Genomic Epidemiology of Candida auris in Qatar Reveals Hospital Transmission Dynamics and a South Asian Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Antifungal Susceptibility Assays
2.2. DNA Extraction and Whole Genome Sequencing
2.3. Data Analysis
3. Results
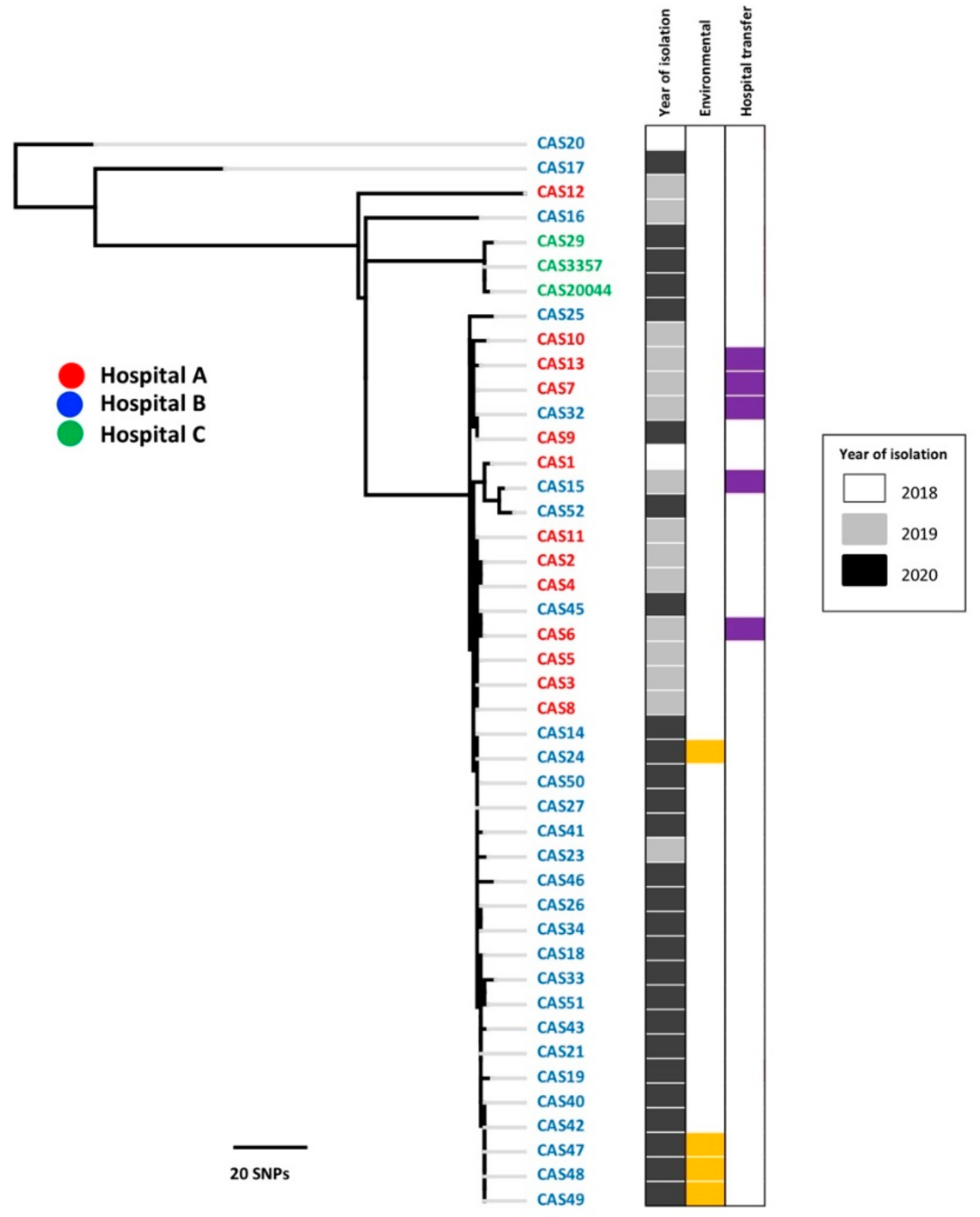
3.1. Genomic Epidemiology
3.2. Antifungal Susceptibility
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Taj-Aldeen, S.J.; Salah, H.; Perez, W.B.; Almaslamani, M.; Motyl, M.; AbdulWahab, A.; Healey, K.R.; Perlin, D.S. Molecular Analysis of Resistance and Detection of Non-Wild-Type Strains Using Etest Epidemiological Cutoff Values for Amphotericin B and Echinocandins for Bloodstream Candida Infections from a Tertiary Hospital in Qatar. Antimicrob. Agents Chemother. 2018, 62, e00214-18. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.A.; Gade, L.; Tsay, S.V.; Forsberg, K.; Greenko, J.A.; Southwick, K.L.; Barrett, P.M.; Kerins, J.L.; Lockhart, S.R.; Chiller, T.M.; et al. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: A molecular epidemiological survey. Lancet Infect. Dis. 2018, 18, 1377–1384. [Google Scholar] [CrossRef]
- Sharma, C.; Kumar, N.; Pandey, R.; Meis, J.F.; Chowdhary, A. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect 2016, 13, 77–82. [Google Scholar] [CrossRef]
- Rhodes, J. Rapid Worldwide Emergence of Pathogenic Fungi. Cell Host Microbe 2019, 26, 12–14. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. MBio 2020, 11, e03364-19. [Google Scholar] [CrossRef]
- Saris, K.; Meis, J.F.; Voss, A. Candida auris. Curr. Opin. Infect. Dis. 2018, 31, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Emergence of Candida auris: An International Call to Arms. Clin. Infect. Dis. 2017, 64, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.A.; de Groot, T.; Badali, H.; Abastabar, M.; Chiller, T.M.; Meis, J.F. Potential Fifth Clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 2019, 25, 1780–1781. [Google Scholar] [CrossRef] [PubMed]
- Al Maani, A.; Paul, H.; Al-Rashdi, A.; Wahaibi, A.A.; Al-Jardani, A.; Al Abri, A.M.A.; AlBalushi, M.A.H.; Al-Abri, S.; Al Reesi, M.; Al Maqbali, A.; et al. Ongoing Challenges with Healthcare-Associated Candida auris Outbreaks in Oman. J Fungi 2019, 5, 101. [Google Scholar] [CrossRef]
- Alfouzan, W.; Dhar, R.; Albarrag, A.; Al-Abdely, H. The emerging pathogen Candida auris: A focus on the Middle-Eastern countries. J. Infect. Public Health 2019, 12, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, R.S.; Albalawi, R.; Mutabagani, M.; Atienza, E.; Aljumaah, S.; Gade, L.; Forsberg, K.; Litvintseva, A.; Althawadi, S. Molecular characterisation and clinical outcomes of Candida auris infection: Single-centre experience in Saudi Arabia. Mycoses 2020, 63, 452–460. [Google Scholar] [CrossRef]
