Daldiniaeschsone A, a Rare Tricyclic Polyketide Having a Chromone Unit Fused to a δ-Lactone and Its Symmetrical Biphenyl Dimer, Daldiniaeschsone B, from an Endophytic Fungus Daldinia eschscholtzii SDBR-CMUNKC745
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
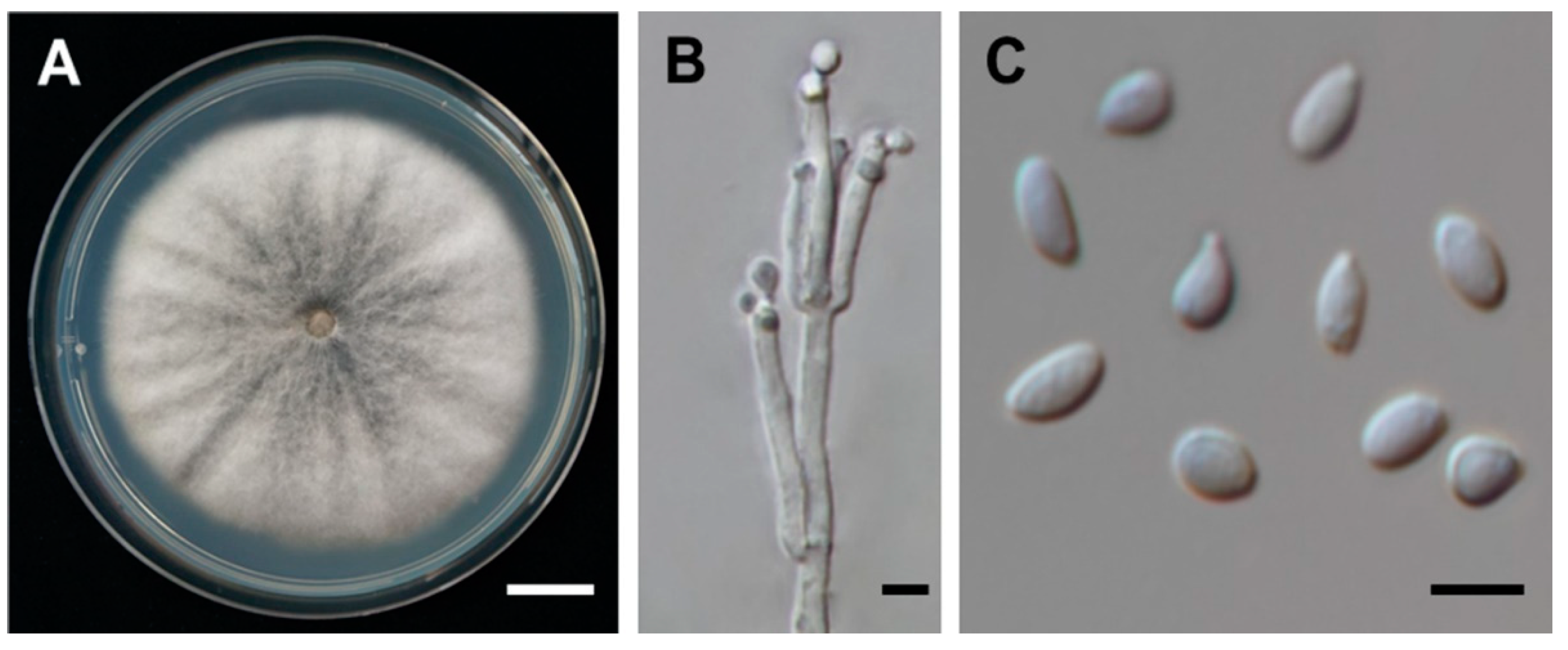
2.2. Fungal Material
2.3. Fermentation, Extraction, and Isolation of Secondary Metabolites
2.4. Bioassays
2.4.1. α-Glucosidase Inhibitory Activity
2.4.2. Nitric Oxide (NO) Production Inhibitory Activity
2.4.3. Cytotoxicity Assay (MTT Assay)
3. Results
3.1. Isolated Compounds from Broth Extract of D. eschscholtzii SDBR-CMUNKC745
3.2. α-Glucosidase and NO Production Inhibitory Activities
4. Discussion
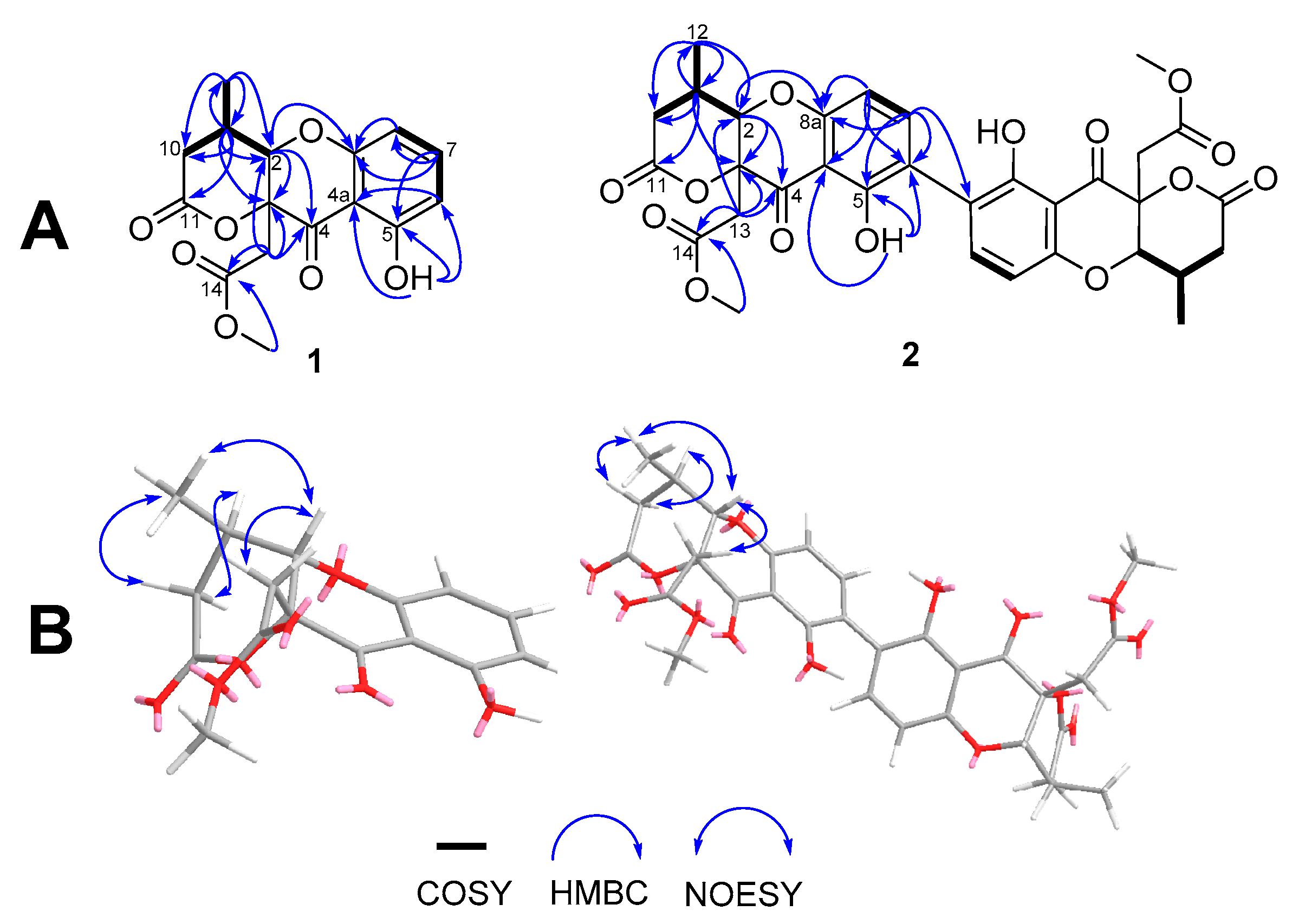
4.1. Structure Elucidation of Two Novel Compounds Isolated from D. eschscholtzii SDBR-CMUNKC745 Broth Extract
4.2. Putative Biosynthesis Pathway of Two Novel Compounds
4.3. α-Glucosidase and NO Production Inhibitory Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, H.X.; Zheng, C.J.; Huang, G.L.; Mei, R.Q.; Nong, X.H.; Shao, T.M.; Chen, G.-Y.; Wang, C.-Y. Bioactive polyketide derivatives from the mangrove-derived fungus Daldinia eschscholtzii HJ004. J. Nat. Prod. 2019, 82, 2211–2219. [Google Scholar] [CrossRef]
- Kamauchi, H.; Shiraishi, Y.; Kojima, A.; Kawazoe, N.; Kinoshita, K.; Koyama, K. Isoindolinones, phthalides, and a naphthoquinone from the fruiting body of Daldinia concentrica. J. Nat. Prod. 2018, 81, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.H.; Gou, J.Y.; Zhao, D.L.; Wang, D.; Liu, J.; Ma, G.Y.; Lia, Y.-Q.; Zhang, C.-S. Phytotoxicity and anti-phytopathogenic activities of marine-derived fungi and their secondary metabolites. RSC Adv. 2018, 8, 37573–37580. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U.A. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Kushveer, J.S.; Khan, M.; Imran, K.; Pagal, S.; Meena, C.K.; Murali, A.; Dhayalan, A.; Sarma, V.V. 2,4-Di-tert-butylphenol isolated from an endophytic fungus, Daldinia eschscholtzii, reduces virulence and quorum sensing in Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 1668. