1. Introduction
Heat-resistant fungi cause spoilage of food products after heat treatments, such as pasteurization. This is the result of the presence of extreme stress resistant ascospores [
1,
2] that are formed in a homothallic (i.e., self-fertile) fashion, as in the case of the model systems
Talaromyces macrosporus and
Aspergillus fischeri (earlier described as
Neosartorya fischeri) [
3,
4] or in a heterothallic fashion (as is described forthe fungi
Paecilomyces variotii and
Aspergillus fumigatus [
5,
6]). These ascospores are characterized by a dormant state that is independent of the presence of nutrients (as e.g., [
7,
8]). This constitutive dormancy (as defined by [
9]) can be broken by extreme triggers as high temperature (85 °C, e.g., [
10]) or high pressure (6000 Bar, [
11,
12]). Spoilage of food products after pasteurization is a consistent problem in food industry [
13,
14,
15].
T. macrosporus is a model system for heat-resistant ascospores. Ascospores are formed inside numerous asci in ascomata, present in large numbers on the growth medium after 40 days of cultivation. Homogenous suspensions with high numbers of spores are easily obtained and can be studied with respect to their properties. Many studies on D-values of ascospores of different species of heat-resistant fungi have been performed in the past [
2]. A D
T-value expresses the time needed, at temperature T, to heat-inactivate 90% of the spores. A comparison of nearly all the data compiled from these studies indicate that ascospores originating from different species exhibit varying heat resistance.
T. macrosporus belongs to the most heat-resistant fungi [
2].
Ascospores of
T. macrosporus are characterized by an extremely thick cell wall and dense cytoplasm containing highly accumulated trehalose [
3,
4,
16,
17]. Germination of ascospores is activated and synchronized bya heat treatment at 80–85 °C for 5–10 min, temperatures that are characteristic for a pasteurization treatment. Kikoku [
18,
19] showed that activation became more pronounced when the temperature increased between 81 and 91 °C. Upon heat activation, germinating ascospores show a very fast degradation of trehalose, accompanied by a decrease in intracellular (micro)viscosity as measured by means of electron spin resonance [
3,
4,
17]. These observations suggest that cytoplasmic viscosity parameters play an important role in prolonged dormancy and that degradation of compatible solutes is an important stage in the renormalization of the ascospore towards a growing fungal vegetative cell. Subsequently, ascospores of
T. macrosporus show a fast shedding of the external thick cell wall, increase of respiration and subsequent swelling of the spore [
17,
20]. Remarkably, shedding of the outer cell wall, could be blocked by the respiration blocker sodium azide. To complicate things more, after washing away azide, trehalose breakdown had occurred and ESR measurements still suggested high micro(viscosity) and low respiration.After shedding, microviscosity dropped and respiration increased strongly.
The breaking of dormancy in heat-resistant fungi is not addressed in great detail. Ascospores of
T. macrosporus do not measurably consume any oxygen [
16] and have more profound blockage of metabolic activity compared to conidia (as is observed in the case of
Aspergillus niger that shows some respiration [
16,
21]). When ascospores are activated by heat and subsequently freeze-dried or frozen (either −20 °C or −196 °C), ascospores remain activated upon rewetting and cultivation [
22]. It is of interest to realize that stress resistance of ascospores remains high only when drying or freezing was applied directly after the heat activation treatment. First, dormancy is broken, then an autonomous process leads to germination characterized by intracellular conditions that conduce high metabolic activity at low concentrations of compatible solutes and low microviscosity. Further investigations into the processes following the heat-activation of these spores may lead to new ways of preventing food spoilage by
T. macrosporus and other heat-resistant fungi.
However, the nature of (heat) activation of extreme stress-resistant fungal ascospores is never addressed. In this contribution, we provide data that suggest a novel mechanism in which the impermeability of the thick outer layer of the cell wall is conveyed by a small protein, ICARUS, that is present either within or around the spore.
2. Materials and Methods
2.1. Fungal Strains
Cultures of the fungus
Talaromyces macrosporus (CBS 130.89 [
23]) were grown on oatmeal agar plates for 40 days at 30 °C. The ascomata were scraped off of the plates and suspended in 20 mL of N-(2-Acetamido)-2-aminoethane-sulfonic acid (ACES) buffer (Sigma, pH 6.8, 10 mM) supplemented with 0.05% TWEEN 80. The ascomata and asci were ruptured by either suction through a 10 mL syringe with a 0.9 mm hypodermic needle or short vortexing with a mixture of 1 mm and 0.1 mm glass beads. After the asci had been loosened, the suspension was filtered through a 30 mL syringe filled with 5–10 mL sterile glass wool at the bottom. Then, the suspension was washed 2–4 times with ACES buffer by centrifugation and the pellet was resuspended in fresh ACES buffer. To count the spores a hemocytometer was used.
2.2. Preparation of a Cell Wall Fraction
Costar tubes (50 mL) were filled with 1 mm glass beads to about 5 to 10 mL. Subsequently, the spore solution (typically 10
8 spores/mL) was added as a volume that did not exceed the level of glass beads. The suspension was vortexed for 3 minwith intermittant cooling on ice. By this method an average of 96% of the spores could be broken as was judged by microscopical examination (see
Supplementary Figure S1).