- Alfouzan, W.; Ahmad, S.; Dhar, R.; Asadzadeh, M.; Almerdasi, N.; Abdo, N.M.; Joseph, L.; de Groot, T.; Alali, W.Q.; Khan, Z.; et al. Molecular Epidemiology of Candida Auris Outbreak in a Major Secondary-Care Hospital in Kuwait. J. Fungi 2020, 6, 307. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Benwan, K.; Purohit, P.; Al-Obaid, I.; Bafna, R.; Emara, M.; Mokaddas, E.; Abdullah, A.A.; Al-Obaid, K.; et al. Invasive Candida auris infections in Kuwait hospitals: Epidemiology, antifungal treatment and outcome. Infection 2018, 46, 641–650. [Google Scholar] [CrossRef]
- Alatoom, A.; Sartawi, M.; Lawlor, K.; AbdelWareth, L.; Thomsen, J.; Nusair, A.; Mirza, I. Persistent candidemia despite appropriate fungal therapy: First case of Candida auris from the United Arab Emirates. Int. J. Infect. Dis. 2018, 70, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Al Ansari, N.; Al Wali, W.; Karic, E.; El Madhoun, I.; Mitwally, H.; Hamed, M.; Alutra-Visan, F. Experience of treating Candida auris cases at a general hospital in the state of Qatar. IDCases 2021, 23, e01007. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Argimón, S.; Abudahab, K.; Goater, R.J.E.; Fedosejev, A.; Bhai, J.; Glasner, C.; Feil, E.J.; Holden, M.T.G.; Yeats, C.A.; Grundmann, H.; et al. Microreact: Visualizing and sharing data for genomic epidemiology and phylogeography. Microb. Genom. 2016, 2, e000093. [Google Scholar] [CrossRef]
- Rybak, J.M.; Muñoz, J.F.; Barker, K.S.; Parker, J.E.; Esquivel, B.D.; Berkow, E.L.; Lockhart, S.R.; Gade, L.; Palmer, G.E.; White, T.C.; et al. Mutations in TAC1B: A Novel Genetic Determinant of Clinical Fluconazole Resistance in Candida auris. MBio 2020, 11, e00365-20. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, A.; Wang, Y.; van Haren, M.H.; Singh, A.; de Groot, T.; Meis, J.F.; Xu, J.; Chowdhary, A. Colonisation and Transmission Dynamics of Candida auris among Chronic Respiratory Diseases Patients Hospitalised in a Chest Hospital, Delhi, India: A Comparative Analysis of Whole Genome Sequencing and Microsatellite Typing. J. Fungi 2021, 7, 81. [Google Scholar] [CrossRef]
- Hou, X.; Lee, A.; Jiménez-Ortigosa, C.; Kordalewska, M.; Perlin, D.S.; Zhao, Y. Rapid Detection of ERG11-Associated Azole Resistance and FKS-Associated Echinocandin Resistance in Candida auris. Antimicrob. Agents Chemother. 2019, 63, e01811-18. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rhodes, J.; Abdolrasouli, A.; Farrer, R.A.; Cuomo, C.A.; Aanensen, D.M.; Armstrong-James, D.; Fisher, M.C.; Schelenz, S. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg. Microbes Infect. 2018, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef]
