Differential Induction Pattern Towards Classically Activated Macrophages in Response to an Immunomodulatory Extract from Pleurotus ostreatus Mycelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Pleurotus ostreatus Strain and Extract Preparation
2.3. Cell Culture
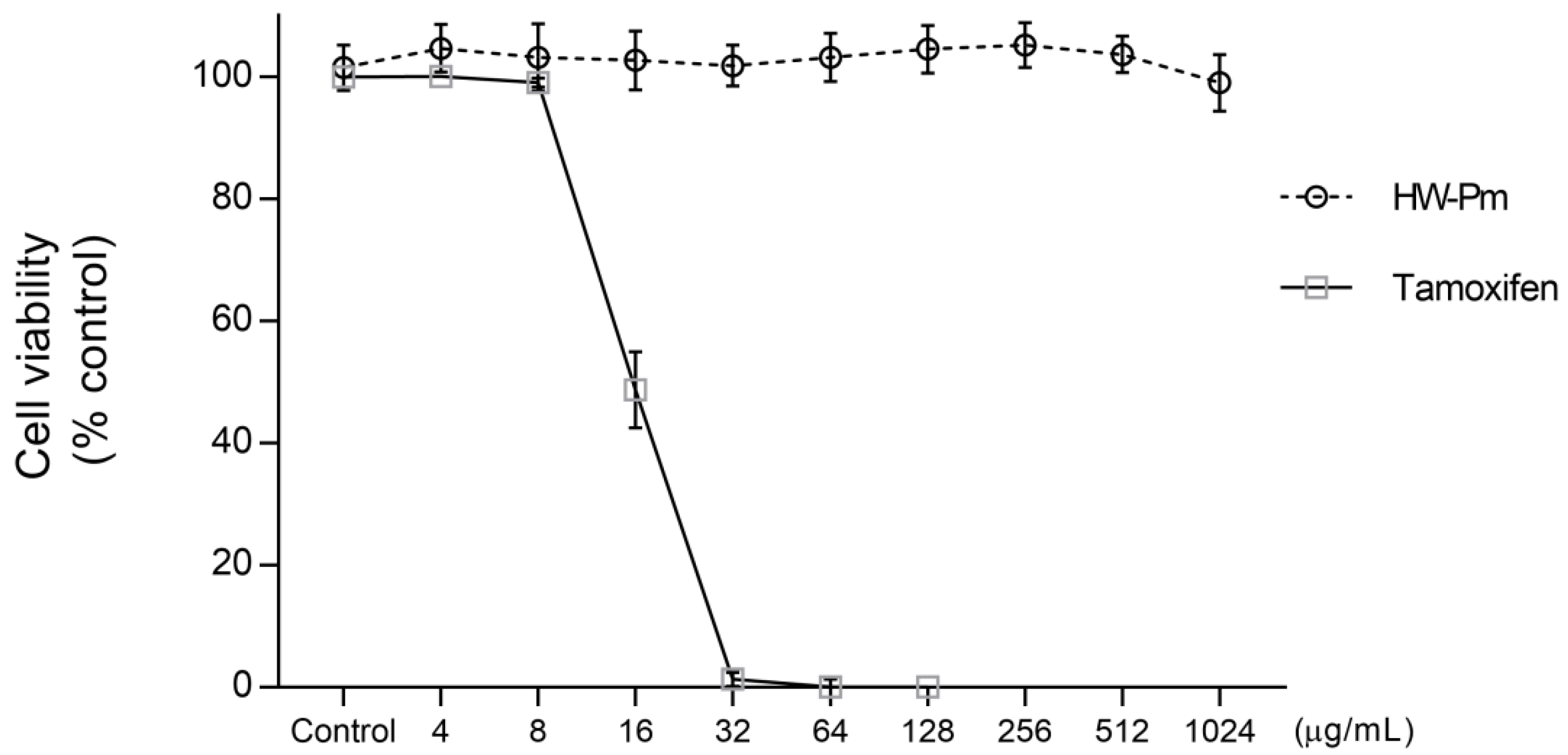
Cell Viability Assay
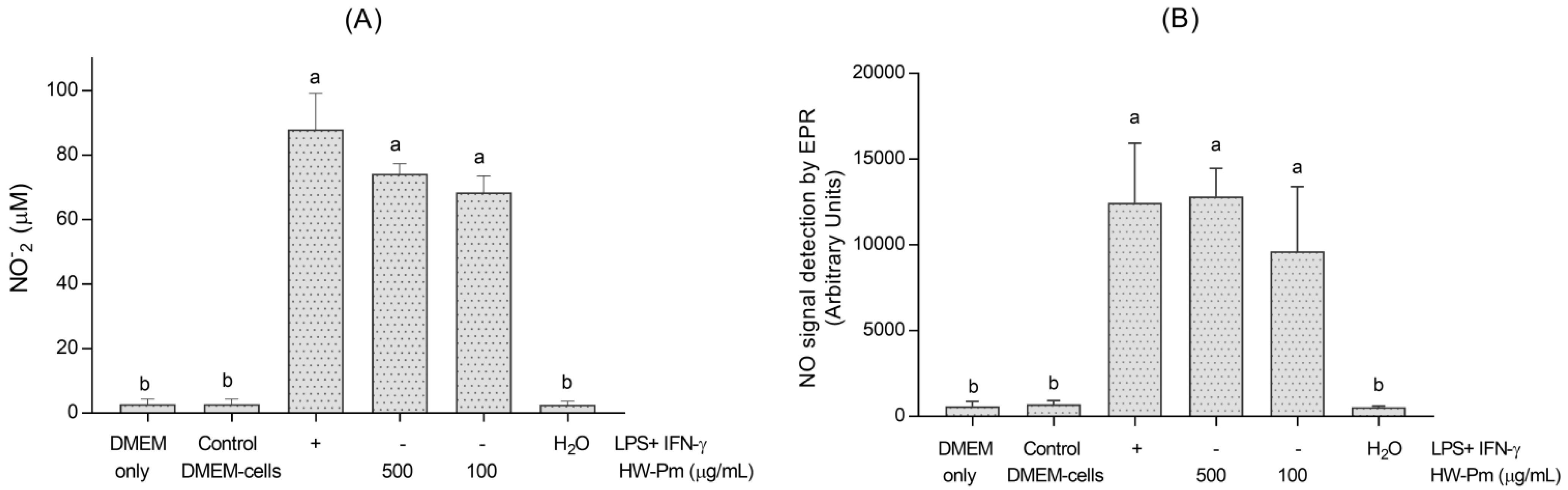
2.4. Nitrites and Cytokine Determination
2.5. Intracellular NO Determination by EPR Spectroscopy
2.6. RNA Extraction and Quantitative Real-Time PCR
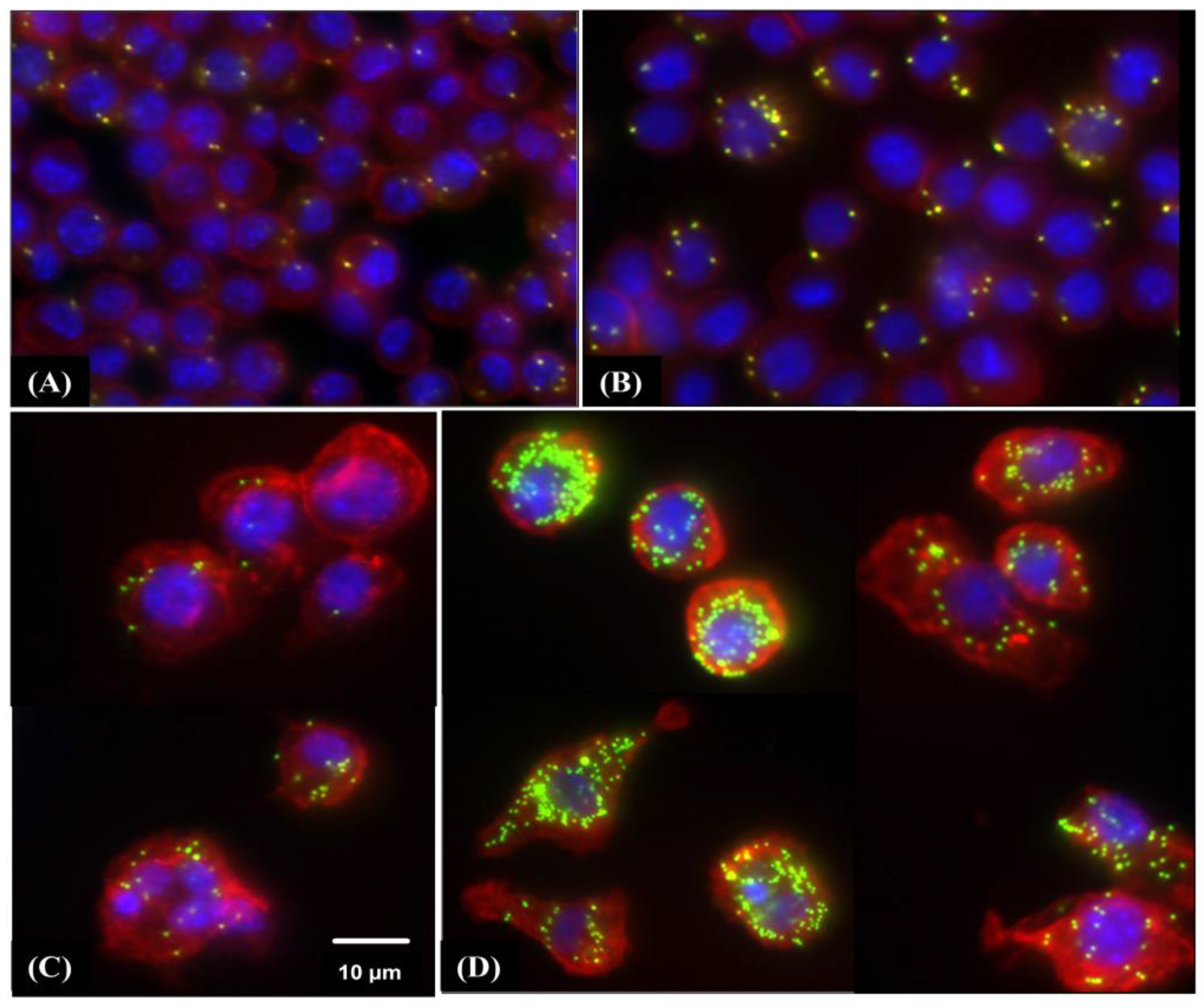
2.7. Phagocytosis Assay
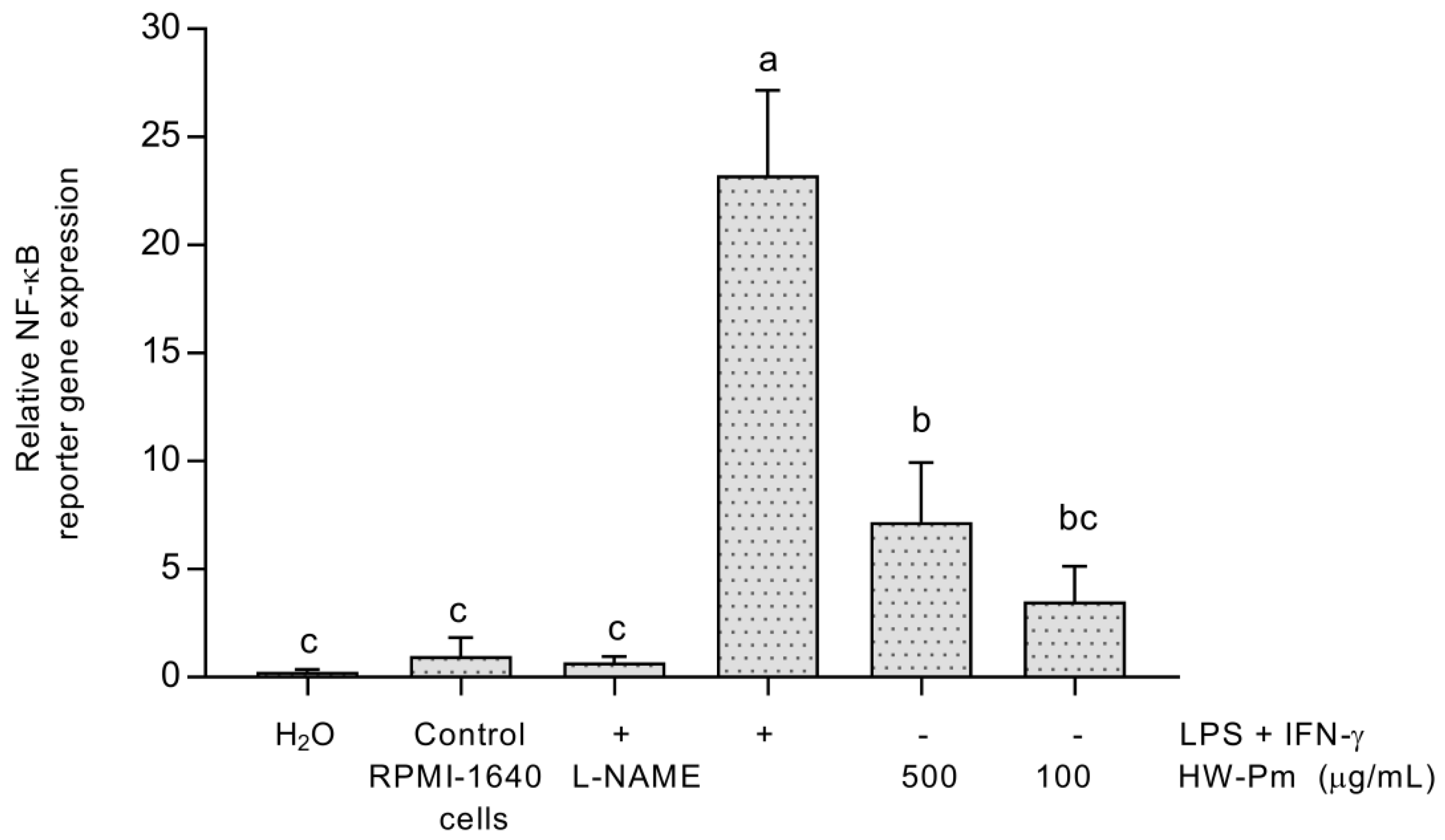
2.8. NF-κB Reporter Gene In Vitro Assay
2.9. Statistical Analysis
3. Results
3.1. Effect of HW-Pm on RAW 264.7 Macrophages Cell Viability
3.2. Effect of HW-Pm on Nitric Oxide Production in RAW 264.7 Macrophages
3.3. HW-Pm Enhanced iNOS mRNA Expression in RAW 264.7 Macrophages
3.4. Effect of HW-Pm on TNF-α and IL-6 Production in RAW 264.7 Macrophages
3.5. Phagocytic Activity of RAW 264.7 Macrophages Is Induced by HW-Pm
3.6. NF-κB Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghonime, M.; Emara, M.; Shawky, R.; Soliman, H.; El-Domany, R.; Abdelaziz, A. Immunomodulation of RAW 264.7 murine macrophage functions and antioxidant activities of 11 plant extracts. Immunol. Invest. 2015, 44, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Shin, S.; Chung, H.; Lee, S.; Lee, Y.; Kim, L.; Lee, T. Immunostimulatory effects of cordycepin-enriched WIB-801CE from Cordyceps militarisin splenocytes and cyclophosphamide-induced immunosuppressed mice. Phytother. Res. 2018, 32, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Rathore, H.; Sharma, S.; Yadav, A.S. Medicinal Mushrooms as a Source of Novel Functional Food. Int. J. Food Sci. Nut. Diet 2015, 4, 221–225. [Google Scholar]