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Liao, H.X.; Bai, M.; Huang, G.L.; Luo, Y.P.; Niu, Y.Y.; Zheng, C.-J.; Wang, C.-Y. One new cytochalasin metabolite isolated from a mangrove-derived fungus Daldinia eschscholtzii HJ001. Nat. Prod. Res. 2018, 32, 208–213. [Google Scholar] [CrossRef]
- Tarman, K.; Palm, G.J.; Porzel, A.; Merzweiler, K.; Arnold, N.; Wessjohann, L.A.; Unterseher, M.; Lindequist, U. Helicascolide C, a new lactone from an Indonesian marine algicolous strain of Daldinia eschscholzii (Xylariaceae, Ascomycota). Phytochem. Lett. 2012, 5, 83–86. [Google Scholar] [CrossRef]
- Nagasawa, H.; Nagura, F.; Mohamad, S.B.; Uto, Y.; Zhu, J.W.; Tsukuda, T.; Hashimoto, T.; Asakawa, T.; Hori, H. Apoptosis induction in HCT116 cells by cytochalasins isolated from the fungus Daldinia vernicosa. Phytomedicine 2000, 6, 403–409. [Google Scholar] [CrossRef]
- Quang, D.N.; Hashimoto, T.; Tanaka, M.; Baumgartner, M.; Stadler, M.; Asakawa, Y. Chemical constituents of the Ascomycete Daldinia concentrica. J. Nat. Prod. 2002, 65, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.D.; Dong, Z.J.; Liu, J.K.; Yang, L.M.; Wang, R.R.; Zheng, Y.T.; Zheng, Q.T. Concentricolide, an anti-HIV Agent from the Ascomycete Daldinia concentrica. Helv. Chim. Acta 2006, 89, 127–133. [Google Scholar] [CrossRef]
- Stadler, M.; Læssøe, T.; Fournier, J.; Decock, C.; Schmieschek, B.; Tichy, H.V.; Peršoh, D.A. polyphasic taxonomy of Daldinia (Xylariaceae). Stud. Mycol. 2014, 77, 1–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Ge, H.M.; Zhao, W.; Dong, H.; Xu, Q.; Li, S.H.; Li, J.; Zhang, J.; Song, Y.C.; Tan, R.X. Unprecedented immunosuppressive polyketides from Daldinia eschscholzii, a mantis-associated fungus. Angew. Chem. Int. Ed. 2008, 47, 5823–5826. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Tan, H.B.; Li, S.N.; Chen, Y.C.; Li, H.H.; Zhang, W.M. Two new metabolites from Daldinia eschscholtzii, an endophytic fungus derived from Pogostemon cablin. J. Asian Nat. Prod. Res. 2019, 21, 150–156. [Google Scholar] [CrossRef]
