Stimulatory Effects of Oleci Acid and Fungal Elicitor on Betulinic Acid Production by Submerged Cultivation of Medicinal Mushroom Inonotus obliquus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Microorganism and Culture Media
2.3. Microorganism and Culture Media
2.4. Preparation of Standard Solutions and Elicitors
2.5. Assay of Mycelia Biomass and the Isolation of BA
2.6. Reverse-Phase HPLC (RP-HPLC) and HPLC-MS Analysis of BA
2.7. Real-Time Fluorescence Quantitative PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Comparison of Different Exogenous Inducing Factors
3.2. The Identification of BA from I. obliquus
3.3. Determination of Optimal Adding Concentration of Oleic Acid, Fungal Elicitors
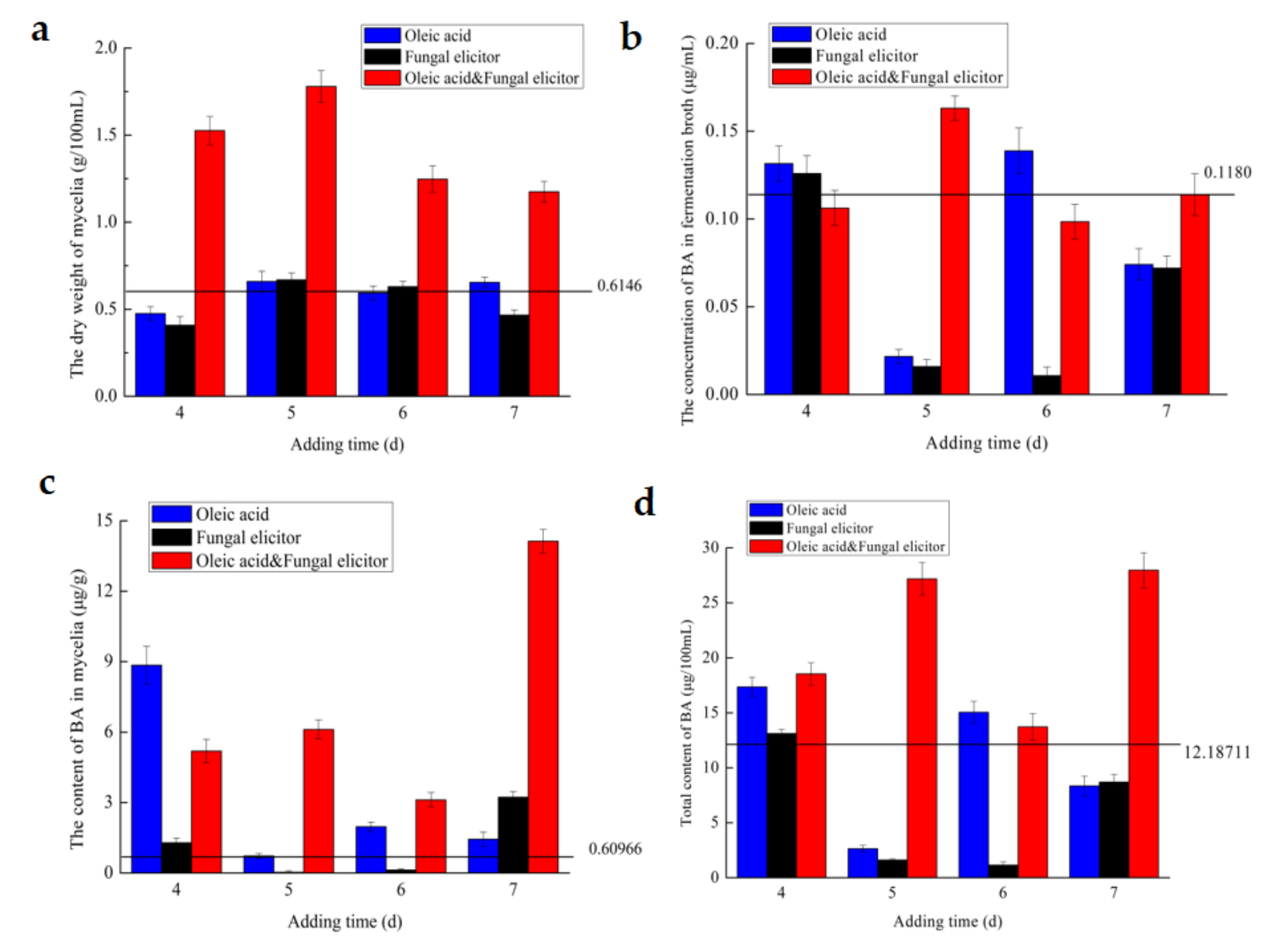
3.4. Effect of Addition Time
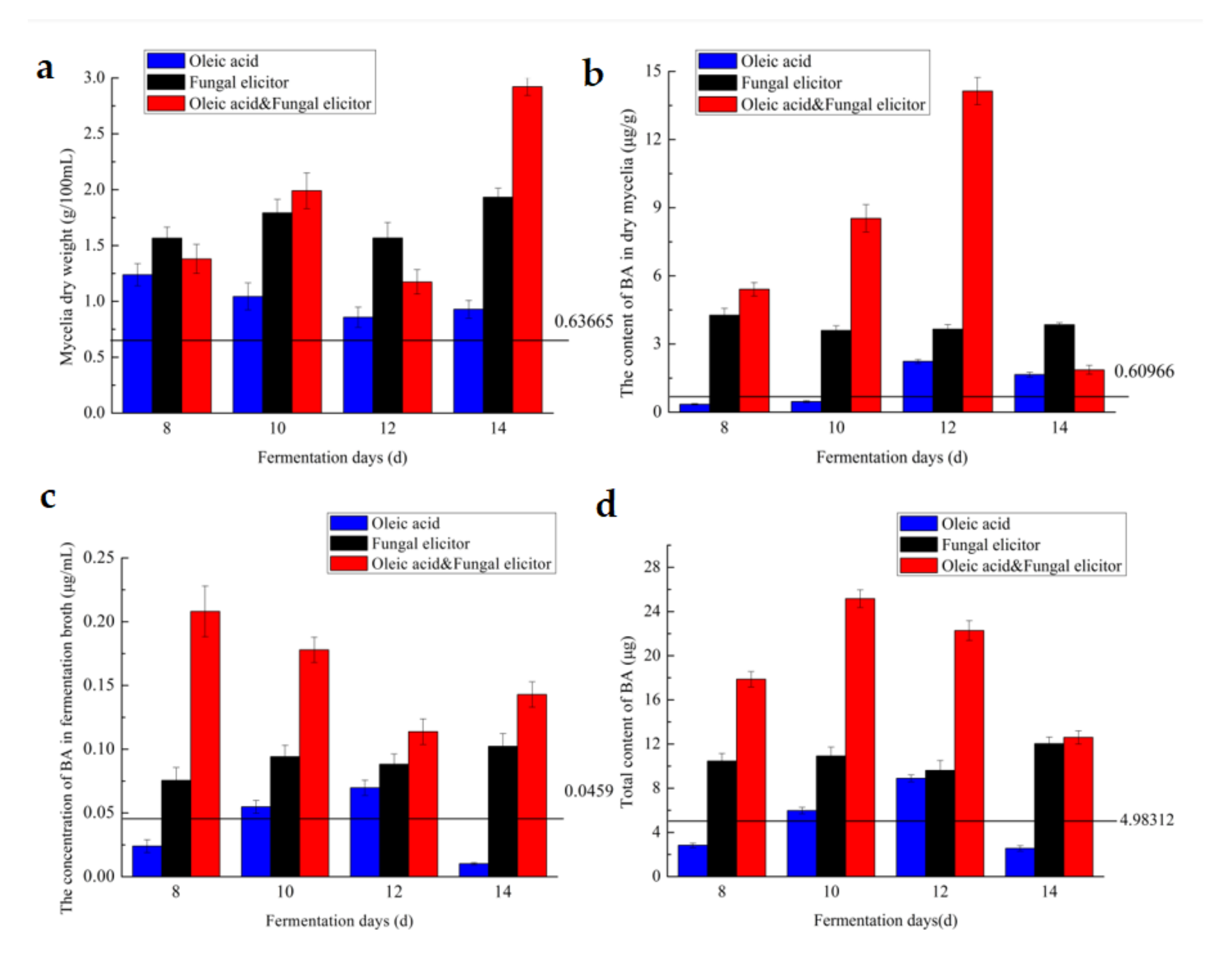
3.5. Effect of Culture Age
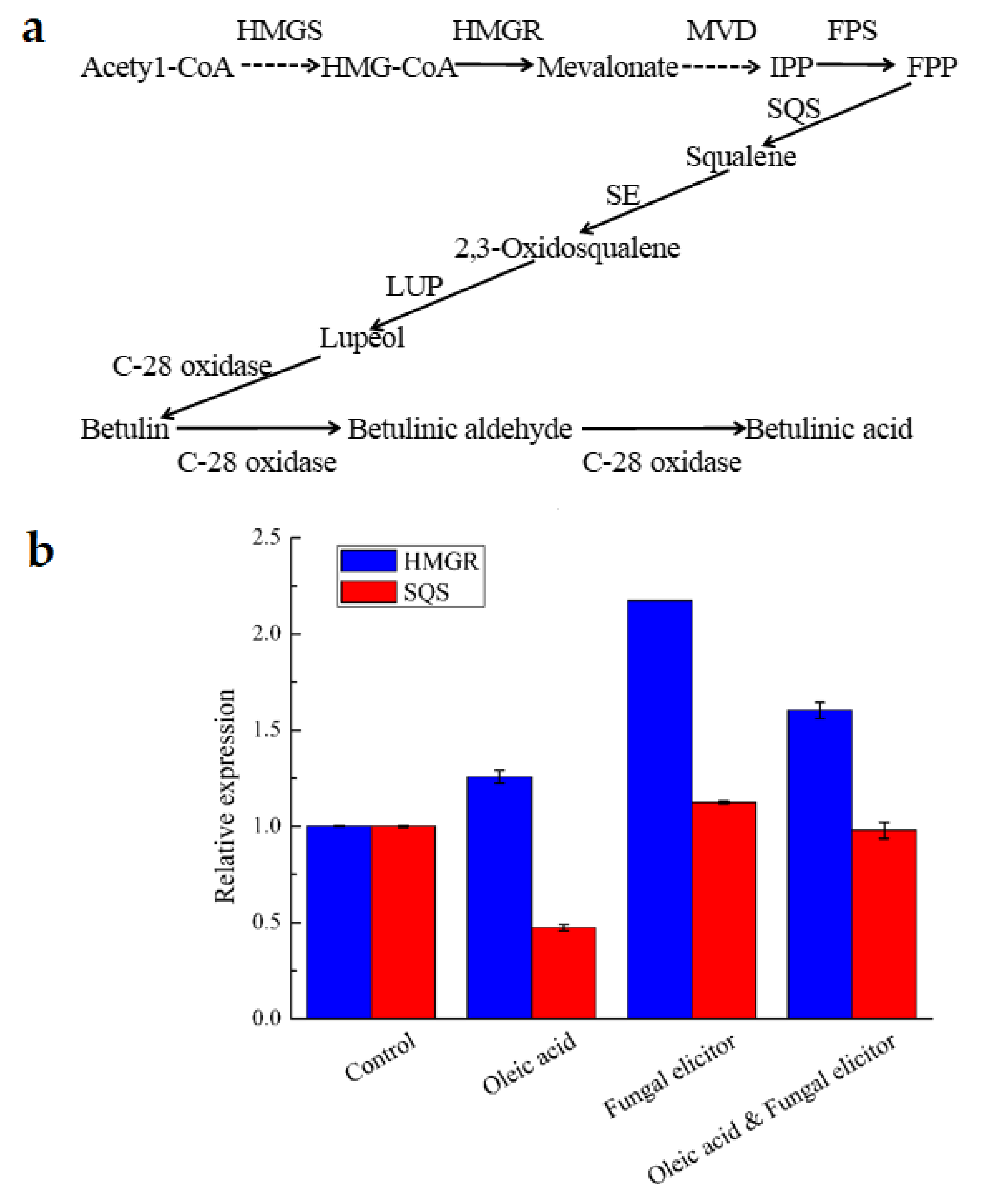
3.6. Transcriptional Responses to Different Stimulators
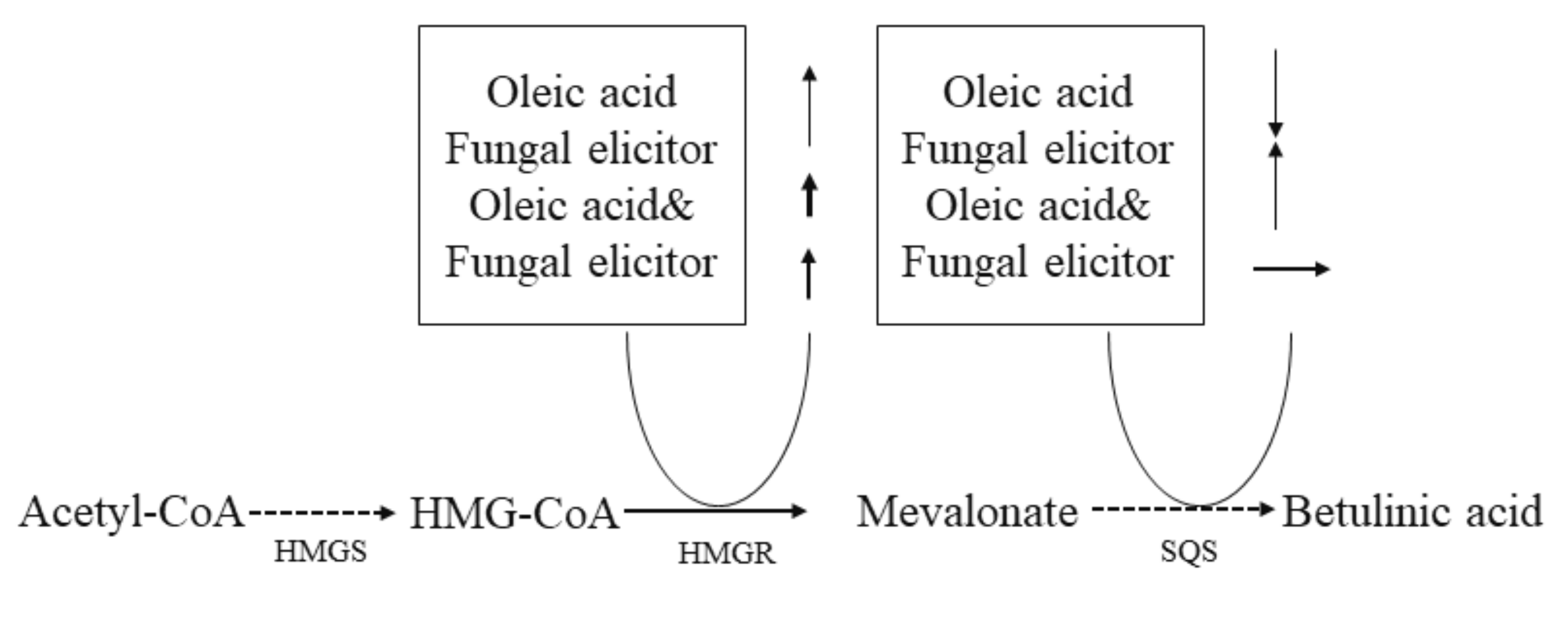
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thurnher, D.; Turhani, D.; Pelzmann, M.; Wannemacher, B.; Knerer, B.; Formanek, M.; Wacheck, V.; Selzer, E. Betulinic acid: A new cytotoxic compound against malignant head and neck cancer cells. Head Neck J. Sci. Spec. Head Neck 2003, 25, 732–740. [Google Scholar] [CrossRef]
- Zheng, W.; Miao, K.; Liu, Y.; Zhao, Y.; Zhang, M.; Pan, S.; Dai, Y. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 2010, 87, 1237–1254. [Google Scholar] [CrossRef]
- Kim, J.; Yang, S.C.; Hwang, A.Y.; Cho, H.; Hwang, K.T. Composition of Triterpenoids in Inonotus obliquus and Their Anti-Proliferative Activity on Cancer Cell Lines. Molecules 2020, 25, 4066. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Tan, Y.; Chen, H.; Mei, R.; Dong, X.; Wu, B. Triterpenoids of Inonotus obliquus. Chin. Tradit. Herb. Drugs 2015, 46, 2355–2360. [Google Scholar]