- Morris, H.J.; Beltrán, Y.; Llauradó, G.; Batista, P.L.; Perraud-Gaime, I.; García, N.; Moukha, S.; Bermúdez, R.C.; Cos, P.; Hernández, E.; et al. Mycelia from Pleurotus sp. (oyster mushroom): A new wave of antimicrobials, anticancer and antioxidant bio-ingredients. Int. J. Phytocos. Nat. Ingred. 2017, 2. [Google Scholar] [CrossRef]
- Elisashvil, V. Submerged cultivation of medicinal mushrooms: Bioprocesses and products (review). Int. J. Med. Mushr. 2012, 14, 211–239. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef] [PubMed]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Deepalakshmi, K.; Mirunalini, S. Pleurotus ostreatus: An oyster mushroom with nutritional and medicinal properties. J. Biochem. Technol. 2014, 5, 718–726. [Google Scholar]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.H.; Magee, A.S.; Danielson, M.D.; et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 2011, 472, 471–476. [Google Scholar] [CrossRef]
- Noss, I.; Doekes, G.; Thorne, P.S.; Heederik, D.J.J.; Wouters, I.M. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate Immun. 2013, 19, 10–19. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000 Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Tomoya, L.; Nakase, H. The phagocytic function of macrophage-enforcing innate Immunity and tissue homeostasis. Int. J. Mol. Sci. 2018, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.M.; Matthias, A.; Banbury, L.; Penman, K.G.; Bone, K.M. Modulation of macrophage immune responses by Echinacea. Molecules 2005, 10, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Li, F.; He, Z.; Jiang, Y.; Hao, R.; Sun, X.; Tong, H. Anti-tumor and macrophage activation induced by alkali-extracted polysaccharide from Pleurotus ostreatus. Int. J. Biol. Macromol. 2014, 69, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW 264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohyd. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef]
