New Dothideomycetes from Freshwater Habitats in Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection and Fungal Isolation
2.2. Phenotypic Study
2.3. DNA Extraction, Amplification, and Sequencing
2.4. Phylogenetic Analysis
3. Results
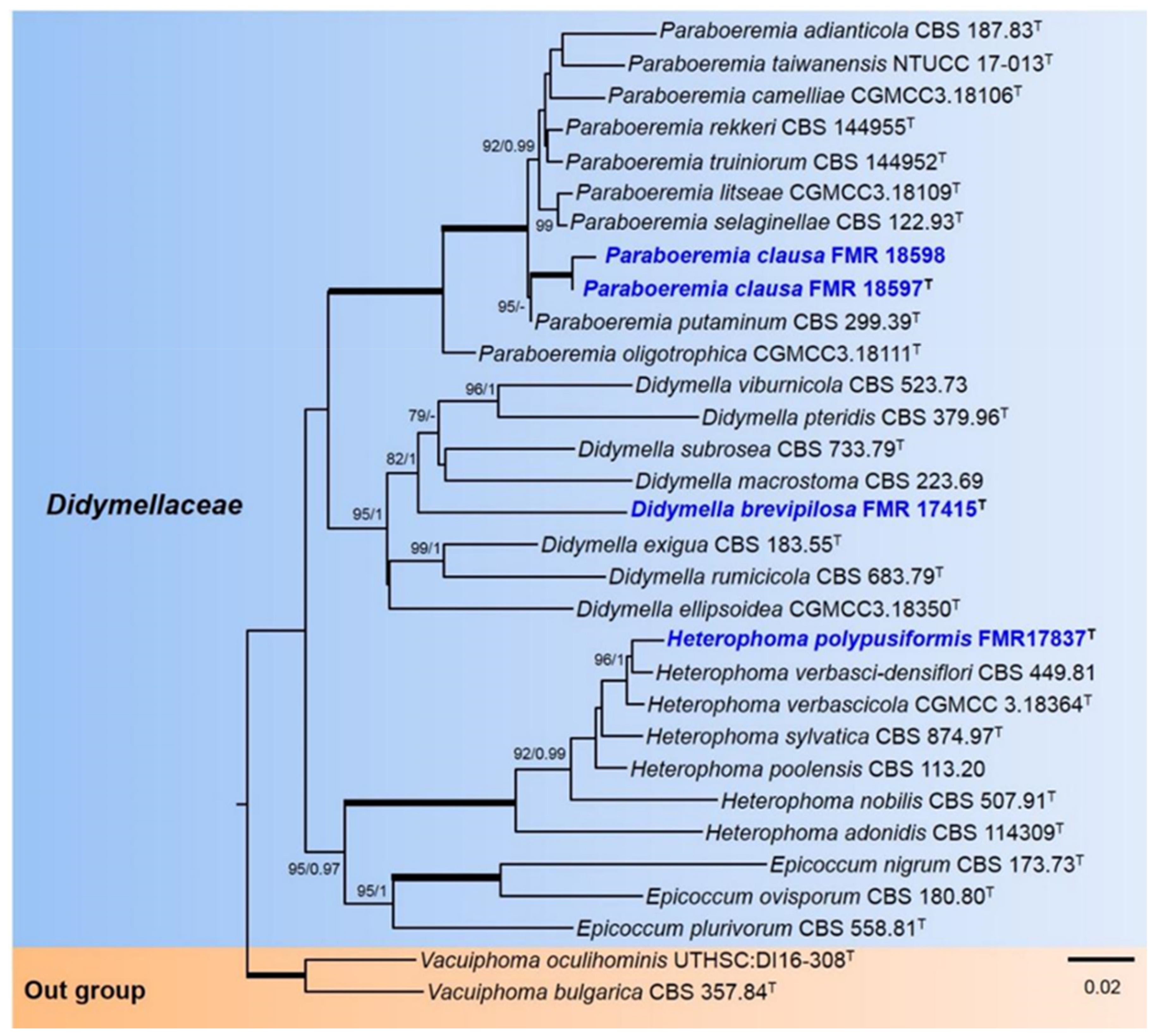
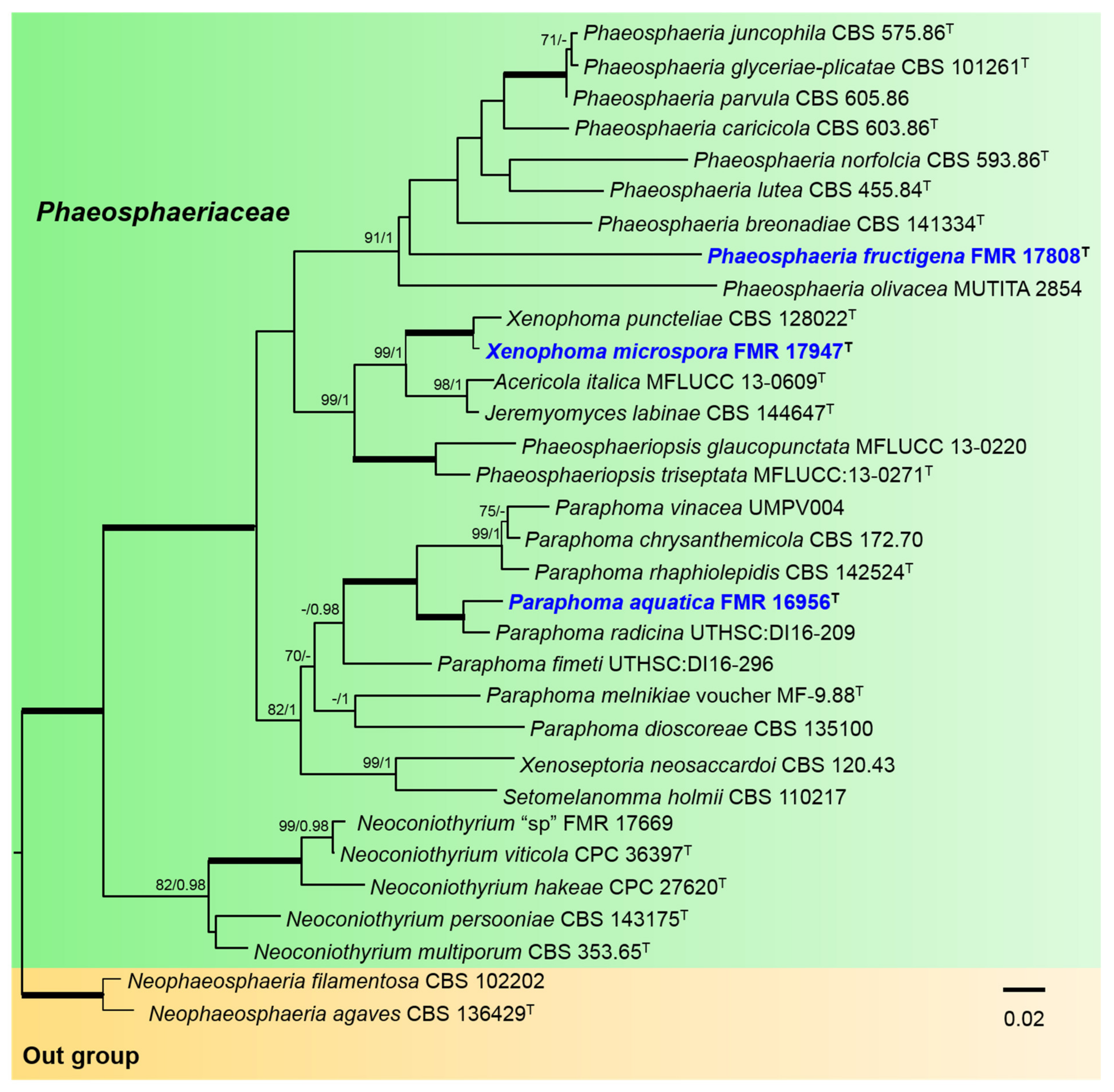
3.1. Phylogeny
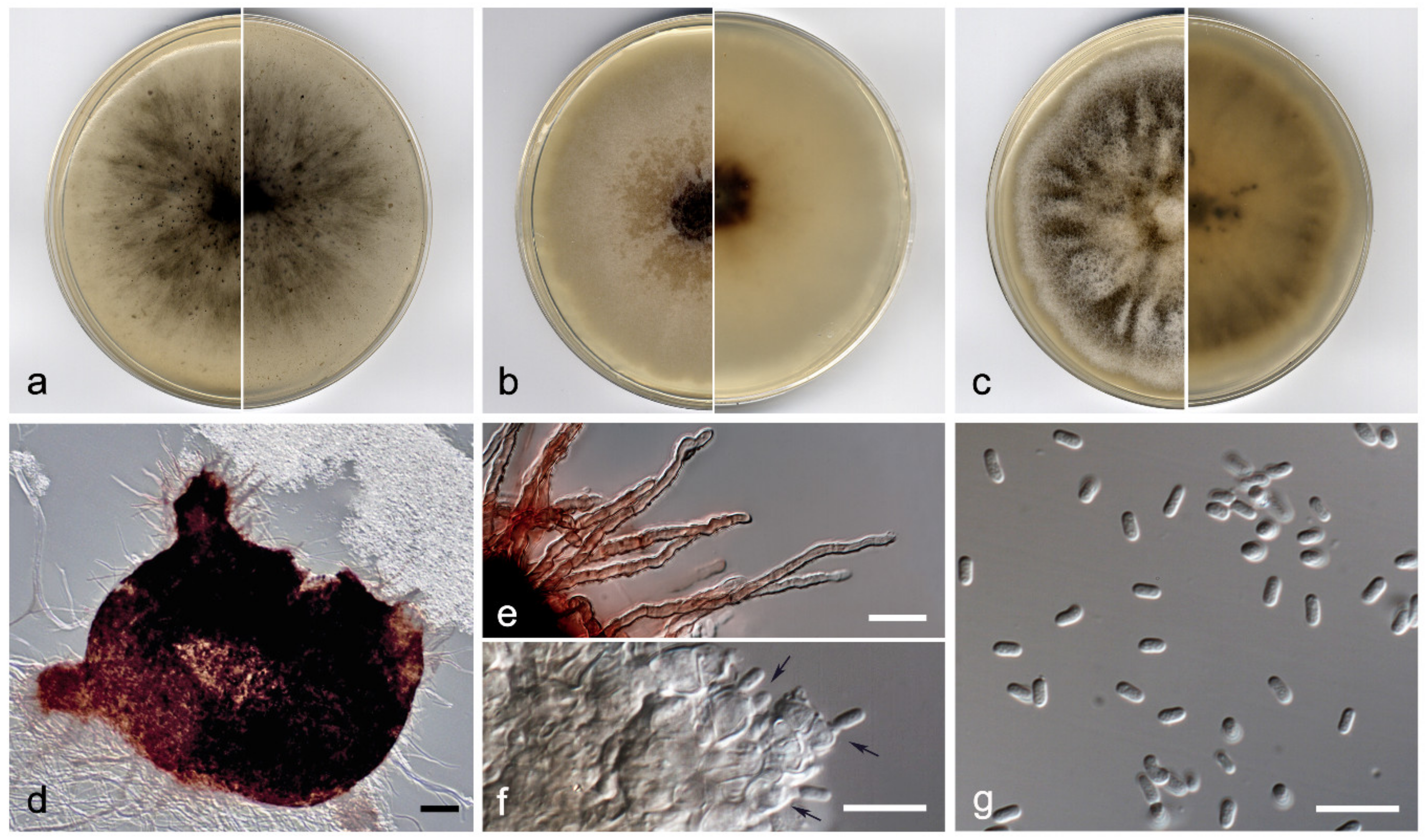
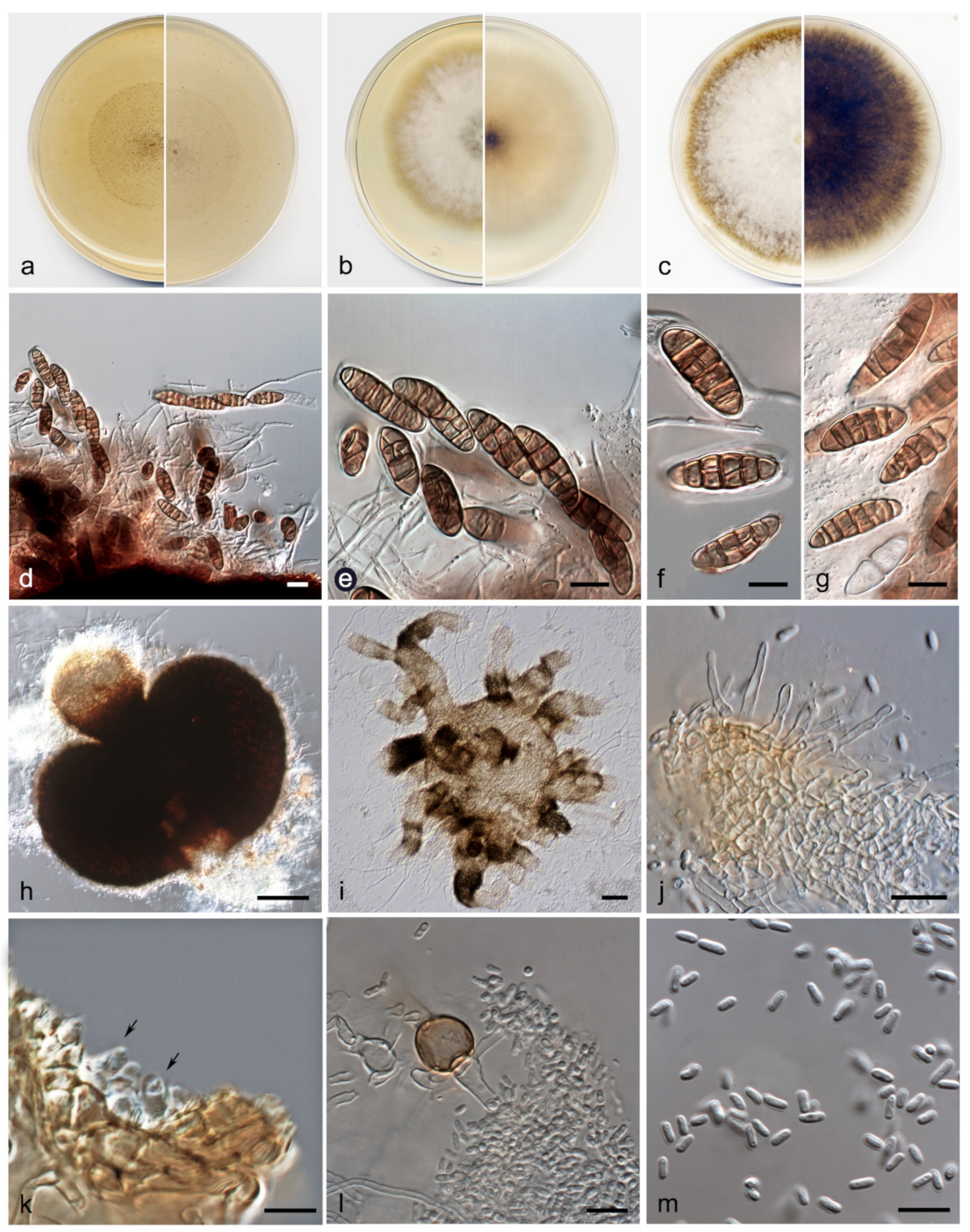
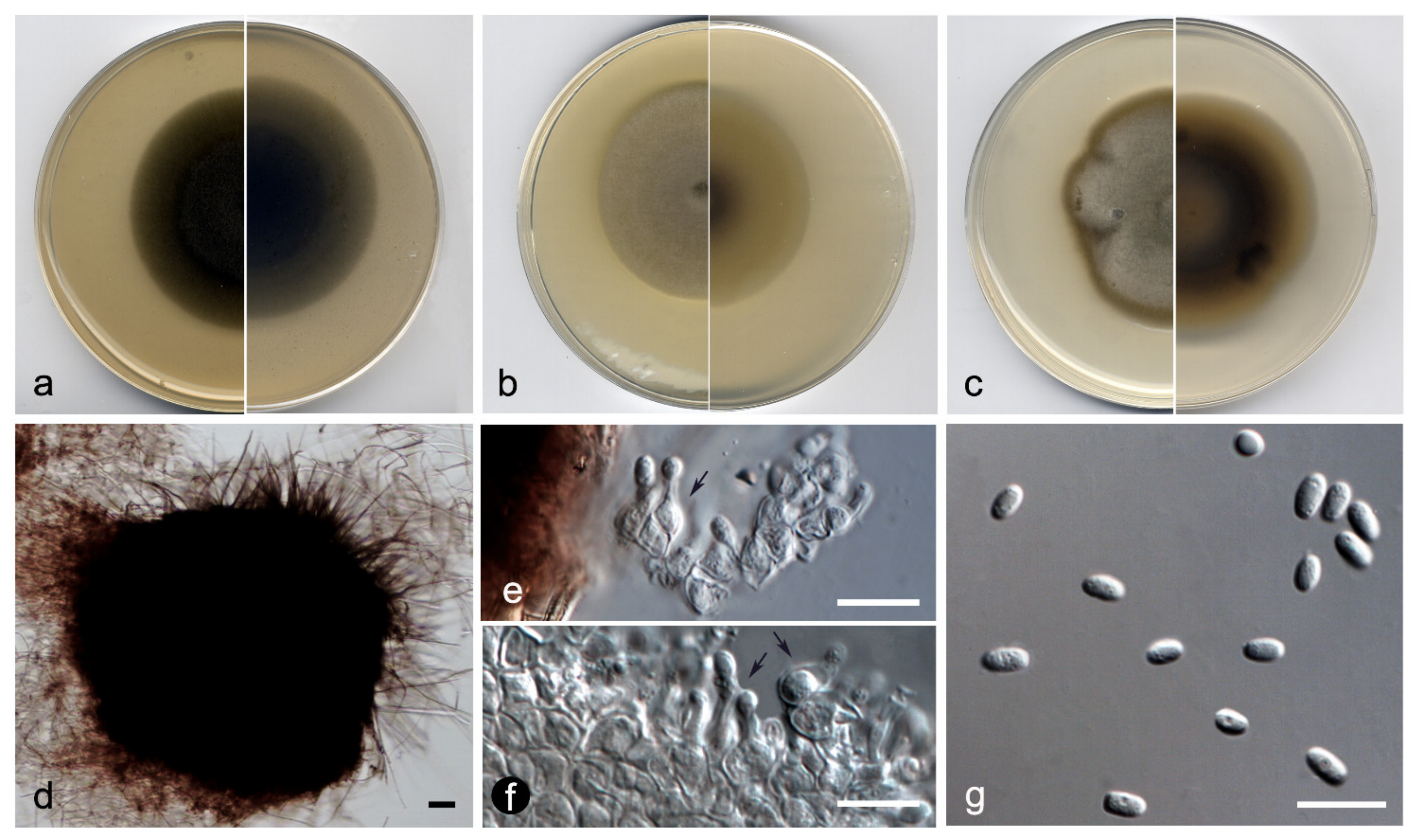