- Kongyen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Sakayaroj, J. A new hydronaphthalenone from the mangrove-derived Daldinia eschscholtzii PSU-STD57. Nat. Prod. Res. 2015, 29, 1995–1999. [Google Scholar] [CrossRef]
- Luo, Y.; Qiu, L.; Deng, Y.; Yuan, X.H.; Gao, P. A new chromone and a new aliphatic ester isolated from Daldinia eschscholtzii. J. Asian Nat. Prod. Res. 2018, 20, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Tan, R.; Jiang, N.; Yusupu, K.; Wang, G.; Wang, X.L.; Tan, R.X. Selesconol, a fungal polyketide that induces stem cell differentiation. Org. Lett. 2016, 18, 5488–5491. [Google Scholar] [CrossRef]
- Lin, L.P.; Yuan, P.; Jiang, N.; Mei, Y.N.; Zhang, W.J.; Wu, H.M.; Zhang, A.H.; Cao, J.M.; Xiong, Z.X.; Lu, Y.; et al. Gene-inspired mycosynthesis of skeletally new indole alkaloids. Org. lett. 2015, 17, 2610–2613. [Google Scholar] [CrossRef]
- Phukhatmuen, P.; Raksat, A.; Laphookhieo, S.; Charoensup, R.; Duangyod, T.; Maneerat, W. Bioassay-guided isolation and identification of antidiabetic compounds from Garcinia cowa leaf extract. Heliyon 2020, 6, e03625. [Google Scholar] [CrossRef]
- Joo, T.; Sowndhararajan, K.; Hong, S.; Lee, J.; Park, S.Y.; Kim, S.; Jhoo, J.W. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi. J. Biol. Sci. 2014, 21, 427–435. [Google Scholar] [CrossRef]
- Dong, M.; Dan, L.; Li, Y.H.; Xuan, Q.C.; Kaim, L.; Yong, M.Z.; Li, R.T. Naphthoquinones from Onosma paniculatum with potential anti-inflammatory activity. Planta Med. 2016, 83, 631–635. [Google Scholar] [CrossRef]
- Polbuppha, I.; Suthiphasilp, V.; Maneerat, T.; Charoensup, R.; Limtharakul, T.; Cheenpracha, S.; Pyne, S.G.; Laphookhieo, S. Macluracochinones AE, antimicrobial flavonoids from Maclura cochinchinensis (Lour.) Corner. Phytochemistry 2021, 187, 112773. [Google Scholar] [CrossRef] [PubMed]
