Favorable Effects of Voriconazole Trough Concentrations Exceeding 1 μg/mL on Treatment Success and All-Cause Mortality: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
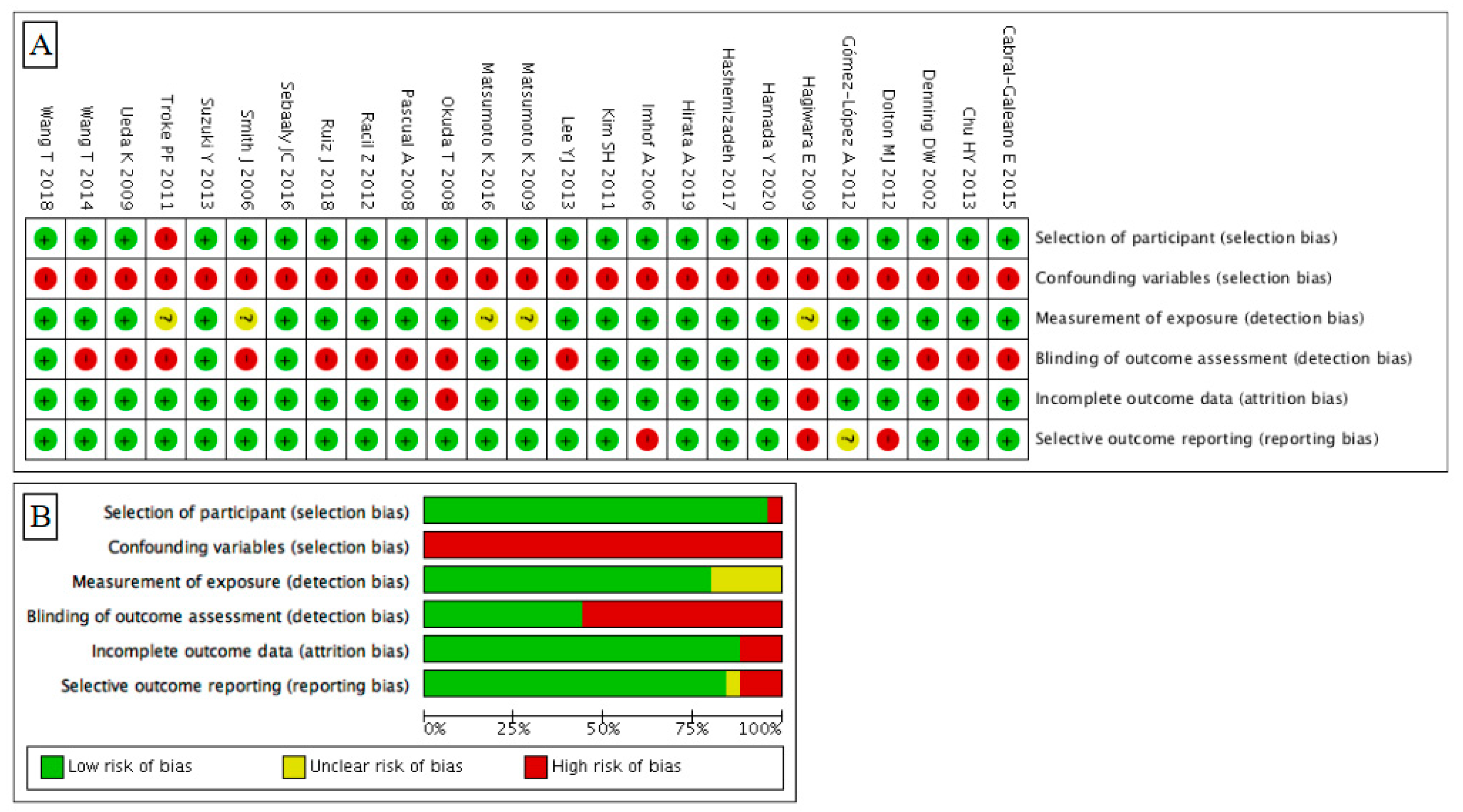
2.3. Quality Assessment
2.4. Outcomes and Definitions
2.5. Statistical Analysis
3. Results
3.1. Studies Retrieved and Characteristics
3.2. Risk of Bias
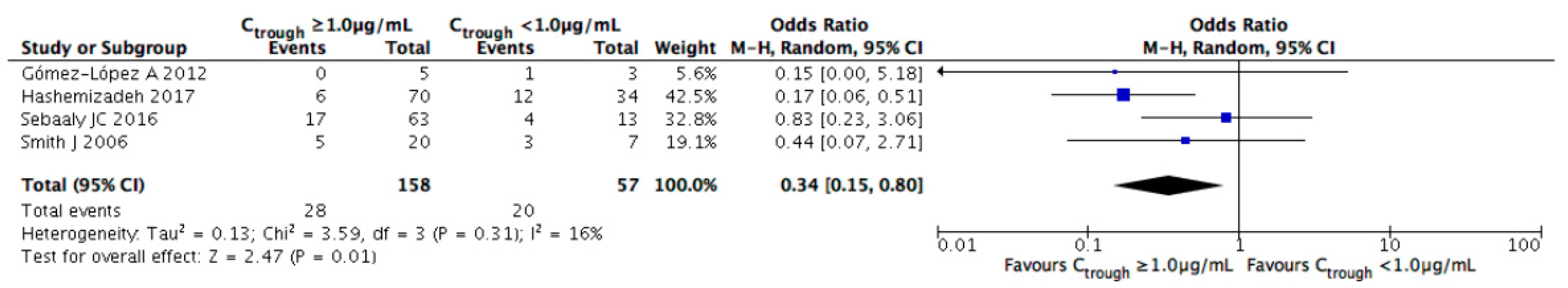
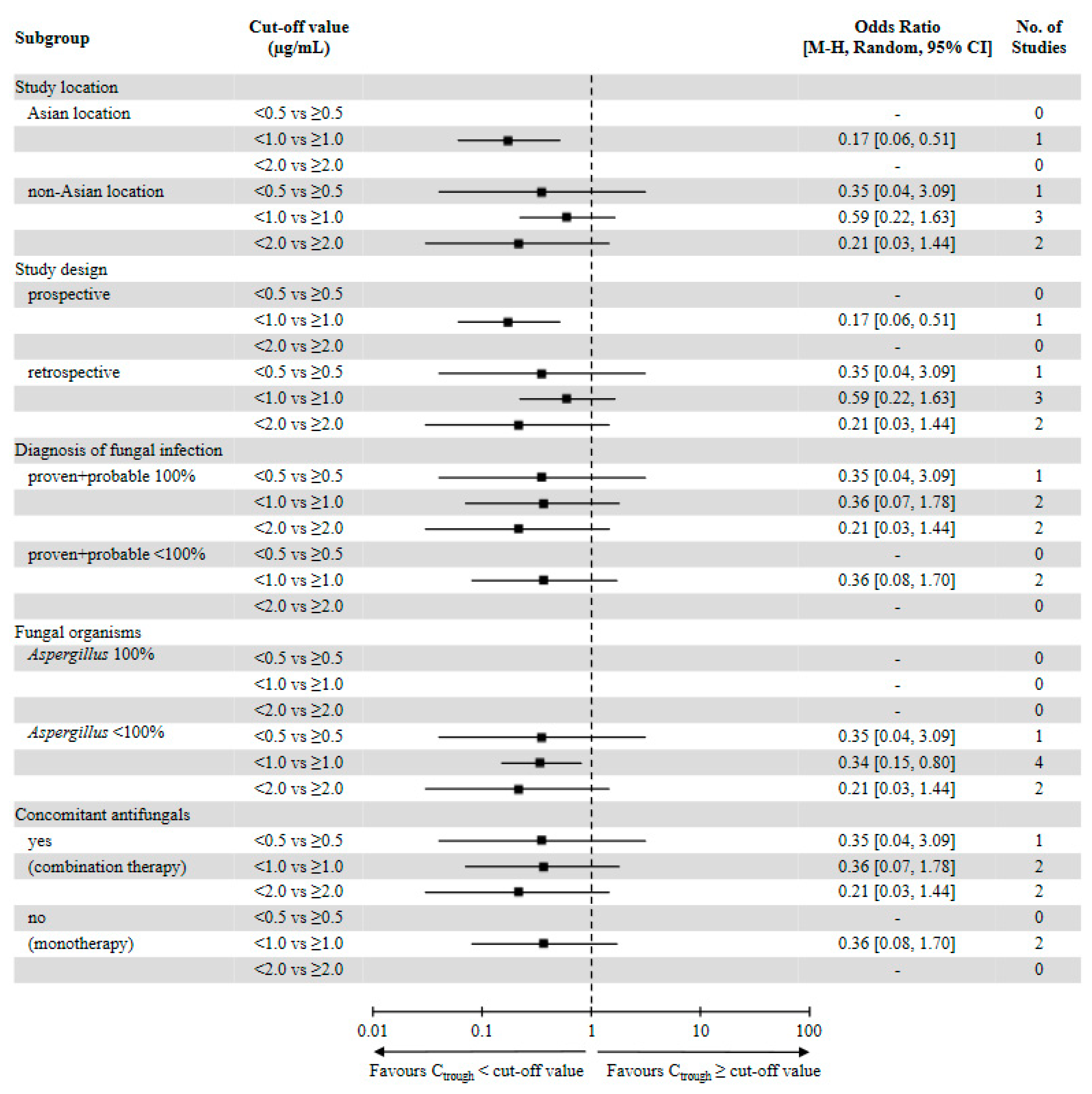
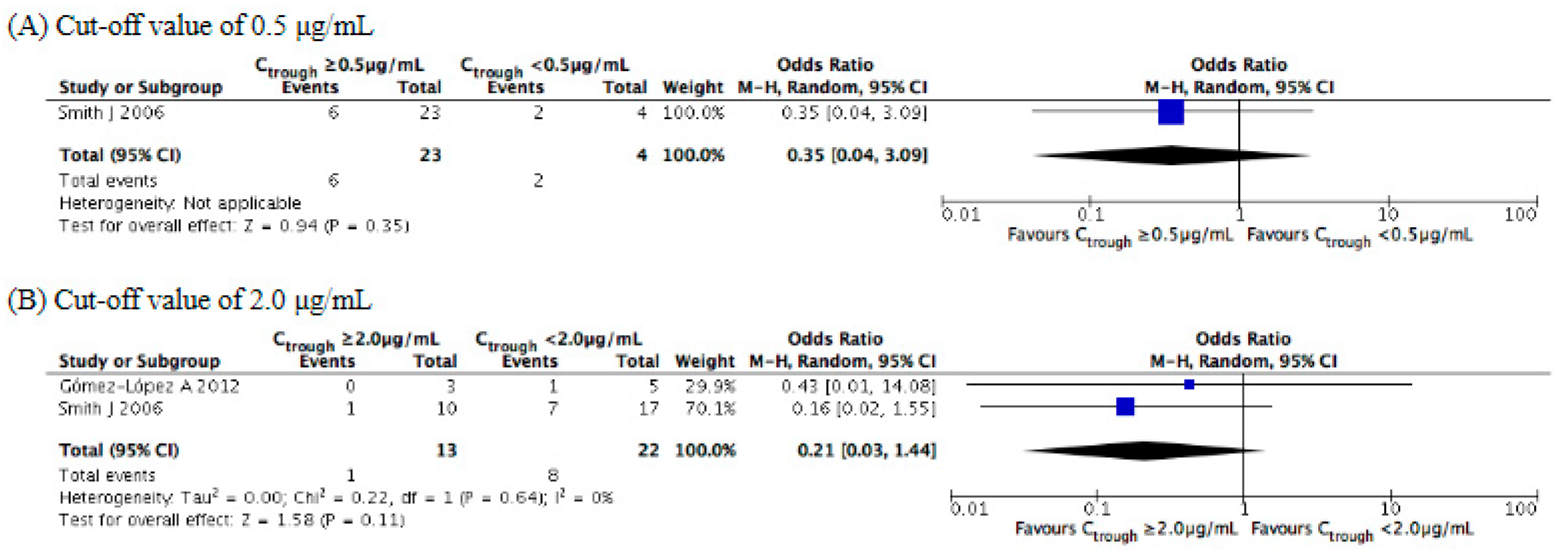
3.3. Evaluation of Efficacy Outcomes
3.4. Evaluation of Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Akova, M.; Herbrecht, R.; Viscoli, C.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 53–67. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, M.; Yan, J.; Gao, L.; Zhou, L.; Wang, Y.; Li, Q.; Wang, J.; Chen, T.; Wang, T.; et al. Voriconazole therapeutic drug monitoring in critically ill patients improves efficacy and safety of antifungal therapy. Basic Clin. Pharmacol. Toxicol. 2020, 127, 495–504. [Google Scholar] [CrossRef]
- Park, W.B.; Kim, N.H.; Kim, K.H.; Lee, S.H.; Nam, W.S.; Yoon, S.H.; Song, K.H.; Choe, P.G.; Kim, N.J.; Jang, I.J.; et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: A randomized controlled trial. Clin. Infect. Dis. 2012, 55, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Ueda, T.; Miyazaki, Y.; Nakajima, K.; Fukunaga, K.; Miyazaki, T.; Nakada-Motokawa, N.; Nagao, M.; Kawamura, H.; Shigemi, A.; et al. Effects of antifungal stewardship using therapeutic drug monitoring in voriconazole therapy on the prevention and control of hepatotoxicity and visual symptoms: A multicentre study conducted in Japan. Mycoses 2020, 63, 779–786. [Google Scholar] [CrossRef]
- Lortholary, O.; Petrikkos, G.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; Cornely, O.A.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Patients with HIV infection or AIDS. Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 68–77. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 19–37. [Google Scholar] [CrossRef]
- Limper, A.H.; Knox, K.S.; Sarosi, G.A.; Ampel, N.M.; Bennett, J.E.; Catanzaro, A.; Davies, S.F.; Dismukes, W.E.; Hage, C.A.; Marr, K.A.; et al. American Thoracic Society Fungal Working Group. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am. J. Respir. Crit. Care Med. 2011, 183, 96–128. [Google Scholar] [CrossRef]
- Hamada, Y.; Tokimatsu, I.; Mikamo, H.; Kimura, M.; Seki, M.; Takakura, S.; Ohmagari, N.; Takahashi, Y.; Kasahara, K.; Matsumoto, K.; et al. Practice guidelines for therapeutic drug monitoring of voriconazole: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 2013, 19, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Seto, Y.; Yago, K.; Kuroyama, M. Investigation and threshold of optimum blood concentration of voriconazole: A descriptive statistical meta-analysis. J. Infect. Chemother. 2012, 18, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Takesue, Y. Optimal trough concentration of voriconazole with therapeutic drug monitoring in children: A systematic review and meta-analysis. J. Infect. Chemother. 2021, 27, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, T.; Falcione, B.A.; Olsen, K.M.; Chen, K.; Tang, H.; Hui, J.; Zhai, S. Trough concentration of voriconazole and its relationship with efficacy and safety: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2016, 71, 1772–1785. [Google Scholar] [CrossRef] [PubMed]
