Kuyper, T.W., Velinga, E.C., Eds.; Taylor & Francis Group; CRC Press: Boca Raton, FL, USA, 2005; Volume 6, pp. 204–221. [Google Scholar]
- Mycological Notes 19: Agrocybe parasitica and Related Species. Available online: https://www.funnz.org.nz/sites/default/files/MycNotes19-AgrocybeParasitica_2.pdf (accessed on 26 April 2021).
- Esser, K.; Semerdžieva, M.; Stahl, U. Genetische Untersuchungen an dem Basidiomyceten Agrocybe aegerita. Theor. Appl. Genet. 1974, 45, 77–85. [Google Scholar] [CrossRef]
- Uhart, M.; Albertó, E. Morphologic characterization of Agrocybe cylindracea (Basidiomycetes, Agaricales) from America, Europe and Asia. Rev. Mex. Micol. 2007, 24, 9–18. [Google Scholar]
- Uhart, M.; Piscera, J.M.; Albertó, E. Utilization of new naturally occurring strains and supplementation to improve the biological efficiency of the edible mushroom Agrocybe cylindracea. J. Ind. Microbiol. Biotechnol. 2008, 35, 595–602. [Google Scholar] [CrossRef]
- Orban, A.; Weber, A.; Herzog, R.; Hennicke, F.; Rühl, M. Transcriptome of different fruiting stages in the cultivated mushroom Cyclocybe aegerita suggests a complex regulation of fruiting and reveals enzymes putatively involved in fungal oxylipin biosynthesis. BMC Genom. 2021, 22, 324. [Google Scholar] [CrossRef]
- Labarère, J.; Noël, T. Mating type switching in the tetrapolar basidiomycete Agrocybe aegerita. Genetics 1992, 131, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Solovyeva, I.; Rühl, M.; Thines, M.; Hennicke, F. Dikaryotic fruiting body development in a single dikaryon of Agrocybe aegerita and the spectrum of monokaryotic fruiting types in its monokaryotic progeny. Mycol. Prog. 2016, 15, 947–957. [Google Scholar] [CrossRef]
- Singer, R. Mycoflora Australis. Beih. Nova Hedw. 1969, 29, 1–405. [Google Scholar]
- Stahl, U.; Esser, K. Genetics of fruit body production in higher basidiomycetes. Mol. Gen. Genet. 1976, 148, 183–197. [Google Scholar] [CrossRef]
- Herzog, R.; Solovyeva, I.; Bölker, M.; Lugones, L.G.; Hennicke, F. Exploring molecular tools for transformation and gene expression in the cultivated edible mushroom Agrocybe aegerita. Mol. Gen. Genom. 2019, 294, 663–677. [Google Scholar] [CrossRef]
- Clémençon, H. Fixierung, Einbettung und Schnittfärbungen für die plectologische Untersuchung von Hymenomyceten mit dem Lichtmikroskop. Mycol. Helv. 1990, 3, 451–466. [Google Scholar]
- Meinhardt, F. Untersuchungen zur Genetik des Fortpflanzungsverhaltens und der Fruchtkörper- und Antibiotikabildung des Basidiomyceten Agrocybe aegerita. Ph.D. Thesis, Ruhr-University Bochum, Bochum, Germany, 1980. [Google Scholar]
- Esser, K.; Saleh, F.; Meinhardt, F. Genetics of fruit body production in higher basidiomycetes. II. Monokaryotic and dikaryotic fruiting in Schizophyllum commune. Curr. Genet. 1979, 1, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Kothe, E. Mating Types and Pheromone Recognition in the Homobasidiomycete Schizophyllum commune. Fungal Genet. Biol. 1999, 27, 146–152. [Google Scholar] [CrossRef]
- Meinhardt, F.; Leslie, J.F. Mating types of Agrocybe aegerita. Curr. Genet. 1982, 5, 65–68. [Google Scholar] [CrossRef]
- Raudaskoski, M.; Vauras, R. Scanning electron microscope study of fruitbody differentiation in Schizophyllum commune. Trans. Br. Mycol. Soc. 1982, 78, 475–481. [Google Scholar] [CrossRef]
- Orban, A.; Hennicke, F.; Rühl, M. Volatilomes of Cyclocybe aegerita during different stages of monokaryotic and dikaryotic fruiting. Biol. Chem. 2020, 401, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Yli-Mattila, T.; Ruiters, M.H.J.; Wessels, J.G.H.; Raudaskoski, M. Effect of inbreeding and light on monokaryotic and dikaryotic fruiting in the homobasidiomycete Schizophyllum commune. Mycol. Res. 1989, 93, 535–542. [Google Scholar] [CrossRef]
- Knabe, N.; Jung, E.M.; Freihorst, D.; Hennicke, F.; Horton, J.S.; Kothe, E. A central role for Ras1 in morphogenesis of the basidiomycete Schizophyllum commune. Eukaryot Cell 2013, 12, 941–952. [Google Scholar] [CrossRef]
- Gupta, D.K.; Rühl, M.; Mishra, B.; Kleofas, V.; Hofrichter, M.; Herzog, R.; Pecyna, M.J.; Sharma, R.; Kellner, H.; Hennicke, F.; et al. The genome sequence of the commercially cultivated mushroom Agrocybe aegerita reveals a conserved repertoire of fruiting-related genes and a versatile suite of biopolymer-degrading enzymes. BMC Genom. 2018, 19, 48. [Google Scholar] [CrossRef]
- Terashima, K.; Yuki, K.; Muraguchi, H.; Akiyama, M.; Kamada, T. The dst1 gene involved in mushroom photomorphogenesis of Coprinus cinereus encodes a putative photoreceptor for blue light. Genetics 2005, 171, 101–108. [Google Scholar] [CrossRef]