- Mosser, D.M.; Zhang, X. Activation of murine macrophages. Curr. Protoc. Immunol. 2008. [Google Scholar] [CrossRef]
- Jedinak, A.; Dudhgaonkar, S.; Wu, Q.; Simon, J.; Sliva, D. Anti-inflammatory activity of edible oyster mushroom is mediated through the inhibition of NK-κB and AP-1 signaling. Nut. J. 2011, 10, 52. [Google Scholar] [CrossRef]
- Carrillo, E.; Jimenez, M.A.; Sanchez, C.; Cunha, J.; Martins, C.; Seva, A.P.; Moreno, J. Protein malnutrition impairs the immune response and influences the severity of infection in a hamster model of chronic visceral Leishmaniasis. PLoS ONE 2014, 9, e89412. [Google Scholar] [CrossRef]
- Peixoto, R.; Correia, É.M.; Oliveira, M.T.; Machado, M. Immune response of severe malnutrition children treated according to the protocol of the World Health Organization. Nutr. Hosp. 2015, 32, 638–644. [Google Scholar]
- Schaible, U.E.; Kaufmann, S. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med. 2007, 4, e115. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohyd. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Tolera, K.D.; Abera, S. Nutritional quality of Oyster Mushroom (Pleurotus Ostreatus) as affected by osmotic pretreatments and drying methods. Food Sci. Nut. 2017, 5, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Mariga, A.M.; Pei, F.; Yang, W.; Zhao, L.; Shao, Y.N.; Mugambi, D.K.; Hu, Q.H. Immunopotentiation of Pleurotus eryngii (DC. ex Fr.) Quel. J. Ethnopharmacol. 2014, 153, 604–614. [Google Scholar] [CrossRef]