- Talapatra, S.K.; Karmacharya, B.; De, S.C.; Talapatra, B. (−)-Regiolone, an α-tetralone from Juglans regia: Structure, stereochemistry and conformation. Phytochemistry 1988, 27, 3929–3932. [Google Scholar] [CrossRef]
- Iwasaki, S.; Muro, H.; Sasaki, K.; Nozoe, S.; Okuda, S.; Sato, Z. Isolations of phytotoxic substances produced by Pyricularia oryzae Cavara. Tetrahedron Lett. 1973, 14, 3537–3542. [Google Scholar] [CrossRef]
- Chacón-Morales, P.; Amaro-Luis, J.M.; Bahsas, A. Isolation and characterization of (+)-mellein, the first isocoumarin reported in Stevia genus. Av. Quím. 2013, 8, 145–151. [Google Scholar]
- Bringmann, G.; Hinrichs, J.; Henschel, P.; Kraus, J.; Peters, K.; Peters, E.M. Atropo-enantioselective synthesis of the natural bicoumarin (+)-isokotanin a via a configurationally stable biaryl lactone. Eur. J. Org. Chem. 2002, 6, 1096–1106. [Google Scholar] [CrossRef]
- Shim, S.H.; Baltrusaitis, J.; Gloer, J.B.; Wicklow, D.T. Phomalevones A−C: Dimeric and pseudodimeric polyketides from a fungicolous hawaiiani of Phoma sp. (Cucurbitariaceae). J. Nat. Prod. 2011, 74, 395–401. [Google Scholar] [CrossRef]
- Rönsberg, D.; Debbab, A.; Mándi, A.; Vasylyeva, V.; Böhler, P.; Stork, B.; Engelke, L.; Hamacher, A.; Sawadogo, R.; Diederich, M.; et al. Pro-apoptotic and immunostimulatory tetrahydroxanthone dimers from the endophytic fungus Phomopsis longicolla. J. Org. Chem. 2013, 78, 12409–12425. [Google Scholar]
- Kurobane, I.; Vining, L.C.; Mcinnes, A.G. Biosynthetic relationships among the secalonic acids isolation of emodin, endocrocin and secalonic Acids from Pyrenochaeta Terrestris and Aspergillus Aculeatus. J. Antibiot. 1979, 32, 1256–1266. [Google Scholar] [CrossRef]
- Padhi, S.; Masi, M.; Cimmino, A.; Tuzi, A.; Jena, S.; Tayung, K.; Evidente, A. Funiculosone, a substituted dihydroxanthene-1,9-dione with two of its analogues produced by an endolichenic fungus Talaromyces funiculosus and their antimicrobial activity. Phytochemistry 2019, 157, 175–183. [Google Scholar] [CrossRef]