- Kamada, T.; Sano, H.; Nakazawa, T.; Nakahori, K. Regulation of fruiting body photomorphogenesis in Coprinopsis cinerea. Fungal Genet. Biol. 2010, 47, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Kuratani, M.; Tanaka, K.; Terashima, K.; Muraguchi, H.; Nakazawa, T.; Nakahori, K.; Kamada, T. The dst2 gene essential for photomorphogenesis of Coprinopsis cinerea encodes a protein with a putative FAD-binding-4 domain. Fungal Genet. Biol. 2010, 47, 152–158. [Google Scholar] [CrossRef]
- He, M.Q.; Zhao, R.L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.C.; Raspé, O.; Kakishima, M.; Sánchez-Ramírez, S.; et al. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar]
- Chen, B.; van Peer, A.F.; Yan, J.; Li, X.; Xie, B.; Miao, J.; Huang, Q.; Zhang, L.; Wang, W.; Fu, J.; et al. Fruiting body formation in Volvariella volvacea can occur independently of its MAT-A-controlled bipolar mating system, enabling homothallic and heterothallic life cycles. G3 (Bethesda) 2016, 6, 2135. [Google Scholar] [CrossRef]
- Chang, S.T.; Ling, K.Y. Nuclear behaviour in the basidiomycete Volvariella volvacea. Am. J. Bot. 1970, 57, 165–171. [Google Scholar] [CrossRef]
- Chang, S.T.; Yau, C.K. Volvariella volvacea and its life history. Am. J. Bot. 1971, 58, 552–561. [Google Scholar] [CrossRef]
- Li, G.S.F. Morphology of Volvariella volvacea. In Tropical Mushrooms: Biological Nature and Cultivation Methods; Chang, S.T., Quimio, T.H., Eds.; The Chinese University Press: Hong Kong, China, 1982; pp. 119–137. [Google Scholar]
- Li, S.X.; Chang, S.T. Study on the spore pattern in basidium of Volvareilla volvacea. In Science and Cultivation of Edible fungi; van Griensven, L.J.L.D., Ed.; Balkema: Rotterdam, The Netherlands, 1991; Volume 2, pp. 115–118. [Google Scholar]
- Chiu, S.W. Evidence for a haploid life-cycle in Volvariella volvacea from microspectrophotometric measurements and observations of nuclear behaviour. Mycol. Res. 1993, 12, 1481–1485. [Google Scholar] [CrossRef]
- Wells, K. Meiotic and mitotic divisions in the Basidiomycotina. In Mechanisms and Control of Cell Division; Rost, T.L., Gifford, E.M., Jr., Eds.; Dowden, Hutchison and Ross: Stroudsburg, PA, USA, 1977; pp. 337–374. [Google Scholar]
- Heitman, J. Evolution of sexual reproduction: A view from the Fungal Kingdom supports an evolutionary epoch with sex before sexes. Fungal Biol. Rev. 2015, 29, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Heitman, J. From two to one: Unipolar sexual reproduction. Fungal Biol. Rev. 2015, 29, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Phadke, S.S.; Feretzaki, M.; Heitman, J. Unisexual reproduction enhances fungal competitiveness by promoting habitat exploration via hyphal growth and sporulation. Eukaryot Cell 2013, 12, 1155–1159. [Google Scholar] [CrossRef]
- Roach, K.C.; Heitman, J. Unisexual reproduction reverses Muller’s ratchet. Genetics 2014, 198, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Esser, K.; Meinhardt, F. A common genetic control of dikaryotic and monokaryotic fruiting in the basidiomycete Agrocybe aegerita. Mol. Gen. Genet. 1977, 155, 113–115. [Google Scholar] [CrossRef]
- Meinhardt, F.; Esser, K. Genetic studies of the basidiomycete Agrocybe aegerita. 2. Genetic control of fruit body formation and its practical implications. Theor. Appl. Genet. 1981, 60, 265–268. [Google Scholar] [CrossRef]
- Almási, E.; Sahu, N.; Krizsán, K.; Bálint, B.; Kovács, G.M.; Kiss, B.; Cseklye, J.; Drula, E.; Henrissat, B.; Nagy, I.; et al. Comparative genomics reveals unique wood-decay strategies and fruiting body development in the Schizophyllaceae. New Phytol. 2019, 224, 902–915. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elders, H.; Hennicke, F. The Pacific Tree-Parasitic Fungus Cyclocybe parasitica Exhibits Monokaryotic Fruiting, Showing Phenotypes Known from Bracket Fungi and from Cyclocybe aegerita. J. Fungi 2021, 7, 394. https://doi.org/10.3390/jof7050394
Elders H, Hennicke F. The Pacific Tree-Parasitic Fungus Cyclocybe parasitica Exhibits Monokaryotic Fruiting, Showing Phenotypes Known from Bracket Fungi and from Cyclocybe aegerita. Journal of Fungi. 2021; 7(5):394. https://doi.org/10.3390/jof7050394
Chicago/Turabian StyleElders, Hannah, and Florian Hennicke. 2021. "The Pacific Tree-Parasitic Fungus Cyclocybe parasitica Exhibits Monokaryotic Fruiting, Showing Phenotypes Known from Bracket Fungi and from Cyclocybe aegerita" Journal of Fungi 7, no. 5: 394. https://doi.org/10.3390/jof7050394
APA StyleElders, H., & Hennicke, F. (2021). The Pacific Tree-Parasitic Fungus Cyclocybe parasitica Exhibits Monokaryotic Fruiting, Showing Phenotypes Known from Bracket Fungi and from Cyclocybe aegerita. Journal of Fungi, 7(5), 394. https://doi.org/10.3390/jof7050394