- Luong, M.L.; Al-Dabbagh, M.; Groll, A.H.; Racil, Z.; Nannya, Y.; Mitsani, D.; Husain, S. Utility of voriconazole therapeutic drug monitoring: A meta-analysis. J. Antimicrob. Chemother. 2016, 71, 1786–1799. [Google Scholar] [CrossRef] [PubMed]
- Drago, M.; Scaltrito, M.M.; Morace, G.; GISIA-2 Group. In vitro activity of voriconazole and other antifungal agents against clinical isolates of Candida glabrata and Candida krusei. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Maesaki, S.; Iwakawa, J.; Higashiyama, Y.; Miyazaki, Y.; Yanagihara, K.; Tomono, K.; Tashiro, T.; Kohno, S. Antifungal activity of a new triazole, voriconazole (UK-109496), against clinical isolates of Aspergillus spp. J. Infect. Chemother. 2000, 6, 101–103. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Ihle, F.; Derendorf, H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 2006, 45, 649–663. [Google Scholar] [CrossRef]
- Posteraro, B.; Spanu, T.; Fiori, B.; De Maio, F.; De Carolis, E.; Giaquinto, A.; Prete, V.; De Angelis, G.; Torelli, R.; D’Inzeo, T.; et al. Antifungal susceptibility profiles of bloodstream yeast isolates by Sensititre YeastOne over nine years at a large Italian teaching hospital. Antimicrob. Agents Chemother. 2015, 59, 3944–3955. [Google Scholar] [CrossRef] [PubMed]
- Trifilio, S.; Singhal, S.; Williams, S.; Frankfurt, O.; Gordon, L.; Evens, A.; Winter, J.; Tallman, M.; Pi, J.; Mehta, J. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant. 2007, 40, 451–456. [Google Scholar] [CrossRef]
- Ueda, K.; Nannya, Y.; Kumano, K.; Hangaishi, A.; Takahashi, T.; Imai, Y.; Kurokawa, M. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int. J. Hematol. 2009, 89, 592–599. [Google Scholar] [CrossRef]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.J.; Sheen, S.S.; Hahn, S.; Jang, B.H.; Son, H.J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- Shimizu, T.; Ochiai, H.; Asell, F.; Shimizu, H.; Saitoh, R.; Hama, Y.; Katada, J.; Hashimoto, M.; Matsui, H.; Taki, K.; et al. Bioinformatics research on inter-racial difference in drug metabolism I. Analysis on frequencies of mutant alleles and poor metabolizers on CYP2D6 and CYP2C19. Drug Metab. Pharmacokinet. 2003, 18, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions Version 6. Available online: http://training.cochrane.org/handbook (accessed on 30 January 2021).
- Smith, J.; Safdar, N.; Knasinski, V.; Simmons, W.; Bhavnani, S.M.; Ambrose, P.G.; Andes, D. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 2006, 50, 1570–1572. [Google Scholar] [CrossRef]