- Xu, X.; Shen, M.; Quan, L. Stimulatory Agents Simultaneously Improving the Production and Antioxidant Activity of Polyphenols from Inonotus obliquus by Submerged Fermentation. Appl. Biochem. Biotechnol. 2015, 176, 1237–1250. [Google Scholar] [CrossRef]
- Zheng, W.; Miao, K.; Zhang, Y.; Pan, S.; Zhang, M.; Jiang, H. Nitric oxide mediates the fungal-elicitor-enhanced biosynthesis of antioxidant polyphenols in submerged cultures of Inonotus obliquus. Microbiology (Reading) 2009, 155, 3440–3448. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Chen, C. Stimulated production of triterpenoids of Inonotus obliquus using methyl jasmonate and fatty acids. Ind. Crop. Prod. 2016, 85, 49–57. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Zhou, X.; Hu, J.; Xue, J.; Liu, X.; Zhang, J.; Liu, P.; Tong, S. Simultaneous Use of Stimulatory Agents to Enhance the Production and Hypoglycaemic Activity of Polysaccharides from Inonotus obliquus by Submerged Fermentation. Molecules 2019, 24, 4400. [Google Scholar] [CrossRef]
- Yang, F.C.; Ke, Y.F.; Kuo, S.S. Effect of fatty acids on the mycelial growth and polysaccharide formation by Ganoderma lucidum in shake flask cultures. Enzyme Microb. Technol. 2000, 27, 295–301. [Google Scholar] [CrossRef]
- Yang, H.; Min, W.; Bi, P.; Zhou, H.; Huang, F. Stimulatory effects of Coix lacryma-jobi oil on the mycelial growth and metabolites biosynthesis by the submerged culture of Ganoderma lucidum. Biochem. Eng. J. 2013, 76, 77–82. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Liu, J.; Zhang, H.-F.; He, G.-Q.; Fu, M.-L. The betulinic acid production from betulin through biotransformation by fungi. Enzyme Microb. Technol. 2009, 45, 175–180. [Google Scholar] [CrossRef]
- Wu, J.; Niu, Y.; Bakur, A.; Li, H.; Chen, Q. Cell-Free Production of Pentacyclic Triterpenoid Compound Betulinic Acid from Betulin by the Engineered Saccharomyces cerevisiae. Molecules 2017, 22, 1075. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Dubey, K.K. An efficient process for the transformation of betulin to betulinic acid by a strain of Bacillus megaterium. 3 Biotech 2017, 7, 157. [Google Scholar] [CrossRef][Green Version]