2.3. ESR and X-ray Analysis
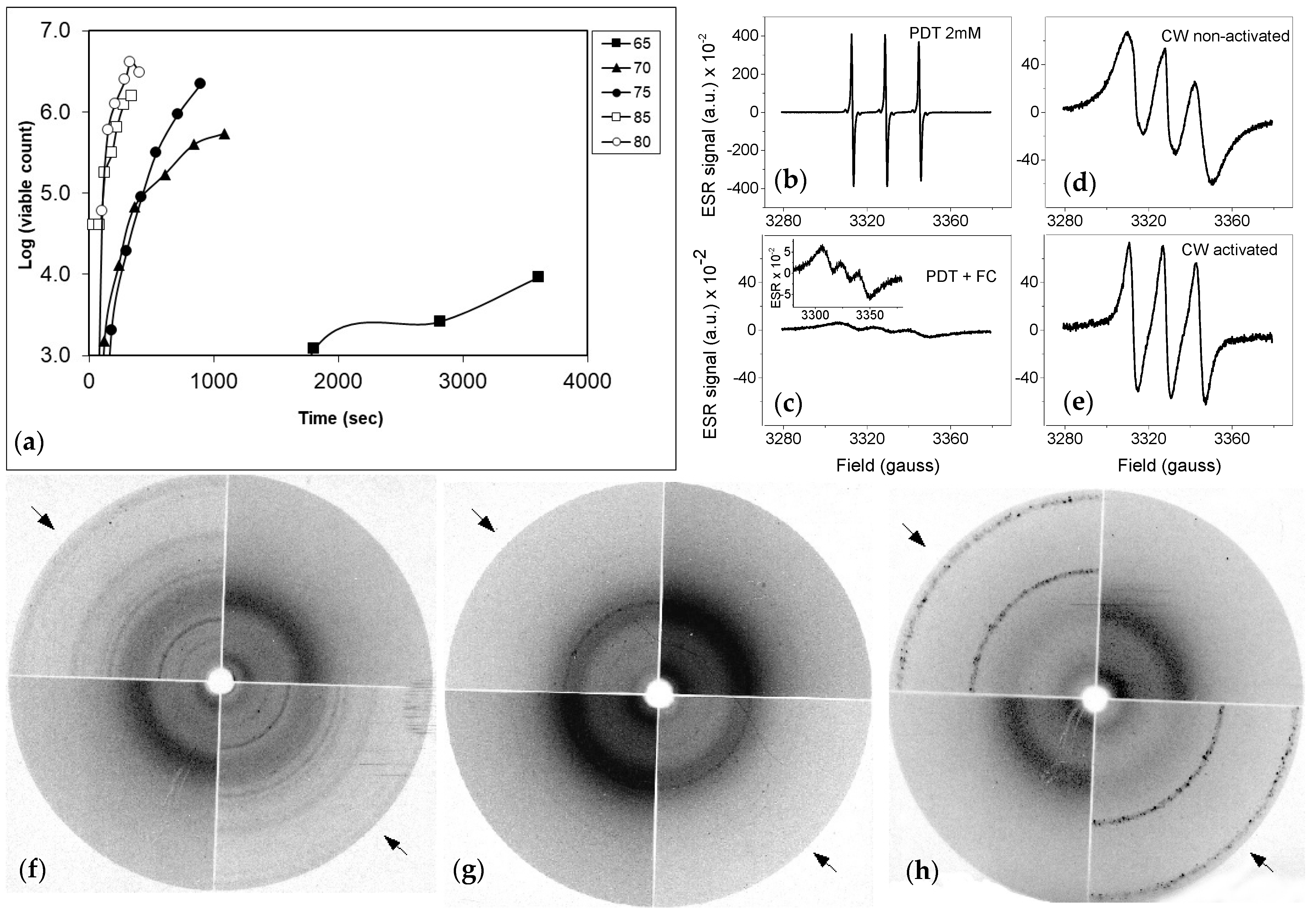
For the ESR sample preparation, 40 day-old cultures were isolated as described above. After the last washing step the spores were suspended in 10 mL ACES buffer. A quantity of 4.5 mL of the spore suspension was activated (7 min at 85 °C). Both heat-activated and non-activated spores were broken as described above. After breaking the spores, they were taken out of the Costar tubes with a 10 mL syringe and distributed over 6 Eppendorf tubes (three for the activated spores and three for the non-activated spores). The pellets were washed 4 times with Milli-Q water (centrifuged for 30 s at 15.000× g in an Eppendorf centrifuge) and resuspended in the vial. Two of the six tubes (one activated and one non-activated) were centrifuged and the pellet was washed with Milli-Q. The other four cups were also centrifuged and resuspended in 500 μL of 2% SDS. After that they were incubated for 10 min at 100 °C. Then, they were washed with Milli-Q again for four times. Two of the four pellets were subsequently resuspended in Milli-Q- while the others were resuspended in 500 µL 1 N KOH and incubated for 20 min at 60 °C. Afterwards the two KOH treated solutions were washed again for four times and then resuspended in 500 uL Milli-Q.
For ESR, all samples were prepared with perdeuterated TEMPONE (PDT,-4-oxo-2,2,6,6-tetramethylpiperidine-N-oxyl, 2 mM, obtained from Prof. Igor Grigoriev, Novosibirsk, Russia) and potassium ferricyanide (120 mM) was added and the spectra were recorded. The samples were placed in a glass capillary (i.d. 2.5 mm) flame-sealed from one end and then the capillary with a sample was transferred into the quartz tube that was fixed in the ESR cavity for spectra recording by means of a ESR spectrometer ELEXYS 500 (Bruker EAS GmbH, Hanau, Germany). ESR spectra were recorded at 2 mW microwave power, the modulation amplitude was 0.2–1 gauss depending on the shape of the spectrum.
For X-ray diffraction studies, powder diffraction recordings [
24] freeze dried cell wallswre packed into small holes in plastic specimens discs, which were mounted on a X-ray collimator and exposed to nickel-filtered copper (CuKa) radiation. The X-ray tube was operated at 38 kV, 23 mA and the exposure time was 30 min. Specimen to film distance was 40 cm.