- Pascual, A.; Calandra, T.; Bolay, S.; Buclin, T.; Bille, J.; Marchetti, O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 2008, 46, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Troke, P.F.; Hockey, H.P.; Hope, W.W. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob. Agents Chemother. 2011, 55, 4782–4788. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, A.; Cendejas-Bueno, E.; Cuesta, I.; García Rodríguez, J.; Rodríguez-Tudela, J.L.; Gutiérrez-Altés, A.; Cuenca-Estrella, M. Voriconazole serum levels measured by high-performance liquid chromatography: A monocentric study in treated patients. Med. Mycol. 2012, 50, 439–445. [Google Scholar] [CrossRef]
- Racil, Z.; Winterova, J.; Kouba, M.; Zak, P.; Malaskova, L.; Buresova, L.; Toskova, M.; Lengerova, M.; Kocmanova, I.; Weinbergerova, B.; et al. Monitoring trough voriconazole plasma concentrations in haematological patients: Real life multicentre experience. Mycoses 2012, 55, 483–492. [Google Scholar] [CrossRef]
- Chu, H.Y.; Jain, R.; Xie, H.; Pottinger, P.; Fredricks, D.N. Voriconazole therapeutic drug monitoring: Retrospective cohort study of the relationship to clinical outcomes and adverse events. BMC Infect. Dis. 2013, 13, 105. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Chun, S.; Kim, D.Y.; Lee, J.H.; Lee, J.H.; Lee, K.H.; et al. Initial voriconazole trough blood levels and clinical outcomes of invasive aspergillosis in patients with hematologic malignancies. Med. Mycol. 2013, 51, 324–330. [Google Scholar] [CrossRef][Green Version]
- Cabral-Galeano, E.; Ruiz-Camps, I.; Len-Abad, O.; Pou-Clavé, L.; Sordé-Masip, R.; Meije-Castillo, Y.; Blanco-Grau, A.; Barba-Suñol, P.; Monforte-Torres, V.; Román-Broto, A.; et al. Clinical usefulness of therapeutic drug monitoring of voriconazole in a university hospital. Enferm. Infecc. Microbiol. Clin. 2015, 33, 298–302. [Google Scholar] [CrossRef]
- Sebaaly, J.C.; MacVane, S.H.; Hassig, T.B. Voriconazole concentration monitoring at an academic medical center. Am. J. Health. Syst. Pharm. 2016, 73, S14–S21. [Google Scholar] [CrossRef]
- Ruiz, J.; Gordon, M.; Villarreal, E.; Peruccioni, M.; Marqués, M.R.; Poveda-Andrés, J.L.; Castellanos-Ortega, Á.; Ramirez, P. Impact of voriconazole plasma concentrations on treatment response in critically ill patients. J. Clin. Pharm. Ther. 2019, 44, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Imhof, A.; Schaer, D.J.; Schanz, U.; Schwarz, U. Neurological adverse events to voriconazole: Evidence for therapeutic drug monitoring. Swiss Med. Wkly. 2006, 136, 739–742. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ikawa, K.; Abematsu, K.; Fukunaga, N.; Nishida, K.; Fukamizu, T.; Shimodozono, Y.; Morikawa, N.; Takeda, Y.; Yamada, K. Correlation between voriconazole trough plasma concentration and hepatotoxicity in patients with different CYP2C19 genotypes. Int. J. Antimicrob. Agents 2009, 34, 91–94. [Google Scholar] [CrossRef]
- Kim, S.H.; Yim, D.S.; Choi, S.M.; Kwon, J.C.; Han, S.; Lee, D.G.; Park, C.; Kwon, E.Y.; Park, S.H.; Choi, J.H.; et al. Voriconazole-related severe adverse events: Clinical application of therapeutic drug monitoring in Korean patients. Int. J. Infect. Dis. 2011, 15, e753–e758. [Google Scholar] [CrossRef]