- Zhu, L.-W.; Zhong, J.-J.; Tang, Y.-J. Significance of fungal elicitors on the production of ganoderic acid and Ganoderma polysaccharides by the submerged culture of medicinal mushroom Ganoderma lucidum. Process Biochem. 2008, 43, 1359–1370. [Google Scholar] [CrossRef]
- Wang, W.J.; Yu, L.J.; Zhou, P.P. Effects of different fungal elicitors on growth, total carotenoids and astaxanthin formation by Xanthophyllomyces dendrorhous. Bioresour. Technol. 2006, 97, 26–31. [Google Scholar] [CrossRef]
- Song, Z.; Ma, Z.; Bechthold, A.; Yu, X. Effects of addition of elicitors on rimocidin biosynthesis in Streptomyces rimosus M527. Appl. Microbiol. Biotechnol. 2020, 104, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Nieto, M.; Millán-Gómez, D.V.; Caro, M.; Galindo, E.; Serrano-Carreón, L. Elicitation and biotransformation of 6-pentyl-α-pyrone in Trichoderma atroviride cultures. Process Biochem. 2019, 82, 68–74. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, X.; Li, C.; Zheng, Z.; Yong, S.; Jiang, J. Cloning and Characterization of a Squalene Synthase Gene from the Chaga Medicinal Mushroom, Inonotus obliquus (Agaricomycetes). Int. J. Med. Mushrooms 2016, 18, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Li, C.; Xiao, J.; Yang, J.; Li, X.; Sun, L.; Wang, S.; Tian, H.; Zhan, Y. Cloning, expression characteristics of a new FPS gene from birch (Betula platyphylla suk.) and functional identification in triterpenoid synthesis. Ind. Crop. Prod. 2020, 154, 112591. [Google Scholar] [CrossRef]
- Li, J.; Goto, M.; Yang, X.; Morris-Natschke, S.L.; Huang, L.; Chen, C.-H.; Lee, K.-H. Fluorinated betulinic acid derivatives and evaluation of their anti-HIV activity. Bioorg. Med. Chem. Lett. 2016, 26, 68–71. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Zhang, W.; Li, Y.; Fan, Z.; Jiang, H.; Luo, J. Identification of lncRNA-mRNA Regulatory Module to Explore the Pathogenesis and Prognosis of Melanoma. Front. Cell Dev. Biol. 2020, 8, 615671. [Google Scholar] [CrossRef]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Ozdemir, Z.; Wimmer, Z. Recent Achievements in Medicinal and Supramolecular Chemistry of Betulinic Acid and Its Derivatives (double dagger). Molecules 2019, 24, 3546. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis. Biomolecules 2019, 9, 257. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- de Melo, C.L.; Queiroz, M.G.R.; Arruda, A.C.V.; Rodrigues, A.M.; de Sousa, D.F.; Almeida, J.G.L.; Pessoa, O.D.L.; Silveira, E.R.; Menezes, D.B.; Melo, T.S.; et al. Betulinic Acid, a Natural Pentacyclic Triterpenoid, Prevents Abdominal Fat Accumulation in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2009, 57, 8776–8781. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.S.; Kim, C.-S.; Kim, J.S. Betulinic Acid has an Inhibitory Effect on Pancreatic Lipase and Induces Adipocyte Lipolysis. Phytother. Res. 2012, 26, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S.N.A. Betulinic Acid: Recent Advances in Chemical Modifications, Effective Delivery, and Molecular Mechanisms of a Promising Anticancer Therapy. Chem. Biol. Drug Des. 2016, 87, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Jung, J.; Jang, D.S.; Kim, J.; Kim, J.H. Induction of Cell Death by Betulinic Acid through Induction of Apoptosis and Inhibition of Autophagic Flux in Microglia BV-2 Cells. Biomol. Ther. 2017, 25, 618–624. [Google Scholar] [CrossRef]
- Shankar, E.; Zhang, A.; Franco, D.; Gupta, S. Betulinic Acid-Mediated Apoptosis in Human Prostate Cancer Cells Involves p53 and Nuclear Factor-Kappa B (NF-kappaB) Pathways. Molecules 2017, 22, 264. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Zhu, R.; Zhang, K.; Li, S.; Chen, Z.; Li, L. Betulinic Acid Induces Apoptosis in Differentiated PC12 Cells Via ROS-Mediated Mitochondrial Pathway. Neurochem. Res. 2017, 42, 1130–1140. [Google Scholar] [CrossRef]