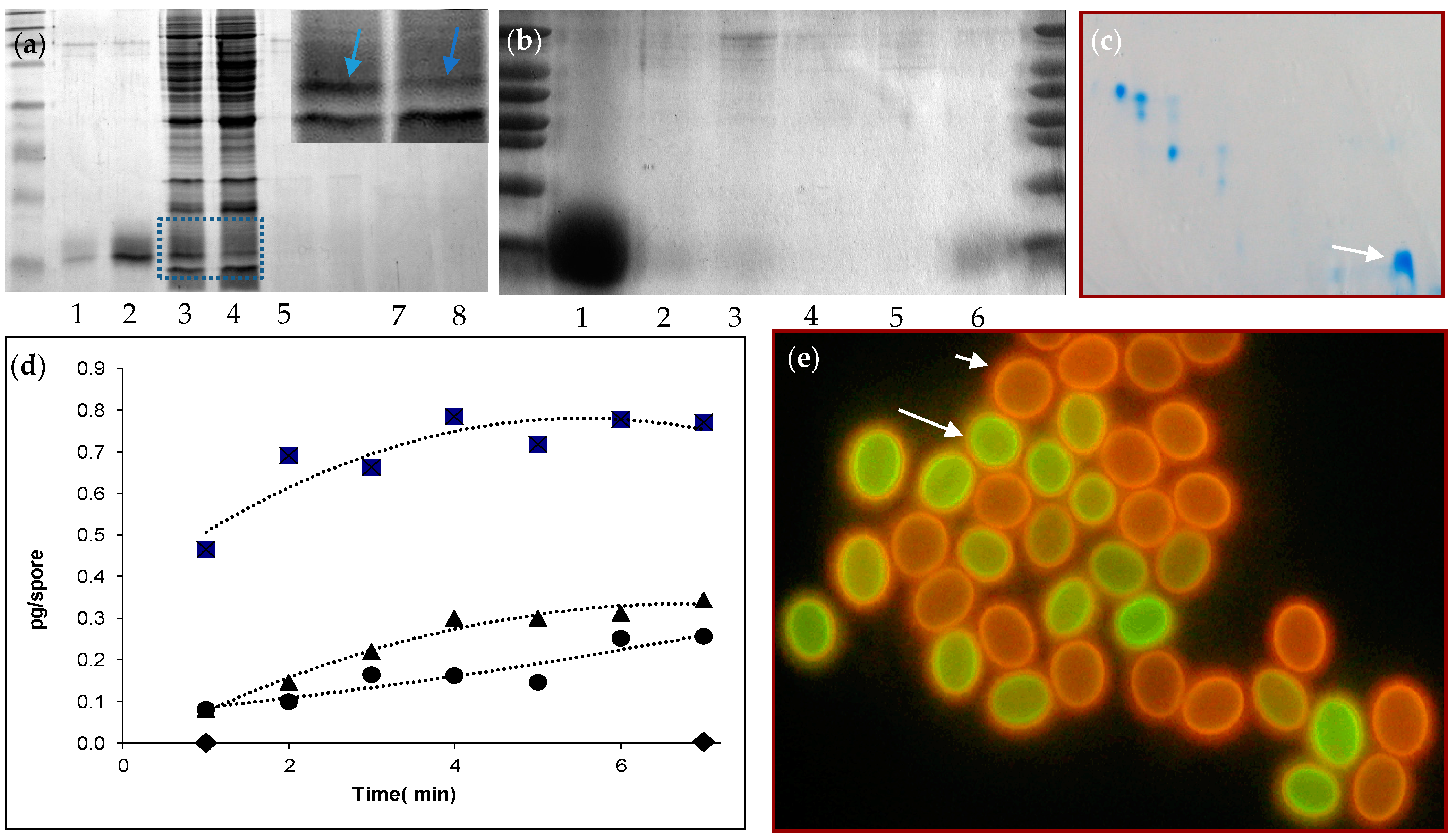
2.4. Protein Release during Heat Activation
A quantity of 375 μL of spore suspension (108 spores/mL) was brought in Eppendorf cups. To activate the spores, the Eppendorf cups were heated at 85 °C for 7 min in a Julabo waterbath (Julabo, GmbH, Seelbach, Germany). Then, the spores were spun down for 20 s at 13.000 rpm. A quantity of 300 μL of denaturing sample buffer was added to the each of the supernatants. The samples were heated to 100 °C for 5 min and run on a 15% SDS-PAGE gel for 50 min at 200 Volts. For N-terminal sequence analysis of the protein, the SDS-PAGE gel was blotted on PVDF membrane for 4.5 min at 60 V. The blotting buffer used was the same as the Electrophoresis buffer, i.e., Tris/glycine/SDS (TGS) but now with 15 mL methanol added. Blots were stained with Coomassie Brilliant Blue R250 in 10% (v/v) acetic acid, 30% (v/v) methanol and de-stained in the same solvent. For staining of the SDS-PAGE gel Coomassie Brilliant Blue was used for one hour. For the subsequent destaining 10% methanol, 10% acetic acid was used. The de-staining was done overnight. The staining of the blot was also done with Coomassie Brilliant Blue, for 5 min. Amino-terminal sequencing was carried out on a cut-out band from the gel blot with a pulse liquid sequenator on-line, connected to a phenylthiohydantoin analyser (Applied Biosystems, Foster City, CA, USA).
The 2D gel electrophoresis was conducted according to [
25].
2.5. Protein Measurement by BCA
Protein levels in supernatant of ascospores suspensions were measured using a BCATM Protein Assay Kit (Pierce, Rockford, IL, USA) and measuring staining of supernatants at 590 nm. Bovine Serum Albumin was used for calibration.
2.6. Staining of Ascospores by Carboxy Fluorescein
Ascospores originating from 33-day-old to 49-day-old cultures were stained in 2.9–9.5 mM carboxy fluorescein in ACES bufferfor 15 min and stained at 30 °C and 160 rpm agitation. Ascospores were washed in buffer and fluorescence was assessed using a Zeis Axioskop (Zeiss, Oberkochen, Germany) equipped with Filterblock II (09), 450–490 nm, FT 510, LP 520. Micrographs were taken with the Axiocam software (Zeiss, Oberkochen, Germany). Cells were observed in liquid, but in cases that the correlation between cell wall staining and germination was assessed, stained ascospores were immobilized on a thin layer of agar on an objective glass and investigated by light microscopy after 6 h. Fluorescence intensity was quantified, using the original pictures, as arbitrary units using the Adobe Photoshop software.