- Yasin, H.; Zahoor, M.; Yousaf, Z.; Aftab, A.; Saleh, N.; Riaz, N.; Shamsheer, B. Ethnopharmacological exploration of medicinal mushroom from Pakistan. Phytomedicine 2019, 54, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Gregori, A.; Švagel, M.; Pohleven, J. Cultivation techniques and medicinal properties of Pleurotus spp. Food Technol. Biotechnol. 2007, 45, 238–249. [Google Scholar]
- Llauradó, G.; Morris, H.J.; Ferrera, L.; Camacho, M.; Castán, L.; Lebeque, Y.; Beltrán, Y.; Cos, P.; Bermúdez, R.C. In-vitro antimicrobial activity and complement/macrophage stimulating effects of a hot-water extract from mycelium of the oyster mushroom Pleurotus sp. Innov. Food Sci. Emerg. Technol. 2015, 30, 177–183. [Google Scholar] [CrossRef]
- Ghazanfari, T.; Roya, Y.; Zohre, F.; Batool, R. Macrophages activation and nitric oxide alterations in mice treated with Pleurotus florida. Immunopharmacol. Immunotoxicol. 2010, 32, 47–50. [Google Scholar] [CrossRef]
- Baskaran, A.; Kek, H.; Vikineswary, S.; Mani, R.R.; Umah, R.K. Pleurotus giganteus (Berk. Karun & Hyde), the giant oyster mushroom inhibits NO production in LPS/H2O2 stimulated RAW 264.7 cells via STAT 3 and COX-2 pathways. BMC Complem. Altern. Med. 2017, 17, 40. [Google Scholar]
- Minato, K.; Ohara, A.; Mizuno, M. A Proinflammatory Effect of the β-Glucan from Pleurotus cornucopiae Mushroom on Macrophage Action. Mediat. Inflamm. 2017, 2017, 8402405. [Google Scholar] [CrossRef]
- Morris, H.J.; Llauradó, G.; Beltrán, Y.; Lebeque, Y.; Fontaine, R.; Bermúdez, R.C.; Rodríguez, S. Procedure to Obtain an Immunoceutical Preparation from Pleurotus spp. Cuban Patent 23717 (1754/2011), 19 July 2011. (In Spanish). [Google Scholar]