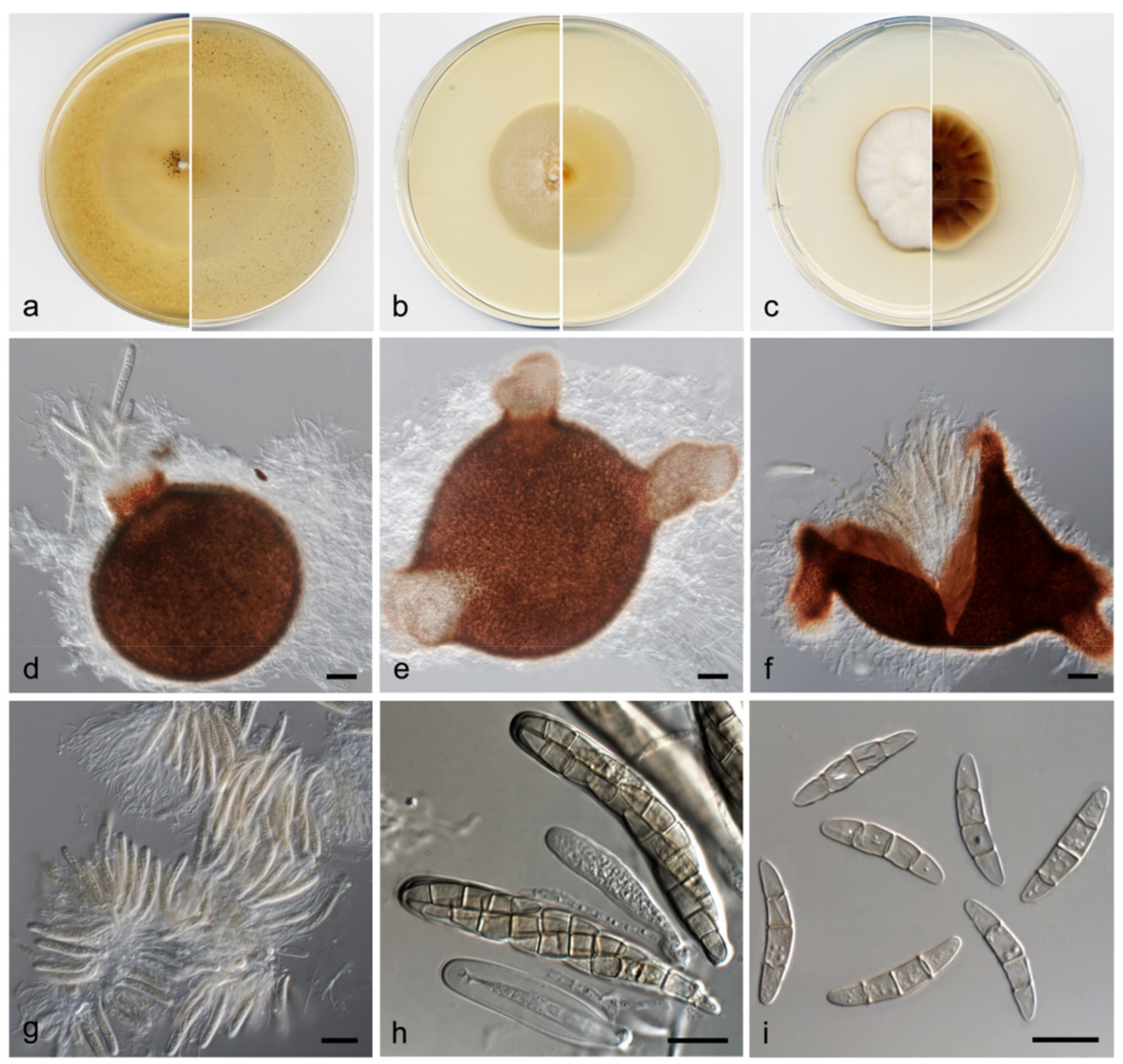
3.2. Taxonomy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goh, T.K.; Hyde, K.D. Biodiversity of freshwater fungi. Ind. Microbiol. Biotechnol. 1996, 17, 328–345. [Google Scholar] [CrossRef]
- Dian-Ming, H.; Cai, L.; Jones, E.G.B.; Zhang, H.; Boonyuen, N.; Hyde, K.D. Taxonomy of filamentous asexual fungi from freshwater habitats, links to sexual morphs and their phylogeny. In Freshwater Fungi: And Fungal-Like Organisms, 1st