2.7. Total RNA Isolation
For RNA isolation, mycelium of 20–30-day-old agar cultures of T. macrosporus was used, frozen in liquid nitrogen and pulverized using a dismembrator (TissueLyser, QIAGEN Benelux B.V., Venlo, The Netherlands) for two times 45 s at 2000 rpm. Subsequently, the broken mycelium was transferred to a cooled Eppendorf cup and total RNA was extracted with TriZol (Invitrogen, ThermoFischer Scientific, Landsmeer, The Netherlands) following the manufacturer’s protocol.
2.8. cDNA Isolation
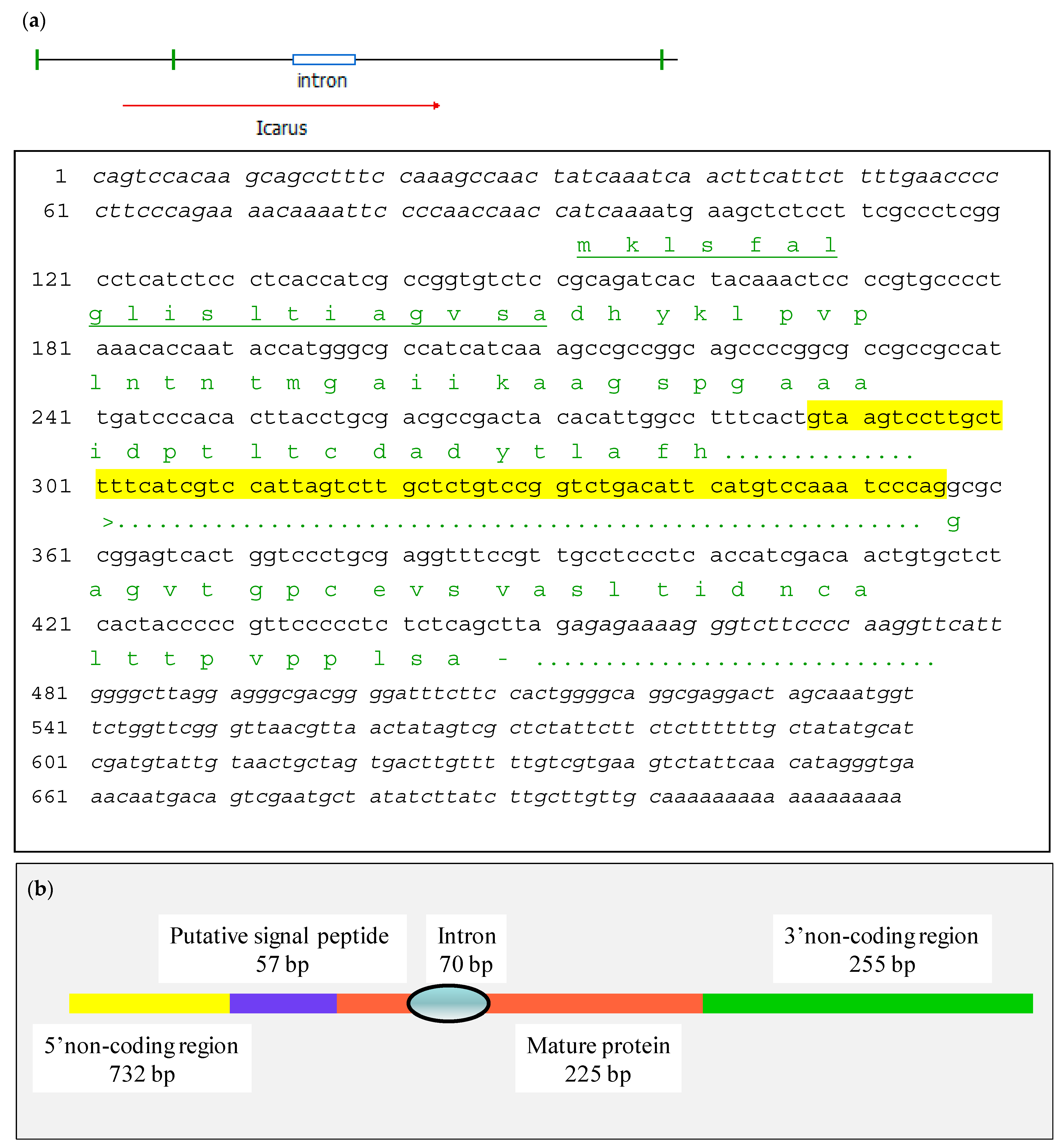
In order to obtain the sequence of ICARUS its cDNA was isolated through RT-PCR.
RT was done on 2 μg total RNA using AMV Reverse transcriptase (Roche, Merck KGaA, Darmstadt, Germany) following the instructions of the manufacturer. As reverse primer for the cDNA synthesis an oligo dT primer was used (5′ TTA ATT TTT TTT TTT TTT TTT TTT TTV 3′).
For the PCR reaction to generate the second strand, a primer derived from the N-terminal sequence of the protein was used (5′ CCY ACY CTS ACB GAY GAY GCB GAY TAY 3′) together with the reverse primer used for first strand synthesis. The thermal cycling program was 94 °C (4 min); {94 °C (20 s); 45 °C (20 s); 72 °C (2 min)} × 35 times; 72 °C (10 min). The obtained band was cloned in pUC20 and sent for sequencing.
2.9. Chromosomal DNA Isolation
Genomic DNA was isolated from T. macrosporus ascospores (5 × 106/mL) that had germinated for 16 h at 30 °C in 100 mL malt extract broth and shaken at 200 rpm. To the culture fluid another 100 mL of fresh medium was added to 10 mL of blended culture and inoculated for an additional 36–48 h. The mycelium was spun down and frozen in liquid nitrogen. The mycelium was pulverized in a Tissuelyser. To approximately 3 mL of pulverized mycelium, 5 mL DNA extraction buffer, 80 μL Proteinase K (10 mg/mL) and 100 μL β-mercapto-ethanol were added and incubated for 30 min at 55 °C. After centrifugation (10 min, 10,000× g), the supernatant was placed in a clean tube and 1 volume isopropanol was added. The formed pellet (5 min, 10,000× g) was washed with 70% ethanol, dried and dissolved in TE (10 mM Tris, 3 mM EDTA, pH 8.0). 100 μg/mL RNAse was added to degrade the RNA (15 min, 37 °C). The amount of DNA was assessed by gel electrophoresis and further purified on a silica column and stored at 4 °C.