- Morris, H.J.; Hernández, E.; Llauradó, G.; Tejedor, M.C.; Sancho, P.; Herraez, A.; Boyano-Adánez, M.C.; García-Pérez, A.I.; Diez, J.C. In Vitro Anti-proliferative Effects on NB4 Human Leukemia Cells and Physicochemical Screening of Pleurotus sp. (Higher Basidiomycetes) Mycelia from Cuba. Int. J. Med. Mushr. 2014, 16, 239–245. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, H.O.; Rosebrough, A.; Farr, L.; Randall, J.R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Kleschyov, A.L.; Mollnau, H.; Oelze, M.; Meinertz, T.; Huang, Y.; Harrison, D.G.; Münzel, T. Spin trapping of vascular nitric oxide using colloid Fe(II)-diethyldithiocarbamate. Biochem. Biophys. Res. Commun. 2000, 275, 672–677. [Google Scholar] [CrossRef]
- Milenkovic, D.; Vanden Berghe, W.; Boby, C.; Leroux, C.; Declerck, K.; Szarc vel Szic, K.; Heyninck, K.; Laukens, K.; Bizet, M.; Defrance, M.; et al. Dietary Flavanols Modulate the Transcription of Genes Associated with Cardiovascular Pathology without Changes in Their DNA Methylation State. PLoS ONE 2014, 9, e95527. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Ike, K.; Kameyama, N.; Ito, A.; Imai, S. Induction of a T-helper 1 (Th1) immune response in mice by an extract from the Pleurotus eryngii (Eringi) mushroom. J. Med. Food 2012, 15, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Pietretti, D.; Vera-Jimenez, N.I.; Hoole, D.; Wiegertjes, G.F. Oxidative burst and nitric oxide responses in carp macrophages induced by zymosan, MacroGard and selective dectin-1 agonists suggest recognition by multiple pattern recognition receptors. Fish. Shellfish Immunol. 2013, 35, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Reighard, K.P.; Schoenfisch, M.H. Antibacterial Action of Nitric Oxide-Releasing Chitosan Oligosaccharides against Pseudomonas aeruginosa under Aerobic and Anaerobic Conditions. Antimicrob. Agents Chemoth. 2015, 59, 6506–6513. [Google Scholar] [CrossRef][Green Version]