- Dolton, M.J.; Ray, J.E.; Chen, S.C.; Ng, K.; Pont, L.G.; McLachlan, A.J. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob. Agents Chemother. 2012, 56, 4793–4799. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tokimatsu, I.; Sato, Y.; Kawasaki, K.; Sato, Y.; Goto, T.; Hashinaga, K.; Itoh, H.; Hiramatsu, K.; Kadota, J. Association of sustained high plasma trough concentration of voriconazole with the incidence of hepatotoxicity. Clin. Chim. Acta 2013, 424, 119–122. [Google Scholar] [CrossRef]
- Matsumoto, K.; Abematsu, K.; Shigemi, A.; Kanazawa, N.; Watanabe, E.; Yokoyama, Y.; Ikawa, K.; Morikawa, N.; Takeda, Y. Therapeutic drug monitoring of voriconazole in Japanese patients: Analysis based on clinical practice data. J. Chemother. 2016, 28, 198–202. [Google Scholar] [CrossRef]
- Wang, T.; Yan, M.; Tang, D.; Xue, L.; Zhang, T.; Dong, Y.; Zhu, L.; Wang, X.; Dong, Y. A retrospective, multicenter study of voriconazole trough concentrations and safety in patients with Child-Pugh class C cirrhosis. J. Clin. Pharm. Ther. 2018, 43, 849–854. [Google Scholar] [CrossRef]
- Hirata, A.; Noto, K.; Ota, R.; Yokoyama, S.; Hosomi, K.; Takada, M.; Matsuoka, H. Voriconazole trough concentration and hepatotoxicity in patients with low serum albumin. Int. J. Clin. Pharmacol. Ther. 2019, 57, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Ribaud, P.; Milpied, N.; Caillot, D.; Herbrecht, R.; Thiel, E.; Haas, A.; Ruhnke, M.; Lode, H. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 2002, 34, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Okuda, A.; Watanabe, N.; Takao, M.; Takayanagi, K. Retrospective serological tests for determining the optimal blood concentration of voriconazole for treating fungal infection. Yakugaku Zasshi 2008, 128, 1811–1818. [Google Scholar] [CrossRef]
- Hagiwara, E.; Shiihara, J.; Matsushima, A.; Enomoto, T.; Tagawa, A.; Sekine, A.; Tsuchiya, N.; Baba, T.; Shinohara, T.; Endo, T.; et al. Usefulness of monitoring plasma voriconazole concentration in patients with chronic necrotizing pulmonary aspergillosis. Nihon Kokyuki Gakkai Zasshi 2009, 47, 93–97. [Google Scholar]
- Wang, T.; Zhu, H.; Sun, J.; Cheng, X.; Xie, J.; Dong, H.; Chen, L.; Wang, X.; Xing, J.; Dong, Y. Efficacy and safety of voriconazole and CYP2C19 polymorphism for optimised dosage regimens in patients with invasive fungal infections. Int. J. Antimicrob. Agents 2014, 44, 436–442. [Google Scholar] [CrossRef]
- Hashemizadeh, Z.; Badiee, P.; Malekhoseini, S.A.; Raeisi Shahraki, H.; Geramizadeh, B.; Montaseri, H. Observational study of associations between voriconazole therapeutic drug monitoring, toxicity, and outcome in liver transplant patients. Antimicrob. Agents Chemother. 2017, 61, e01211-17. [Google Scholar] [CrossRef] [PubMed]
- Mitsani, D.; Nguyen, M.H.; Shields, R.K.; Toyoda, Y.; Kwak, E.J.; Silveira, F.P.; Pilewski, J.M.; Crespo, M.M.; Bermudez, C.; Bhama, J.K.; et al. Prospective, observational study of voriconazole therapeutic drug monitoring among lung transplant recipients receiving prophylaxis: Factors impacting levels of and associations between serum troughs, efficacy, and toxicity. Antimicrob. Agents Chemother. 2012, 56, 2371–2377. [Google Scholar] [CrossRef]