2.10. DNA and RNA Hybridizations
DNA and RNA hybridizations were conducted according to [
26].
2.11. PCR Walking with Splinkerette’s
PCR-walking with splinkerettes [
27] was used to obtain the flanking regions of the ICARUS gene. Splinkerette bottom and top (
Supplementary Table S1) were annealed in a 100 μL annealing mixture in water consisting of 8 μM splinktop primer; 8 μM splinkbottom primer; 10 mM Tris (pH 7.5) and 5 mM MgCl
2. The mixture was incubated at 94 °C for 4 min and slowly chilled till room temperature.
Digested gDNA of
T. macrosporus was ligated with a 15 times molar excess of splinkerettes in 20 μL for 4.5 h at room temperature. Then, a primary and a secondary PCR were performed. The first PCR was done on 1μL ligation mixture (30 ng) with primers (
Supplementary Table S1) n1 (50 pmol), which annealed on the splinkerette and playipr1 or playipf1 (10 pmol), which annealed on each of the ends of the known sequence. A touchdown cycling protocol was used (30 s, 95 °C and 15 s during each cycle, annealing for 1 min at 71 °C, but decreasing by 2 °C per cycle until 61 °C, 2 min at 72 °C (cycles 1–10), then 4 min (cycles 11–20) and finally, 6 min (cycles 21–30). A second PCR was done using 1 μL primary PCR mixture and nested primers (n2 and playif2 or playir2 depending on which of the two specific primers had worked in the first PCR) using the same parameters as in the first PCR. Amplified DNA was separated on a gel and fragments with the right size were cut out and purified, cloned and sent for sequencing.
2.12. Construction of a Deletion Plasmid
The ICARUS gene was disrupted by replacing the gene with an incomplete copy, which missed the promoter and the first part of the coding region, including an encoded signal peptide for secretion (as shown in
Supplementary Figures S3 and S5). Primers for amplifying the flanks upstream and downstream of the region to be deleted were designed based on the sequence of the fragment isolated through the splinkerette protocol. Amplified flanks were cloned in vector pUC20. Both fragments were cut out of the plasmids with the proper restrictions enzymes and ligated in the vector Pan7–1 using the same restriction enzymes, which allowed for directional cloning of both fragments. The upstream flank was ligated at the end of the hygromycin resistance cassette using the restriction sites HindIII and XbaI. The downstream flank was ligated 500 bp upstream of hygromycin resistance cassette start, using the restriction sites NheI and BglII.
2.13. Protoplast Preparation
Protoplast isolation and transformation were done based on [
28]. Mycelium was grown in malt extract medium (MEB) inoculated with 5 × 10
6 heat-activated ascospores and grown for 2 days (30 °C, 250 rpm). The culture was homogenized for 30 s in a Waring blender at the low stand and the homogenate was used to inoculate fresh malt extract medium. The new culture was further grown at 30 °C for 16 h under shaking (250 rpm). 50 mL culture were transferred to a Falcon tube and spun down at 4500×
g. The pellet was washed twice with 1 M MgSO
4 and resuspended in cell wall lysing buffer (Lysing Enzymes, Applied Plant Research, Wageningen University, 10 mg) dissolved in 20 mL 1 M MgSO
4 and 200 μL 0.5 M malate buffer (pH 5.8) The mixture was incubated at 26 °C on a shaker at 70 rpm for approximately 3 h. The protoplast suspension was then filtered through sterile glass wool to remove the mycelium debris. To the clean protoplast suspension cold STC (1.2 M sorbitol, 10 mM Tris-HCl pH 7.5, 10 mM CaCl
2) was added to a total volume of 45 mL. The protoplasts were pelleted by centrifugation at 800×
g at 4 °C in a swing out rotor (20 min) and washed with 45 mL cold STC. The protoplasts were resuspended in STC buffer to a final concentration of 10
8/mL.
2.14. Transformation of T. macrosporus Protoplasts
For each transformation, 200 μL of the protoplast suspension were used. To the protoplasts, 1 μg DNA was added. After the addition of 50 μL PEG buffer (25% PEG-6000, 50 mM CaCl2, 10 mM Tris/HCl pH 7.5) the mixture was gently shaken and incubated at RT (room temperature) for 20 min. After the incubation, 2 mL of PEG buffer was added and gently mixed. The mixture was incubated for another 5 min at RT. Subsequently, 4 mL of STC was added. Finally, selective malt extract-top agar (malt extract, pH 6.0, 0.95 M sucrose, 0.6% Low Melting Point Agarose, Hygromycin 200 μg/μL) was added to a total volume of 15 mL. After mixing the suspension was poured on selective plates (malt extract pH 6.0, 0.95 M sucrose, 1.2% Agar, Hygromycin 200 μg/μL). The plates with protoplasts were incubated upside down at 30 °C for 3 days.