- Paur, I.; Austenaa, L.M.; Blomhoff, R. Extracts of dietary plants are efficient modulators of nuclear factor kappa B. Food Chem. Toxicol. 2008, 46, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nishimura, M.; Sato, Y.; Sato, H.; Nishihira, J. Enhancement of the Th1-phenotype immune system by the intake of Oyster mushroom (Tamogitake) extract in a double-blind, placebo-controlled study. J. Tradit. Complem. Med. 2016, 6, 424–430. [Google Scholar] [CrossRef]
- Im, K.H.; Nguyen, T.K.; Shin, D.B.; Lee, K.R.; Lee, T.S. Appraisal of Antioxidant and Anti-Inflammatory Activities of Various Extracts from the Fruiting Bodies of Pleurotus florida. Molecules 2014, 19, 3310–3326. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.J.; Adams, E.L.; Ozment-Skelton, T.; Gonzales, A.; Goldman, M.P.; Lockhart, B.E.; Barker, L.A.; Breuel, K.F.; DePonti, W.K.; Kalbfleisch, J.H.; et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 2005, 314, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Llauradó, G.; Morris, H.J.; Lebeque, Y.; Venet, G.; Fong, O.; Marcos, J.; Fontaine, R.; Cos, P.; Bermúdez, R.C. Oral administration of an aqueous extract from the oyster mushroom Pleurotus ostreatus enhances the immunonutritional recovery of malnourished mice. Biomed. Pharmacother. 2016, 83, 1456–1463. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.F.; Abdullah, N.; Aminudin, N. Chemical composition of the Tiger’s Milk Mushroom, Lignosus rhinocerotis (Cooke) Ryvarden, from different developmental stages. J. Agric. Food Chem. 2013, 61, 4890–4897. [Google Scholar] [CrossRef]

| M1 Subset | M2 Subset | ||
|---|---|---|---|
| iNOS | Arg-1 | FIZZ | |
| Control | 0.44 ± 0.04 c | 0.01 | - (nd) |
| LPS + IFN-γ | 5.04 ± 0.59 ab | 0.03 | - |
| HW-Pm (500 µg/mL) | 5.98 ± 0.51 a | 0.01 | - |
| HW-Pm (100 µg/mL) | 4.17 ± 0.54 b | 0.03 | - |