- Yanni, S.B.; Annaert, P.P.; Augustijns, P.; Ibrahim, J.G.; Benjamin, D.K., Jr.; Thakker, D.R. In vitro hepatic metabolism explains higher clearance of voriconazole in children versus adults: Role of CYP2C19 and flavin-containing monooxygenase 3. Drug Metab. Dispos. 2010, 38, 25–31. [Google Scholar] [CrossRef]
- Michael, C.; Bierbach, U.; Frenzel, K.; Lange, T.; Basara, N.; Niederwieser, D.; Mauz-Körholz, C.; Preiss, R. Voriconazole pharmacokinetics and safety in immunocompromised children compared to adult patients. Antimicrob. Agents Chemother. 2010, 54, 3225–3232. [Google Scholar] [CrossRef]
- Karlsson, M.O.; Lutsar, I.; Milligan, P.A. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob. Agents Chemother. 2009, 53, 935–944. [Google Scholar] [CrossRef]
- Driscoll, T.A.; Yu, L.C.; Frangoul, H.; Krance, R.A.; Nemecek, E.; Blumer, J.; Arrieta, A.; Graham, M.L.; Bradfield, S.M.; Baruch, A.; et al. Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised children and healthy adults. Antimicrob. Agents Chemother. 2011, 55, 5770–5779. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Ten Hoevel, M.M.; Burhenne, J.; Walter-Sack, I.; Hoffmann, M.M.; Rengelshausen, J.; Haefeli, W.E.; Mikus, G. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J. Clin. Pharmacol. 2009, 49, 196–204. [Google Scholar] [CrossRef] [PubMed]

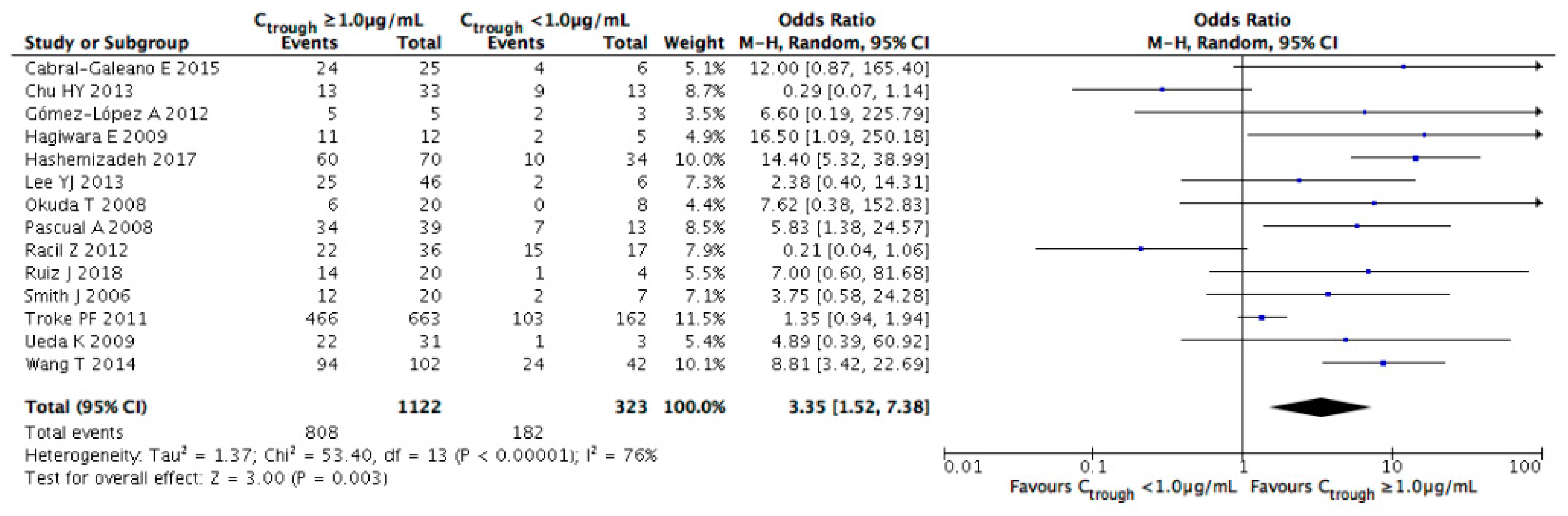
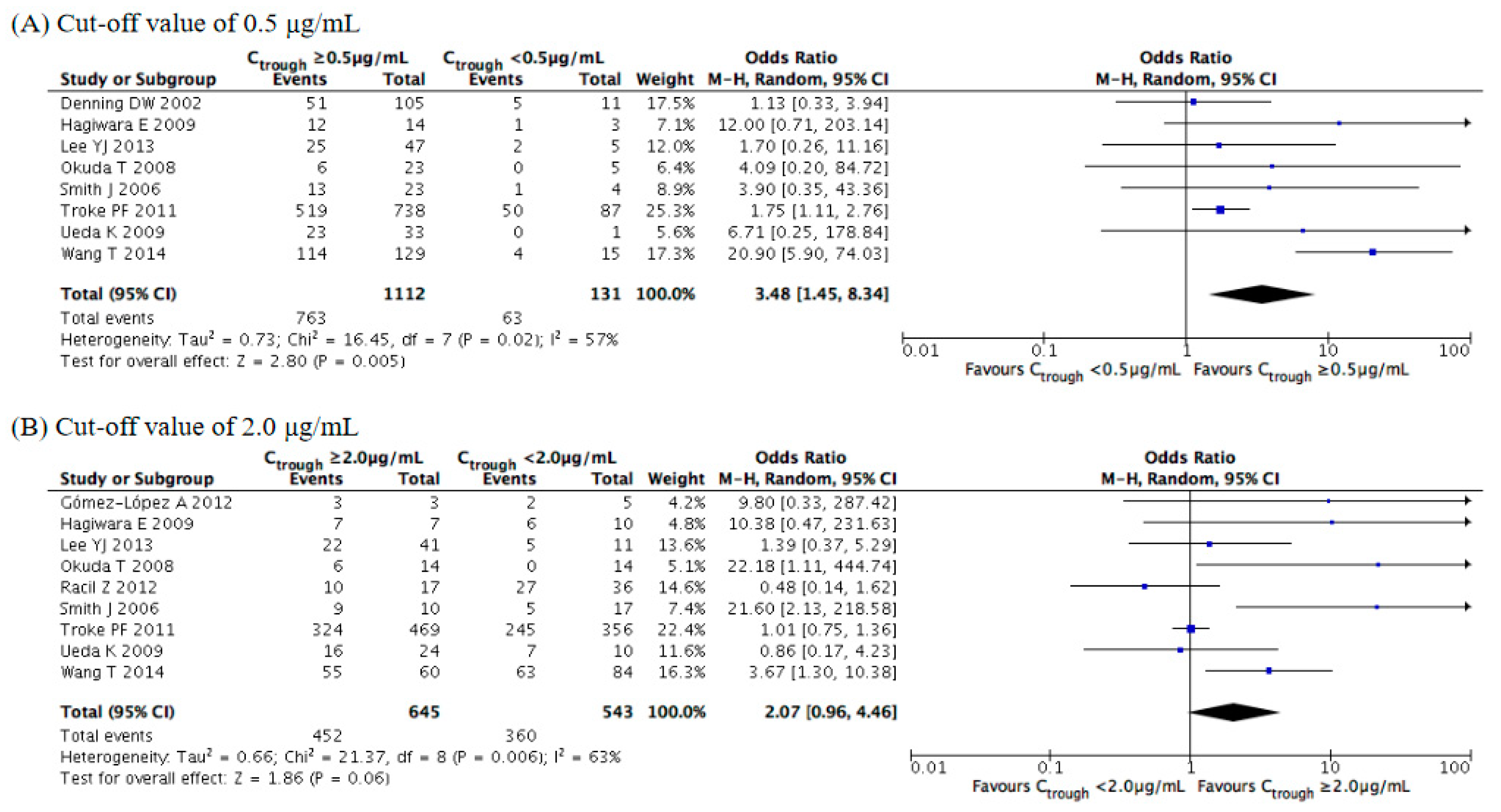
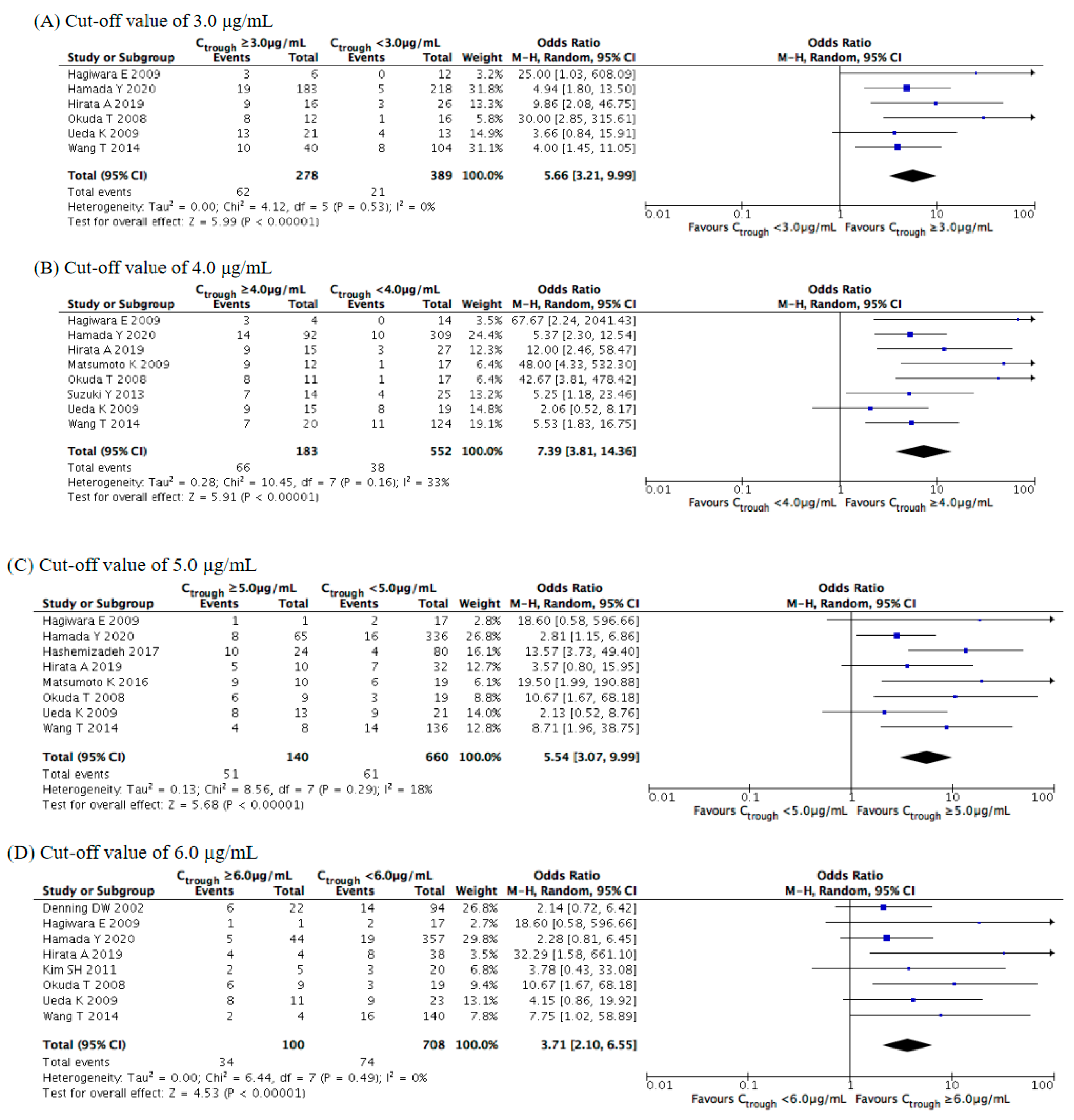
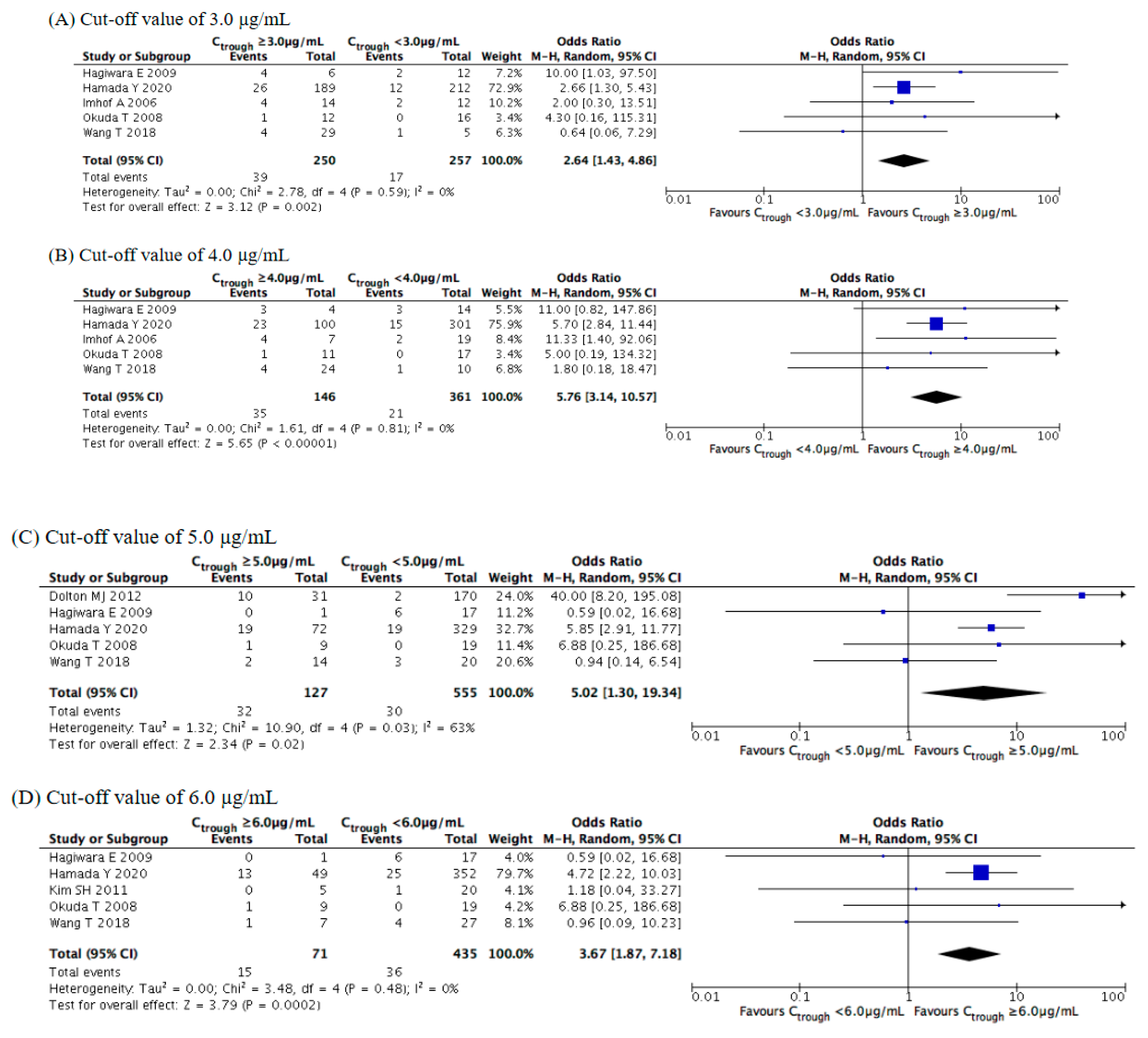
| Study | Year | Study Location | Study Design | Number of Cases | Age (Years) | Main Underlying Disease (%) | Indication of Therapy | Diagnosis of Fungal Infection (%) | Fungal Organisms (%) | Duration of Therapy (Days) | Concomitant Antifungals |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Denning DW | 2002 | UK | MCP | 116 | median: 52 range: 18–79 | haematological disorder (58) allogeneic HSCT (20) | targeted | proven (41) probable (59) | Aspergillus (100) | NR | yes |
| Smith J a | 2006 | USA | SCR | 27 | median: 40 range: 16–70 | solid organ transplantation (59) bone marrow transplantation (19) | targeted | proven or probable (100) | Aspergillus (85) Candida (4) | NR | yes |
| Imhof A | 2006 | Switzerland | SCR | 26 | median: 47.5 range: 22–61 | acute myeloid leukaemia (89) | targeted | proven (27) probable (19) possible (54) | Aspergillus or Candida (100) | NR | no |
| Pascual A | 2008 | Switzerland | SCP | 52 | median: 58.5 range: 23–78 | haematological malignancy (61) solid organ transplantation (6) | targeted | proven or probable (69) possible (21) | Aspergillus (50) Candida (15) | median: 50 range: 4–1130 | no |
| Okuda T b | 2008 | Japan | SCR | 23 | median: 64 range: 18–85 | myelodysplastic syndrome (35) acute myeloid leukaemia (17) acute lymphatic leukaemia (9) | targeted | proven or probable (65) | Aspergillus (48) | NR | yes |
| Ueda K | 2009 | Japan | SCR | 34 | median: 57.5 range: 19–81 | acute myeloid leukaemia (56) non-hodgkin lymphoma (24) | targeted | proven (2) probable (15) possible (83) | NR | NR | no |