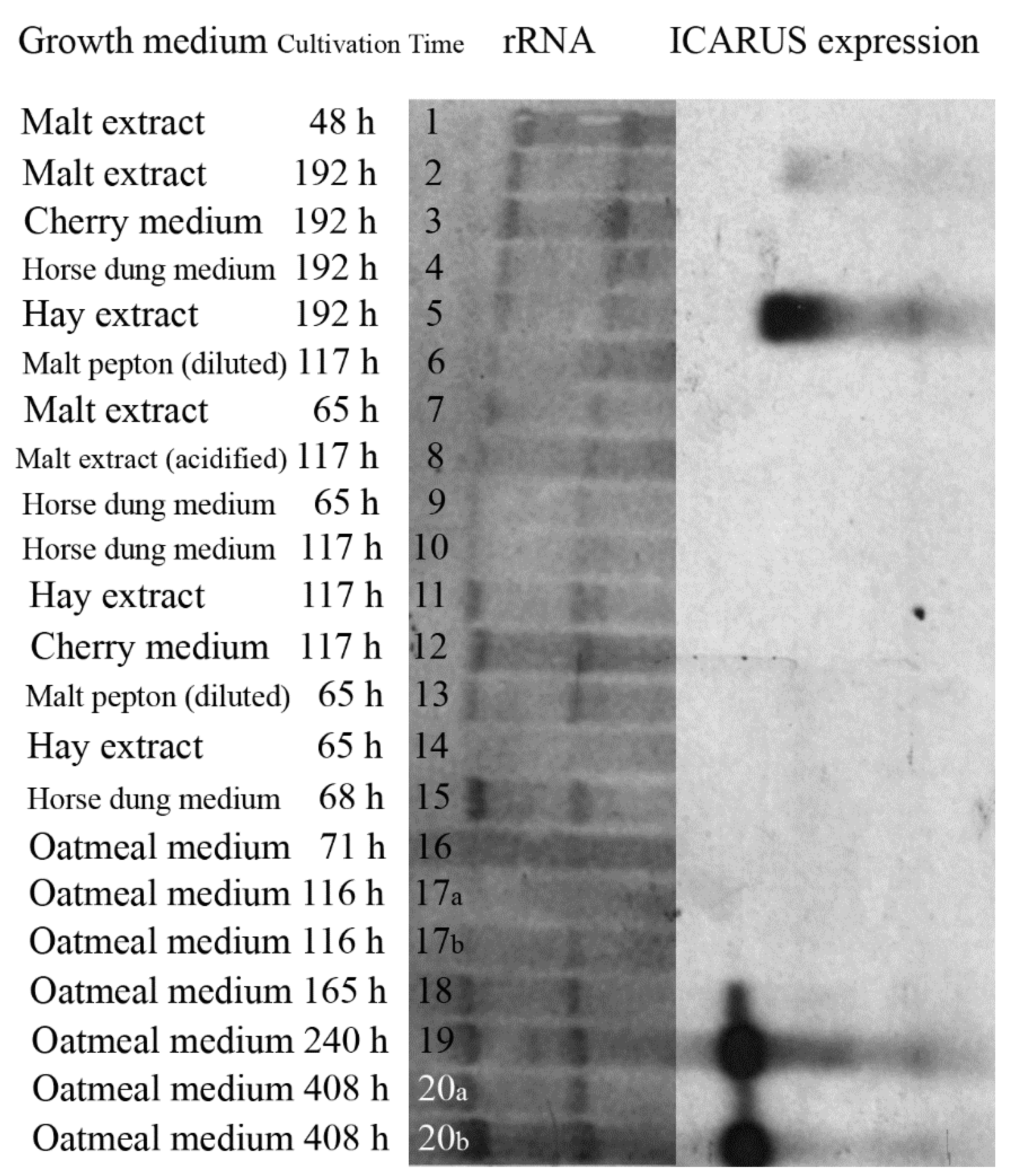
2.15. Expression of ICARUS under Several Growth Conditions
Heat-activated ascospores were inoculated on different growth media. RNA was isolated from fungal hyphae and ascomata. Total RNA was ran on denaturing gel, blotted, and hybridized with probes specific for ICARUS by a Northern blotting procedure. As growth media were used: Oatmeal agar, Hay extract, Horse dung medium, Cherry medium and Malt extract medium as described in [
29]. For development of structures in the dark, microtiter plates containing the liquid media were wrapped in tin foil and kept within a closed carboard box in order to study the effect of darkness on ascomata formation.
2.16. Scanning Electron Microscopy and Cryo-Planing
cryoSEM was conducted according to [
2] and cryo-planing was conducted as described in [
17].
4. Discussion
Dormant fungal cells exhibit a lowered metabolism, accumulate protective compounds and delineate their protoplasts with a thick outer cell wall. Two types of dormancy are mentioned in literature including endogenous (or also called constitutive) dormancy [
9,
30] or exogenous (or environmental) dormancy.
According to [
9] and later discussed by [
30,
31], dormancy is: “Any rest period or reversible interruption of the phenotypic development of the organism.” Constitutive or endogenous dormancy is: “A condition wherein development is delayed due to an innate property of the dormant stage such as a barrier to the penetration of nutrients, a metabolic block, or the production of a self-inhibitor”. Exogenous dormancy is: “A condition wherein development is delayed because of unfavorable chemical or physical conditions of the environment”.
Ascospores of
T. macrosporus do only germinate in very low, if any, numbers when present in or on a rich nutrient liquid or agar medium [
16] and therefore can be defined as constitutively dormant spores. It would be of interest to evaluate in detail, if these spores do not germinate at all in these media and that sparsely present conidia or hyphal cells do account for the low colony counts observed. Further, these cells are triggered into massive synchronized germination after being provoked by an extreme trigger, which, presumably, takes the barrier for germination away. The nature of the cause of delayed germination is unknown. The data in this study provide evidence that the thick, multi-layered (see also [
20]) cell wall of
T. macrosporus ascospores changes after a heat treatment including: (i.) a change in its structure as shown with EPR and X-ray diffraction; (ii.) a release of an abundant protein into the supernatant after heat activation, which is proportional to the extent of heat activation; (iii.) a change in the permeability of the cell wall as judged by fluorescence studies in which staining of the interior of the cell wall correlated with individual germination of individual ascospores.