- Khan, I.; Guru, S.K.; Rath, S.K.; Chinthakindi, P.K.; Singh, B.; Koul, S.; Bhushan, S.; Sangwan, P.L. A novel triazole derivative of betulinic acid induces extrinsic and intrinsic apoptosis in human leukemia HL-60 cells. Eur. J. Med. Chem. 2016, 108, 104–116. [Google Scholar] [CrossRef]
- Chakraborty, B.; Dutta, D.; Mukherjee, S.; Das, S.; Maiti, N.C.; Das, P.; Chowdhury, C. Synthesis and biological evaluation of a novel betulinic acid derivative as an inducer of apoptosis in human colon carcinoma cells (HT-29). Eur. J. Med. Chem. 2015, 102, 93–105. [Google Scholar] [CrossRef]
- Petit, P.X.; Lecoeur, H.; Zorn, E.; Dauguet, C.; Mignotte, B.; Gougeon, M.L. ALTERATIONS IN MITOCHONDRIAL STRUCTURE AND FUNCTION ARE EARLY EVENTS OF DEXAMETHASONE-INDUCED THYMOCYTE APOPTOSIS. J. Cell Biol. 1995, 130, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zamzami, N.; Marchetti, P.; Castedo, M.; Zanin, C.; Vayssiere, J.L.; Petit, P.X.; Kroemer, G. REDUCTION IN MITOCHONDRIAL POTENTIAL CONSTITUTES AN EARLY IRREVERSIBLE STEP OF PROGRAMMED LYMPHOCYTE DEATH IN-VIVO. J. Exp. Med. 1995, 181, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.B.; Yazan, L.S.; Tor, Y.S.; Wibowo, A.; Ismail, N.; How, C.W.; Armania, N.; Loh, S.P.; Ismail, I.S.; Cheah, Y.K.; et al. Induction of cell cycle arrest and apoptosis by betulinic acid-rich fraction from Dillenia suffruticosa root in MCF-7 cells involved p53/p21 and mitochondrial signalling pathway. J. Ethnopharmacol. 2015, 166, 270–278. [Google Scholar] [CrossRef]
- Zhao, G.L.; Yan, W.D.; Cao, D. Simultaneous determination of betulin and betulinic acid in white birch bark using RP-HPLC. J. Pharm. Biomed. Anal. 2007, 43, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.G.; Cho, K.H.; Chung, S.M.; Graham, J.; Das Gupta, T.K.; Pezzuto, J.M. Determination of betulinic acid in mouse blood, tumor and tissue homogenates by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B 1999, 732, 331–336. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Singh, M.K.; Awasthi, A.; Singh, A.T.; Jaggi, M.; Ahmad, F.J. Application of a liquid chromatography-electrospray mass spectrometry (LC/MS) method to the biodistribution and excretion studies of novel 5′-chloro-2, 3-didehydroindolo (2′, 3′: 2, 3) betulinic acid (DRF-4012) in tumour-bearing mice. Xenobiotica 2013, 43, 548–560. [Google Scholar] [CrossRef]