| Hagiwara E c | 2009 | Japan | SCR | 18 | median: 67 range: 53–80 | respiratory disease (100) | targeted | proven (33) probable or possible (67) | Aspergillus (33) | NR | no |
| Matsumoto K | 2009 | Japan | SCR | 29 | mean: 57.3 SD: ±19.3 | NR | targeted | NR | NR | NR | no |
| Troke PF | 2011 | UK | MCR | 825 | median: 44 | haematological malignancy | targeted | NR | NR | NR | no |
| Kim SH | 2011 | Korea | SCP | 25 | median: 45 IQR: 38–54 | acute myeloid leukaemia (56) acute lymphatic leukaemia (24) | targeted | NR | NR | median: 8 IQR: 7–14 | no |
| Gómez-López A d | 2012 | Spain | SCR | 8 | median: 70 range: 17–75 | haematological malignancy (63) solid organ transplantation (13) | targeted | proven (37) probable (63) | Aspergillus (63) | median: 33 range: 9–243 | yes |
| Racil Z e | 2012 | Czech Republic | MCR | 53 | range: 18–77 | NR | targeted | proven (21) probable (79) | Aspergillus (100) | median: 32 range: 5–160 | yes |
| Dolton MJ | 2012 | Australia | SCR | 201 | median: 54 range: 18–88 | haematological malignancy (59) solid organ transplantation (13) | targeted or prophylactic | proven (22) probable (11) possible (29) | Aspergillus (19) Candida (5) | NR | no |
| Chu HY | 2013 | USA | SCR | 108 | median: 53 IQR: 38–64 | HSCT (44) haematological malignancy (34) solid organ transplantation (9) | targeted | proven (7) probable (36) possible (40) | Aspergillus (81) Candida (8) | median: 35 range: 13–92 | yes |
| Lee YJ | 2013 | Korea | SCR | 52 | range: 16–81 | acute myeloid leukaemia (60) acute lymphatic leukaemia (13) myelodysplastic syndrome (10) | targeted | proven (4) probable (56) possible (40) | Aspergillus (100) | range: 23–131 | no |
| Suzuki Y | 2013 | Japan | SCR | 39 | mean: 55.9 SD: ±19.5 | NR | NR | NR | NR | mean: 58.4 SD: ±32.0 | no |
| Wang T | 2014 | China | SCR | 144 | median: 60.6 range: 18–99 | bronchitis (24) liver diseae (22) asthma (19) haematological malignancy (15) solid organ transplantation (7) | targeted | proven (61) probable (39) | Aspergillus (62) Candida (32) | median: 35 range: 16–81 | no |
| Cabral-Galeano E f | 2015 | Spain | SCR | 52 | median: 55 IQR: 37.5–60.7 | lung transplantation (48) haematological disorder (27) cystic fibrosis (10) | targeted or prophylactic | proven (90) | NR | median: 8 IQR: 3–14 | yes |
| Sebaaly JC g | 2016 | USA | SCR | 88 | mean: 52.7 SD: ±14.8 | haematological malignancy (40) solid organ transplantation (23) stem cell transplantation (17) pulmonary disease (15) | targeted | NR | NR | median: 18 IQR: 11–26 | no |
| Matsumoto K | 2016 | Japan | SCR | 29 | mean: 58.6 range: 16–77 | haematological malignancy (52) solid tumour (21) respiratory disease (14) | NR | proven (59) probable or possible (21) | Aspergillus (59) Candida (14) | median: 22 range: 6–171 | no |
| Hashemizadeh Z h | 2017 | Iran | SCP | 104 | mean: 36 range: 18–62 | solid organ transplantation (100) | targeted | proven (8) probable (40) possible (52) | Aspergillus (40) Candida (8) | mean: 54 range: 29–98 | no |
| Ruiz J | 2018 | Spain | SCP | 24 | mean: 55.3 SD: ±12.6 | NR | targeted | proven or probable (75) possible (25) | Aspergillus (100) | NR | yes |