- Ruiz-Gaitan, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-Lopez, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonization and candidemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef]
- Tsay, S.; Welsh, R.M.; Adams, E.H.; Chow, N.A.; Gade, L.; Berkow, E.L.; Poirot, E.; Lutterloh, E.; Quinn, M.; Chaturvedi, S.; et al. Notes from the Field: Ongoing Transmission of Candida auris in Health Care Facilities—United States, June 2016–May 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Eilertson, B.; Cadnum, J.L.; Whitlow, C.S.; Jencson, A.L.; Safdar, N.; Krein, S.L.; Tanner, W.D.; Mayer, J.; Samore, M.H.; et al. Environmental Contamination with Candida Species in Multiple Hospitals Including a Tertiary Care Hospital with a Candida auris Outbreak. Pathog. Immun. 2019, 4, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Escandón, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular Epidemiology of Candida auris in Colombia Reveals a Highly Related, Countrywide Colonization With Regional Patterns in Amphotericin B Resistance. Clin. Infect. Dis. 2019, 68, 15–21. [Google Scholar] [CrossRef]
- Alshamrani, M.M.; El-Saed, A.; Mohammed, A.; Alghoribi, M.F.; Al Johani, S.M.; Cabanalan, H.; Balkhy, H.H. Management of Candida auris outbreak in a tertiary-care setting in Saudi Arabia. Infect. Control Hosp. Epidemiol. 2021, 42, 149–155. [Google Scholar] [CrossRef]
- Muñoz, J.F.; Gade, L.; Chow, N.A.; Loparev, V.N.; Juieng, P.; Berkow, E.L.; Farrer, R.A.; Litvintseva, A.P.; Cuomo, C.A. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat. Commun. 2018, 9, 5346. [Google Scholar] [CrossRef]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef]
| Patient Number | Isolate Code | Date of Isolation | Specimen | Hospital | Unit | Nationality | Transfer between Hospitals in Qatar | Admission to Hospital Abroad (within 6 Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | CAS20 | 12-May-18 | Urine | B | Medical ward | Omani | no | Yes (Oman) |
| 2 | CAS1 | 21-Dec-18 | ETT a | A | ICU b | Pakistani | no | no |
| 3 | CAS2 | 27-Feb-19 | Wound swab | A | ICU | Qatari | no | no |
| 4 | CAS3 | 17-Mar-19 | Wound swab | A | ICU | Qatari | no | no |
| 5 | CAS4 | 26-Jun-19 | Nasal swab | A | ICU | Indian | no | no |
| 6 | CAS5 | 09-Jun-19 | Nasal swab | A | High dependency unit | Palestinian | no | no |