- Zhao, F.; Mai, Q.; Ma, J.; Xu, M.; Wang, X.; Cui, T.; Qiu, F.; Han, G. Triterpenoids from Inonotus obliquus and their antitumor activities. Fitoterapia 2015, 101, 34–40. [Google Scholar] [CrossRef]
- Fu, M.-L.; Liu, J.; Dong, Y.-C.; Feng, Y.; Fang, R.-S.; Chen, Q.-H.; Liu, X.-J. Effect of ionic liquid-containing system on betulinic acid production from betulin biotransformation by cultured Armillaria luteo-virens Sacc cells. Eur. Food Res. Technol. 2011, 233, 507–515. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.; Liu, J.; Xu, T.-Y.; Fang, R.-S.; Chen, Q.-H.; He, G.-Q. A novel one-step microbial transformation of betulin to betulinic acid catalysed by Cunninghamella blakesleeana. Food Chem. 2013, 136, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Quan, L.; Shen, M. Effect of chemicals on production, composition and antioxidant activity of polysaccharides of Inonotus obliquus. Int. J. Biol. Macromol. 2015, 77, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Nano, J.-L.; Nobili, C.; Girard-Pipau, F.; Rampal, P. Effects of fatty acids on the growth of Caco-2 cells. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 207–215. [Google Scholar] [CrossRef]
- Zhang, B.B.; Cheung, P.C. A mechanistic study of the enhancing effect of Tween 80 on the mycelial growth and exopolysaccharide production by Pleurotus tuber-regium. Bioresour. Technol. 2011, 102, 8323–8326. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, W.; Liang, C.; Zhu, L.; Li, Y.; Mo, Q.; Xu, S.; Chu, A.; Zhang, L.; Ding, Z.; et al. Overproduction of alpha-Farnesene in Saccharomyces cerevisiae by Farnesene Synthase Screening and Metabolic Engineering. J. Agric. Food Chem. 2021. [Google Scholar] [CrossRef]