| Wang T | 2018 | China | MCR | 34 | range: 22–82 | NR | NR | NR | NR | NR | no |
| Hirata A | 2019 | Japan | SCR | 42 | mean: 61.9 SD: ±16.9 | haematological malignancy (90) | targeted or prophylactic | proven (40) probable or possible (33) | Aspergillus (36) Candida (5) | NR | no |
| Hamada Y i | 2020 | Japan | MCR | 401 | mean: 61.8 range: 18–91 | haematological malignancy (39) collagen disease (22) solid organ malignancy (10) | targeted or prophylactic | proven (25) probable or possible (52) | Aspergillus (9) Candida (11) | median: 30 IQR: 14–117 | yes |
| Scheme | Year | Reported Outcome | Definition of Treatment Success | Definition of Hepatotoxicity | Definition of Neurotoxicity |
|---|---|---|---|---|---|
| Denning DW | 2002 | treatment success hepatotoxicity | complete, partial or stable response based on clinical and radiological evidence | AST or ALT > 5 times ULN, ALP > 3 times ULN, TBIL > 3 times ULN | - |
| Smith J | 2006 | treatment success all-cause mortality | absence of progression of lesions on follow-up imaging | - | - |
| Imhof A | 2006 | neurotoxicity | - | - | neuropathy, hallucinations, confusion, anxiety, asthenia, visual disturbance, dysarthria or insomnia |
| Pascual A | 2008 | treatment success | absence of persistence or progression of fungal infection (based on clinical and radiological evidence) and proven or persumed eradication of the fungal pathogen | - | - |
| Okuda T | 2008 | treatment success hepatotoxicity neurotoxicity | β-D-glucan or Aspergillus antigen decrease below standard at follow-up | any deviation in AST or ALT from the normal range | hallucination |
| Ueda K | 2009 | treatment success hepatotoxicity | absence of at least 2 of the following 3 types of criteria: clinical (development of new fever or persistent fever), radiologic (>25% expansion of abnormal shadow area in CT image), or mycological (a rise within the abnormal range or positive conversion of serum markers (either β-D-glucan or galactomannan)) failure | AST, ALT, GGT or TBIL was in gredes 2–4 according to NCI criteria | - |
| Hagiwara E | 2009 | treatment success hepatotoxicity neurotoxicity | complete or partial response based on clinical and radiologic evidence | liver function test > 3 times ULN | any visual symptoms |
| Matsumoto K | 2009 | hepatotoxicity | - | AST, ALT, ALP, GGT or TBIL was in gredes 1–4 according to NCI criteria | - |
| Troke PF | 2011 | treatment success | complete or partial response based on EORTC/MSG criteria | - | - |
| Kim SH | 2011 | hepatotoxicity neurotoxicity | - | NCI, grades 3–5 was referred to as severe adverse events | NCI, grade 3–5 |
| Gómez-López A | 2012 | treatment success all-cause mortality | complete or partial response based on EORTC/MSG criteria | - | - |
| Racil Z | 2012 | treatment success | complete or partial response based on EORTC/MSG criteria | - | - |
| Dolton MJ | 2012 | neurotoxicity | - | - | visual/auditory hallucinations |
| Chu HY | 2013 | treatment success | complete or partial response based on clinical, radiologic and microbiologic evidence | - | - |