| 7 | CAS6 | 18-Jun-19 | Nasal swab | A | ICU | Filipino | B/A | no |
| 8 | CAS7 | 18-Jun-19 | Screening swab c | A | Medical ward | Qatari | A/B | no |
| 9 | CAS8 | 28-Jun-19 | Skin swab | A | Medical ward | Qatari | no | no |
| 10 | CAS9 | 28-Jun-19 | Nasal swab | A | Medical ward | Qatari | no | no |
| 11 | CAS10 | 28-Jun-19 | Nasal swab | A | Medical ward | Pakistani | no | no |
| 12 | CAS11 | 02-Jul-19 | Skin swab | A | LTCU d | Nepalese | A/LTCU * | no |
| 13 | CAS12 | 11-Jul-19 | Screening swab | A | Medical ward | Indian | no | no |
| 14 | CAS13 | 30-Jul-19 | Groin swab | A | Medical ward | Qatari | A/B | no |
| 15 | CAS16 | 04-Aug-19 | Urine | B | Cardiac ICU | Palestinian | no | no |
| 16 | CAS23 | 01-Sep-19 | Groin swab | B | Medical ward | Qatari | no | no |
| 17 | CAS32 | 09-Sep-19 | Urine | B | Surgical ward | Qatari | B/A | no |
| 17 | CAS15 | 14-Sep-19 | Urine | B | Surgical ward | Qatari | B/A | no |
| 18 | CAS14 | 04-Jan-20 | ETT | B | Medical ward | Qatari | no | no |
| (18) | CAS24 | 15-Jan-20 | Bedside table (patient 18) | B | ICU (203-1) | N/A | N/A | N/A |
| 19 | CAS18 | 08-Jan-20 | Nasal swab | B | ICU | Qatari | no | no |
| 20 | CAS21 | 08-Jan-20 | Groin swab | B | Medical ward | Qatari | no | no |
| 21 | CAS19 | 09-Jan-20 | Axilla swab | B | ICU | Syrian | no | Yes (Syria) |
| 22 | CAS26 | 20-Feb-20 | BAL e | B | ICU | Nepalese | no | no |
| 22 | CAS33 | 20-Feb-20 | Pleural fluid | B | ICU | Nepalese | no | no |
| 23 | CAS25 | 24-Feb-20 | Pus | B | LTCU | Qatari | no | no |
| 24 | CAS27 | 17-Mar-20 | Blood | B | Medical ward | Indian | no | no |
| 25 | CAS34 | 17-Jun-20 | Blood | B | ICU | Bangladeshi | no | no |
| 26 | CAS40 | 08-Jul-20 | Axilla swab | B | Medical ward | Qatari | no | no |
| 27 | CAS50 | 20-Jul-20 | Nasal swab | B | LTCU | Indian | no | no |
| 28 | CAS41 | 18-Aug-20 | Urine | B | Medical ward | Palestinian | no | no |
| 29 | CAS52 | 25-Aug-20 | Nasal swab | B | LTCU | Qatari | no | no |
| 30 | CAS17 | 28-Aug-20 | Axilla swab | B | Medical ward | Qatari | no | no |
| 31 | CAS42 | 14-Sep-20 | Groin swab | B | Medical ward | Omani | no | no |
| (31) | CAS47 | 16-Sep-20 | Bed (patient 31) | B | Medical ward | N/A | N/A | N/A |
| (31) | CAS48 | 16-Sep-20 | Couch (patient 31) | B | Medical ward | N/A | N/A | N/A |
| (31) | CAS49 | 16-Sep-20 | Cabinet (patient 31) | B | Medical ward | N/A | N/A | N/A |
| 32 | CAS43 | 14-Sep-20 | Axilla swab | B | Medical ward | Iranian | no | no |
| 33 | CAS51 | 14-Sep-20 | Axilla swab | B | LTCU | Qatari | no | no |
| 34 | CAS45 | 14-Oct-20 | Groin swab | B | LTCU | Qatari | no | no |