- Fradj, N.; Goncalves Dos Santos, K.C.; de Montigny, N.; Awwad, F.; Boumghar, Y.; Germain, H.; Desgagne-Penix, I. RNA-Seq de Novo Assembly and Differential Transcriptome Analysis of Chaga (Inonotus obliquus) Cultured with Different Betulin Sources and the Regulation of Genes Involved in Terpenoid Biosynthesis. Int. J. Mol. Sci. 2019, 20, 4334. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, P.; Song, J.; Qi, F.; Tian, W. A fungal elicitor induces Sclerotium rolfsii sacc resistance in Atractylodis maceocephalae koidz. Physiol. Mol. Plant Pathol. 2017, 99, 25–30. [Google Scholar] [CrossRef]
- Tang, H.B.; Ye, Z.W.; Liu, C.; Guo, L.Q.; Lin, J.F.; Wan, H.; Yun, F.; Kang, L.Z. Increasing of the Contain of Carotenoids in Caterpillar Mushroom, Cordyceps militaris (Ascomycetes) by Using the Fungal Elicitors Cultivation. Int. J. Med. Mushrooms 2019, 21, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yuan, J.; Gu, S.; Shi, Q. Influence of fungal elicitors on biosynthesis of natamycin by Streptomyces natalensis HW-2. Appl. Microbiol. Biotechnol. 2013, 97, 5527–5534. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Wang, D.; Wei, L.; Zhang, Y. Fungal elicitor-induced transcriptional changes of genes related to branched-chain amino acid metabolism in Streptomyces natalensis HW-2. Appl. Microbiol. Biotechnol. 2020, 104, 4471–4482. [Google Scholar] [CrossRef]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of plants and microbial cell systems. Biotechnol. Appl. Biochem. 2003, 37, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, Z.-F.; Li, C.-T. Effects of Exogenous Elicitors on Triterpenoids Accumulation and Expression of Farnesyl Diphosphate Synthase Gene in Inonotus obliquus. Biotechnol. Bioprocess Eng. 2020, 25, 580–588. [Google Scholar] [CrossRef]
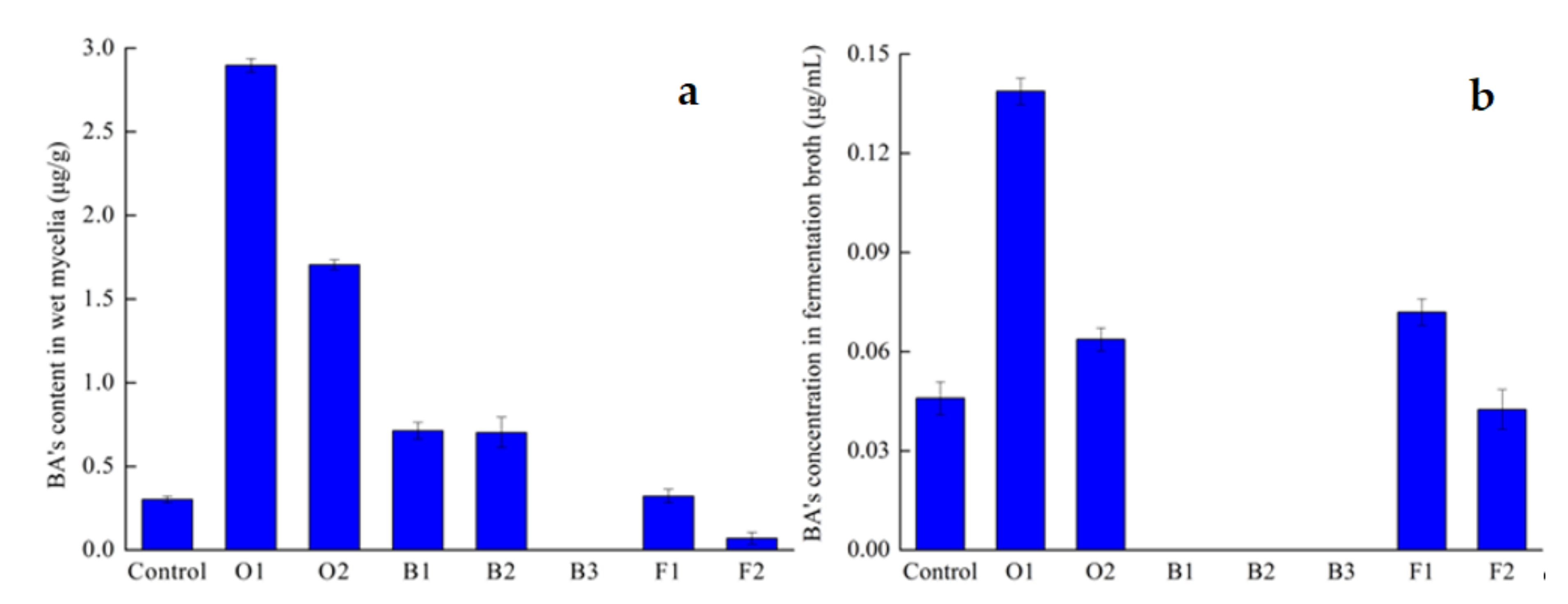

 BA’s concentration in fermentation broth;
BA’s concentration in fermentation broth;  BA’s content in dry mycelia;
BA’s content in dry mycelia;  Mycelia’s dry weight.