| Lee YJ | 2013 | treatment success | complete or partial response based on EORTC/MSG criteria | - | - |
| Suzuki Y | 2013 | hepatotoxicity | - | AST, ALT, ALP, GGT or TBIL was in gredes 2–4 according to NCI criteria | - |
| Wang T | 2014 | treatment success hepatotoxicity | absence of persistence or progression of fungal infection (based on clinical and radiological evidence) and proven or persumed eradication of the fungal pathogen | AST, ALT, ALP or TBIL was in gredes 3–4 according to CTCAE criteria | - |
| Cabral-Galeano E | 2015 | treatment success | absence of persistence or progression of fungal infection based on clinical and radiological evidence | - | - |
| Sebaaly JC | 2016 | all-cause mortality | - | - | - |
| Matsumoto K | 2016 | hepatotoxicity | - | AST, ALT, ALP, GGT or TBIL was in gredes 1–4 according to CTCAE criteria | - |
| Hashemizadeh Z | 2017 | treatment success all-cause mortality hepatotoxicity | complete or partial response based on EORTC/MSG criteria | AST, ALT, ALP or TBIL was in gredes 2–4 according to CTCAE criteria | - |
| Ruiz J | 2018 | treatment success | complete or partial response based on clinical and radiologic evidence | - | - |
| Wang T | 2018 | neurotoxicity | - | - | dizziness, tremor, hallucinations, encephalopathy, consciousness disturbance |
| Hirata A | 2019 | hepatotoxicity | - | AST, ALT, ALP or TBIL was in gredes 2–4 according to CTCAE criteria | - |
| Hamada Y | 2020 | hepatotoxicity neurotoxicity | - | AST or ALT > 3 times ULN or >3 times the baseline if AST or ALT baseline was abnormal | any visual symptoms (colour perception, blurred vision, bright spots, wavy lines and photophobia) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Miyazaki, Y.; Takesue, Y. Favorable Effects of Voriconazole Trough Concentrations Exceeding 1 μg/mL on Treatment Success and All-Cause Mortality: A Systematic Review and Meta-Analysis. J. Fungi 2021, 7, 306. https://doi.org/10.3390/jof7040306
Hanai Y, Hamada Y, Kimura T, Matsumoto K, Takahashi Y, Fujii S, Nishizawa K, Miyazaki Y, Takesue Y. Favorable Effects of Voriconazole Trough Concentrations Exceeding 1 μg/mL on Treatment Success and All-Cause Mortality: A Systematic Review and Meta-Analysis. Journal of Fungi. 2021; 7(4):306. https://doi.org/10.3390/jof7040306
Chicago/Turabian StyleHanai, Yuki, Yukihiro Hamada, Toshimi Kimura, Kazuaki Matsumoto, Yoshiko Takahashi, Satoshi Fujii, Kenji Nishizawa, Yoshitsugu Miyazaki, and Yoshio Takesue. 2021. "Favorable Effects of Voriconazole Trough Concentrations Exceeding 1 μg/mL on Treatment Success and All-Cause Mortality: A Systematic Review and Meta-Analysis" Journal of Fungi 7, no. 4: 306. https://doi.org/10.3390/jof7040306
APA StyleHanai, Y., Hamada, Y., Kimura, T., Matsumoto, K., Takahashi, Y., Fujii, S., Nishizawa, K., Miyazaki, Y., & Takesue, Y. (2021). Favorable Effects of Voriconazole Trough Concentrations Exceeding 1 μg/mL on Treatment Success and All-Cause Mortality: A Systematic Review and Meta-Analysis. Journal of Fungi, 7(4), 306. https://doi.org/10.3390/jof7040306