| 35 | CAS46 | 02-Nov-20 | Axilla swab | B | Medical ward | Indian | Home care/B | no |
| 36 | CAS20044 | 12-May-20 | BAL | C | Oncology ward | Sudanese | no | Yes (Sudan, India) |
| 36 | CAS3357 | 20-May-20 | Screening swab | C | Oncology ward | Sudanese | no | Yes (Sudan, India) |
| 36 | CAS29 | 17-Jun-20 | Tracheal aspirate | C | Oncology ward | Sudanese | no | Yes (Sudan, India) |
| Isolate | FLC | ITC | POS | VOR | AMB | 5FC | CAS | ANI | MICA | ERG11 Mutations |
|---|---|---|---|---|---|---|---|---|---|---|
| CAS12 | 256 | 0.25 | 0.12 | 1 | 1 | 0.12 | 8 | 0.5 | 0.5 | Y132F |
| CAS14 | 32 | 0.06 | 0.03 | 0.12 | 2 | 0.06 | 0.25 | 0.25 | 0.12 | Y132F |
| CAS16 | 128 | 0.5 | 0.25 | 8 | 2 | 0.06 | 0.5 | 0.5 | 0.5 | Y132F |
| CAS17 | 128 | 0.25 | 0.12 | 1 | 2 | 0.06 | 0.12 | 0.25 | 0.12 | K143R |
| CAS20 | 256 | 0.5 | 0.25 | 2 | 4 | 0.12 | 0.25 | 0.12 | 0.12 | K143R |
| CAS25 | 128 | 16 | 8 | 8 | 2 | 0.12 | 0.5 | 0.25 | 0.12 | Y132F |
| CAS27 | 64 | 0.25 | 1 | 1 | 2 | 0.12 | 0.5 | 0.25 | 0.12 | Y132F |
| CAS33 | 128 | 0.12 | 0.12 | 8 | 2 | 0.06 | 0.5 | 0.25 | 0.25 | Y132F |
| CAS34 | 128 | 0.12 | 0.03 | 0.25 | 2 | 0.06 | 0.25 | 0.12 | 0.12 | Y132F |
| CAS41 | 32 | 0.06 | 0.015 | 0.12 | 2 | 0.06 | 0.06 | 0.12 | 0.06 | Y132F |
| CAS20044 | 128 | 0.12 | 0.06 | 0.5 | 2 | 0.12 | 0.25 | 0.12 | 0.12 | Y132F |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salah, H.; Sundararaju, S.; Dalil, L.; Salameh, S.; Al-Wali, W.; Tang, P.; Ben Abid, F.; Tsui, C.K.M. Genomic Epidemiology of Candida auris in Qatar Reveals Hospital Transmission Dynamics and a South Asian Origin. J. Fungi 2021, 7, 240. https://doi.org/10.3390/jof7030240
Salah H, Sundararaju S, Dalil L, Salameh S, Al-Wali W, Tang P, Ben Abid F, Tsui CKM. Genomic Epidemiology of Candida auris in Qatar Reveals Hospital Transmission Dynamics and a South Asian Origin. Journal of Fungi. 2021; 7(3):240. https://doi.org/10.3390/jof7030240
Chicago/Turabian StyleSalah, Husam, Sathyavathi Sundararaju, Lamya Dalil, Sarah Salameh, Walid Al-Wali, Patrick Tang, Fatma Ben Abid, and Clement K. M. Tsui. 2021. "Genomic Epidemiology of Candida auris in Qatar Reveals Hospital Transmission Dynamics and a South Asian Origin" Journal of Fungi 7, no. 3: 240. https://doi.org/10.3390/jof7030240
APA StyleSalah, H., Sundararaju, S., Dalil, L., Salameh, S., Al-Wali, W., Tang, P., Ben Abid, F., & Tsui, C. K. M. (2021). Genomic Epidemiology of Candida auris in Qatar Reveals Hospital Transmission Dynamics and a South Asian Origin. Journal of Fungi, 7(3), 240. https://doi.org/10.3390/jof7030240