Mycelia’s dry weight.
 BA’s concentration in fermentation broth;
BA’s concentration in fermentation broth;  BA’s content in dry mycelia;
BA’s content in dry mycelia;  Mycelia’s dry weight.
Mycelia’s dry weight.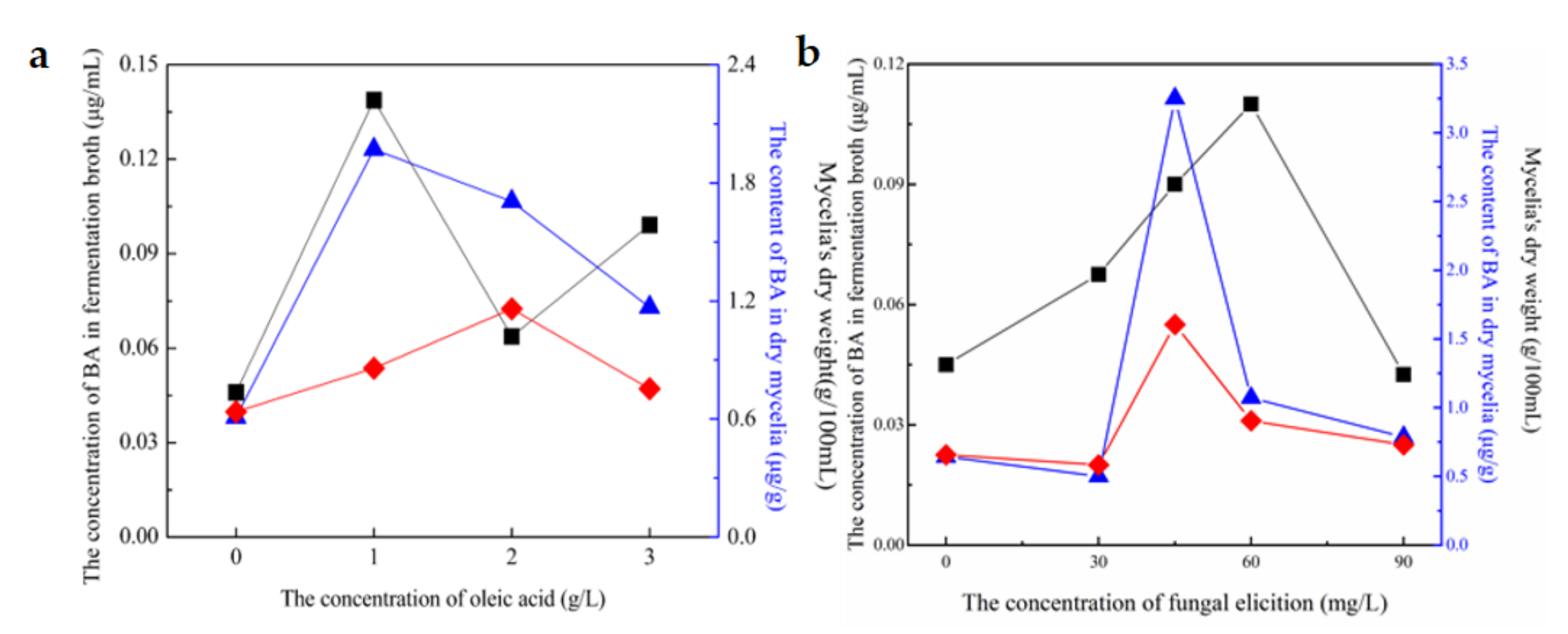
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, H.; Li, H.; Wei, T.; Chen, Q. Stimulatory Effects of Oleci Acid and Fungal Elicitor on Betulinic Acid Production by Submerged Cultivation of Medicinal Mushroom Inonotus obliquus. J. Fungi 2021, 7, 266. https://doi.org/10.3390/jof7040266
Lou H, Li H, Wei T, Chen Q. Stimulatory Effects of Oleci Acid and Fungal Elicitor on Betulinic Acid Production by Submerged Cultivation of Medicinal Mushroom Inonotus obliquus. Journal of Fungi. 2021; 7(4):266. https://doi.org/10.3390/jof7040266
Chicago/Turabian StyleLou, Hanghang, Hao Li, Tianyu Wei, and Qihe Chen. 2021. "Stimulatory Effects of Oleci Acid and Fungal Elicitor on Betulinic Acid Production by Submerged Cultivation of Medicinal Mushroom Inonotus obliquus" Journal of Fungi 7, no. 4: 266. https://doi.org/10.3390/jof7040266
APA StyleLou, H., Li, H., Wei, T., & Chen, Q. (2021). Stimulatory Effects of Oleci Acid and Fungal Elicitor on Betulinic Acid Production by Submerged Cultivation of Medicinal Mushroom Inonotus obliquus. Journal of Fungi, 7(4), 266. https://doi.org/10.3390/jof7040266