| Number of Beads/50 Cells | Phagocytic Ratio (%) | |
|---|---|---|
| Control | 72.3 ± 4.8 d | 42 ± 4.3 c |
| LPS + IFN-γ | 357 ± 6.8 b | 58.3 ± 3.9 b |
| HW-Pm (500 µg/mL) | 454 ± 30.8 a | 75.3 ± 2.5 a |
| HW-Pm (100 µg/mL) | 239 ± 13.9 c | 60 ± 4.9 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llauradó Maury, G.; Morris-Quevedo, H.J.; Heykers, A.; Lanckacker, E.; Cappoen, D.; Delputte, P.; Vanden Berghe, W.; Salgueiro, Z.; Cos, P. Differential Induction Pattern Towards Classically Activated Macrophages in Response to an Immunomodulatory Extract from Pleurotus ostreatus Mycelium. J. Fungi 2021, 7, 206. https://doi.org/10.3390/jof7030206
Llauradó Maury G, Morris-Quevedo HJ, Heykers A, Lanckacker E, Cappoen D, Delputte P, Vanden Berghe W, Salgueiro Z, Cos P. Differential Induction Pattern Towards Classically Activated Macrophages in Response to an Immunomodulatory Extract from Pleurotus ostreatus Mycelium. Journal of Fungi. 2021; 7(3):206. https://doi.org/10.3390/jof7030206
Chicago/Turabian StyleLlauradó Maury, Gabriel, Humberto J. Morris-Quevedo, Annick Heykers, Ellen Lanckacker, Davie Cappoen, Peter Delputte, Wim Vanden Berghe, Zelene Salgueiro, and Paul Cos. 2021. "Differential Induction Pattern Towards Classically Activated Macrophages in Response to an Immunomodulatory Extract from Pleurotus ostreatus Mycelium" Journal of Fungi 7, no. 3: 206. https://doi.org/10.3390/jof7030206
APA StyleLlauradó Maury, G., Morris-Quevedo, H. J., Heykers, A., Lanckacker, E., Cappoen, D., Delputte, P., Vanden Berghe, W., Salgueiro, Z., & Cos, P. (2021). Differential Induction Pattern Towards Classically Activated Macrophages in Response to an Immunomodulatory Extract from Pleurotus ostreatus Mycelium. Journal of Fungi, 7(3), 206. https://doi.org/10.3390/jof7030206