| Position | 1 | Position | 2 | ||
|---|---|---|---|---|---|
| δc | δH (J in Hz) | δc | δH (J in Hz) | ||
| 2 | 83.1, CH | 4.79 (d, 7.5) | 2/2′ | 82.6, CH | 4.81 (d, 7.4) |
| 3 | 84.8, C | 3/3′ | 84.5, C | ||
| 4 | 194.3, C | 4/4′ | 194.1, C | ||
| 4a | 107.9, C | 4a/4a′ | 107.5, C | ||
| 5 | 162.3, C | 5/5′ | 159.2, C | ||
| 6 | 110.5, CH | 6.56 (dd, 8.3, 0.9) | 6/6′ | 117.7, C | |
| 7 | 139.1, CH | 7.42 (t, 8.3) | 7/7′ | 141.2, CH | 7.52 (d, 8.5) |
| 8 | 108.0, CH | 6.54 (dd, 8.3, 0.9) | 8/8′ | 107.4, CH | 6.63 (d, 8.5) |
| 8a | 159.3, C | 8a/8a′ | 158.4, C | ||
| 9 | 33.9, CH | 2.96 (hept, 7.5) | 9/9′ | 33.5, CH | 2.98 (hept, 7.4) |
| 10a 10b | 37.1, CH2 | 2.70 (dd, 17.3, 8.3) 2.47 (dd, 17.3, 8.0) | 10a/10a′ 10b/10b′ | 36.7, CH2 | 2.70 (dd, 17.3, 8.3) 2.48 (dd, 17.3, 8.0) |
| 11 | 175.3, C | 11/11′ | 174.9, C | ||
| 12 | 15.2, CH3 | 1.32 (d, 7.5) | 12/12′ | 14.9, CH3 | 1.34 (d, 7.4) |
| 13a 13b | 40.2, CH2 | 3.25 (d, 17.3) 3.18 (d, 17.3) | 13a/13a′ 13b/13b′ | 39.8, CH2 | 3.27 (d, 17.3) 3.20 (d, 17.3) |
| 14 | 169.5, C | 14/14′ | 169.1, C | ||
| CO2Me-15 | 54.0, CH3 | 3.73 (s) | CO2Me-15/CO2Me-15′ | 53.8, CH3 | 3.77 (s) |
| OH-5 | 11.44 (s) | OH-5/OH-5′ | 11.91 (s) | ||
| Compounds | α-Glucosidase Inhibitory Activity | Anti-Inflammatory (NO Production Inhibitory Activity) | % Cell Viability at 50 µg/mL | ||
|---|---|---|---|---|---|
| % Inhibition at 200 µg/mL | IC50 (mM) | % Inhibition at 50 µg/mL | IC50 (µM) | ||
| Crude extract | 99.4 | 5.96 ± 0.06 µg/mL | 99.5 | 19.85 ± 0.14 µg/mL | 75.52 ± 1.77 |
| 1 | 98.7 | 0.16 ± 0.11 | 26.5 | inactive | 88.52 ± 3.97 |
| 2 | 96.8 | 0.23 ± 0.76 | 36.9 | inactive | 100.24 ± 1.75 |
| 3 | 97.8 | 0.85 ± 1.19 | not tested | not tested | not tested |
| 4 | 97.8 | 0.55 ± 1.33 | not tested | not tested | not tested |
| 5 | 97.5 | 0.76 ± 0.09 | not tested | not tested | not tested |
| Acarbose | 95.6 | 0.08 ± 0.04 | not tested | not tested | not tested |