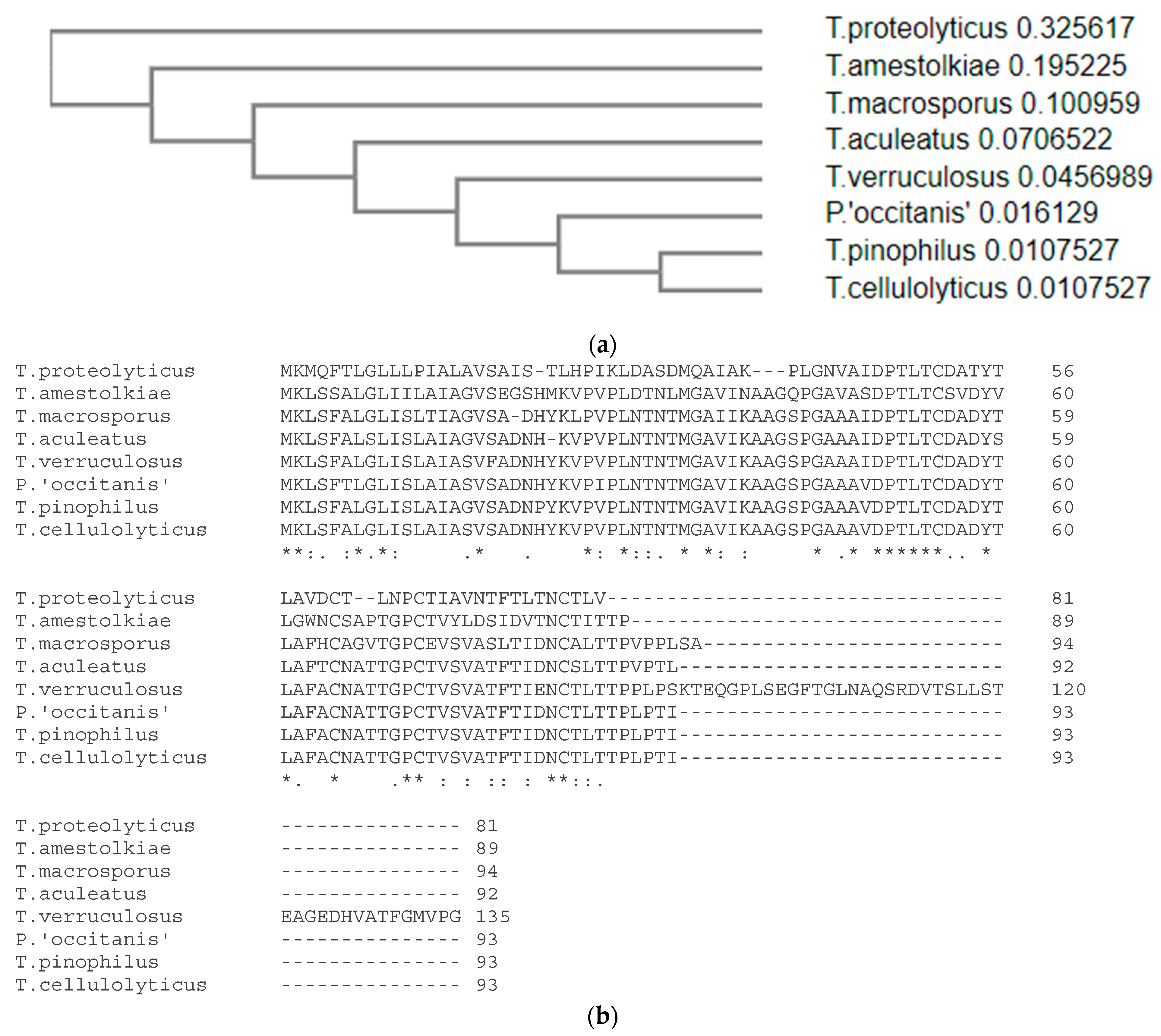
The gene encoding the protein, dubbed ICARUS, was studied in detail and was expressed under growth conditions that showed intense ascomata (fruit body) formation and therefore ascospore formation. It is a small 7–14 kD protein that is not comparable to anything we know (this work was initiated long ago and has now been approached as a “cold case”). At the time of isolation of ICARUS, no homologies could be found in the DNA or protein databases. Recently, new blast jobs were attempted and this action rendered 7 orthologues in 6 other members of the genus
Talaromyces and in a
Penicillium species (
Figure 6). The predicate “hypothetical protein” given to these 7 proteins stressed the importance of publishing all data accumulated on ICARUS. Especially when attending at its properties and its role in the interesting process it is involved in.
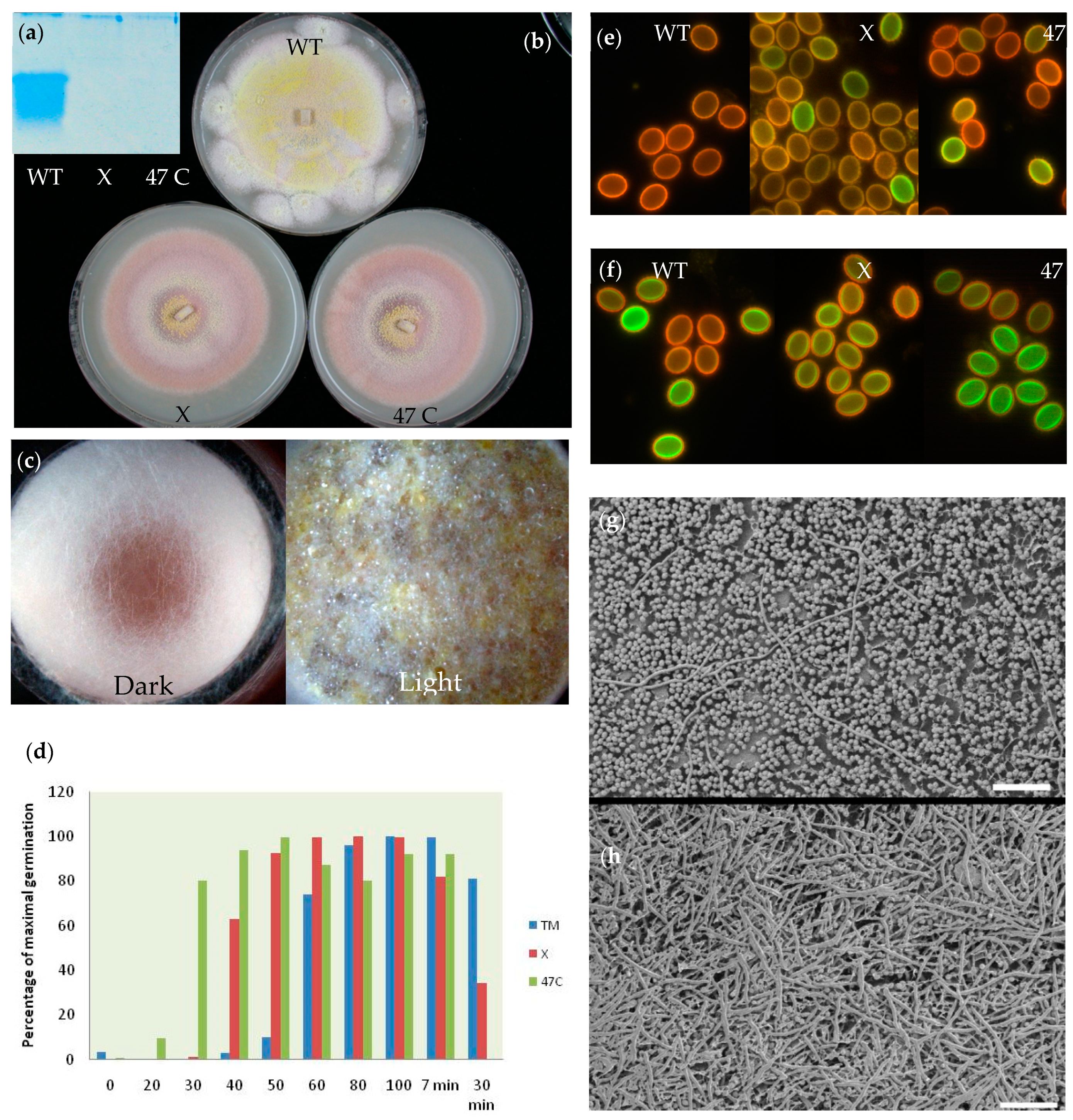
Mutant strains show a delayed ascomata formation, release of pigments into the agar medium or liquid medium, changes in the formation of fruiting bodies in the dark and a decreased dormancy of the spores, which need a shorter heat treatment for activation. All these observations suggest that the protein plays a role in dormancy and permeability of the ascospore cell wall. It is not yet clear if ICARUS is an integral part of the entire cell wall or present as an outer layer, deposited on the ascospores during maturation within the ascus mother cell. The changes in structure and permeability of the cell wall after release of the protein suggest that the protein might be related to the integral structure of the ascospore cell. Electron microscopical techniques applied at high magnification, however, do not show marked differences between the wild type and mutant cell wall structure or the nature of the ornamentation, indicating that the change is one on a very small scale (
Supplementary Figures S6 and S7). A high temperature is needed to provoke the release of the protein, with ascospores that are repetitively washed in buffer, indicating that the protein is not very loosely correlated to the ascospore.
The ICARUS mutant strains release red pigment in the growth medium. The orange/red color of ascospores is a hallmark of
T. macrosporus and the absence of the protein may affect pigment localization in the cell wall during early ascoma formation, which is accompanied with loss of pigment in the medium as if it could be less effectively incorporated or retained in ascomata and ascospores. This is possibly the red pigment mitorubrin, but this fungus also forms and abundant secondary metabolite named duclauxin [
32]. The monomer of duclauxin has some resemblance to the melanin monomers and could, based on its structure, very well fluoresce redly and may have a function in cell wall structure. However, there are quite some species that show constitutive dormancy including unpigmented species as
Aspergillus fischeri (with a neosartorya morph) and
Paecilomyces niveus (with a byssochlamys morph). If constitutive dormancy is conveyed by abundant small proteins as ICARUS, pigment binding must be different. These are aspects that needs to be studied further. These unpigmented species also show extensive heat resistance, equal to
T. macrosporus [
3]. A tantalizing observation even suggests that the function of the protein expands to signaling during fruit body formation as ascomata are not formed in mutant strains in the dark. For instance, in playing a role in light sensing via the pigment that is signaled downstream to evoke the onset of sexual spore formation.
There is clearly more research needed on this including the following research questions: (i.) Is a similar mechanism observed with other constitutively dormant ascospores [
2,
3,
4,
6,
15,
20]? These include the unpigmented species as stated above. (ii.) Are there other factors involved as there is still some heat activation needed? Analysis of cell wall preparations by means of Fourier Transformed Infrared Spectroscopy (FTIR) show that the CH asymmetric stretching vibrations, that originate from CH
2 and CH
3 of hydrocarbons, show a marked change between 60 and 80 °C, which are temperatures near to those that activate the ascospores. This could mean that temperature-related changes occur in the carbohydrate backbone of cell walls (
Supplementary Figure S8 and Table S3,
Appendix A). (iii.) Is impermeability of the cell wall the main mechanism in constitutive dormancy? Sussman and coworkers suggest [
9] that impermeability is not leading in dormancy of
Neurospora ascospores. Indeed, the cytoplasm of ascospores of
Talaromyces and
Aspergillus species with a neosartorya morph is a fluid, indicating that water is present in the cell [
4,
17]. This might point towards a role of ICARUS that is more complex as solely a barrier function, but this aspect surely needs more detailed research.
However, upon heat activation the thick outer cell wall must be shed [
17,
20] (as illustrated by
Supplementary Figure S3 in this manuscript) followed by a strong increase in respiration, which was not measurable in a dense suspension of ascospores before this shedding stage [
16]. The inner cell exhibits swelling and subsequent germ tube formation leading to the formation of a mycelium. There, the transformation of a highly stress-resistant dormant spore towards a growing vegetative cell is completed.