| Indomethacin | not tested | not tested | 71.0 | 19.61 ± 0.62 | 84.96 ± 2.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wutthiwong, N.; Suthiphasilp, V.; Pintatum, A.; Suwannarach, N.; Kumla, J.; Lumyong, S.; Maneerat, T.; Charoensup, R.; Cheenpracha, S.; Limtharakul, T.; et al. Daldiniaeschsone A, a Rare Tricyclic Polyketide Having a Chromone Unit Fused to a δ-Lactone and Its Symmetrical Biphenyl Dimer, Daldiniaeschsone B, from an Endophytic Fungus Daldinia eschscholtzii SDBR-CMUNKC745. J. Fungi 2021, 7, 358. https://doi.org/10.3390/jof7050358
Wutthiwong N, Suthiphasilp V, Pintatum A, Suwannarach N, Kumla J, Lumyong S, Maneerat T, Charoensup R, Cheenpracha S, Limtharakul T, et al. Daldiniaeschsone A, a Rare Tricyclic Polyketide Having a Chromone Unit Fused to a δ-Lactone and Its Symmetrical Biphenyl Dimer, Daldiniaeschsone B, from an Endophytic Fungus Daldinia eschscholtzii SDBR-CMUNKC745. Journal of Fungi. 2021; 7(5):358. https://doi.org/10.3390/jof7050358
Chicago/Turabian StyleWutthiwong, Natnicha, Virayu Suthiphasilp, Aknarin Pintatum, Nakarin Suwannarach, Jaturong Kumla, Saisamorn Lumyong, Tharakorn Maneerat, Rawiwan Charoensup, Sarot Cheenpracha, Thunwadee Limtharakul, and et al. 2021. "Daldiniaeschsone A, a Rare Tricyclic Polyketide Having a Chromone Unit Fused to a δ-Lactone and Its Symmetrical Biphenyl Dimer, Daldiniaeschsone B, from an Endophytic Fungus Daldinia eschscholtzii SDBR-CMUNKC745" Journal of Fungi 7, no. 5: 358. https://doi.org/10.3390/jof7050358
APA StyleWutthiwong, N., Suthiphasilp, V., Pintatum, A., Suwannarach, N., Kumla, J., Lumyong, S., Maneerat, T., Charoensup, R., Cheenpracha, S., Limtharakul, T., Pyne, S. G., & Laphookhieo, S. (2021). Daldiniaeschsone A, a Rare Tricyclic Polyketide Having a Chromone Unit Fused to a δ-Lactone and Its Symmetrical Biphenyl Dimer, Daldiniaeschsone B, from an Endophytic Fungus Daldinia eschscholtzii SDBR-CMUNKC745. Journal of Fungi, 7(5), 358. https://doi.org/10.3390/jof7050358