ed.; Jones, E.G.B., Hyde, K.D., Pang, K.L., Eds.; De Gruyter: Berlín, Germany, 2014; pp. 109–131. [Google Scholar]
- Wong, M.K.M.; Goh, T.K.; Hodgkiss, I.J.; Hyde, K.D.; Ranghoo, V.M.; Tsui, C.K.M.; Ho, W.-H.; Wong, W.S.W.; Yuen, T.-K. Role of fungi in freshwater ecosystems. Biodivers. Conserv. 1998, 7, 1187–1206. [Google Scholar] [CrossRef]
- Shearer, C.A.; Raja, H.A.; Miller, A.N.; Nelson, P.; Tanaka, K.; Hirayama, K.; Marvanová, L.; Hyde, K.D.; Zhang, Y. The molecular phylogeny of freshwater Dothideomycetes. Stud. Mycol. 2009, 64, 145–153. [Google Scholar] [CrossRef]
- Shearer, C.A.; Pang, K.L.; Suetrong, S.; Raja, H.R. Phylogeny of the Dothideomycetes and other classes of freshwater fissitunicate Ascomycota. In Freshwater Fungi: And Fungal-Like Organisms, 1st ed.; Jones, E.G.B., Hyde, K.D., Pang, K.L., Eds.; De Gruyter: Berlín, Germany, 2014; pp. 25–46. [Google Scholar]
- Hyde, K.D.; Jones, E.B.G.; Liu, J.K.; Ariyawansa, H.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.-Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Dong, W.; Wang, B.; Hyde, K.D.; McKenzie, E.H.C.; Raja, H.A.; Tanaka, K.; Abdel-Wahab, M.A.; Abdel-Aziz, F.A.; Doilom, M.; Phookamsak, R.; et al. Freshwater Dothideomycetes. Fungal Divers. 2020, 105, 319–575. [Google Scholar] [CrossRef]
- Schoch, C.L.; Crous, P.W.; Groenewald, J.Z.; Boehm, E.W.A.; Burgess, T.I.; De Gruyter, J.; De Hoog, G.S.; Dixon, L.J.; Grube, M.; Gueidan, C.; et al. A class-wide phylogenetic assessment of Dothideomycetes. Stud. Mycol. 2009, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hongsanan, S.; Hyde, K.D.; Pookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.C.; Sarma, V.V.; Boonmee, S.; Lücking, R.; Bhat, D.J.; Liu, N.G.; et al. Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 2020, 11, 1553–2107. [Google Scholar] [CrossRef]
- Liu, J.K.; Hyde, K.D.; Jeewon, R.; Phillips, A.J.; Maharachchikumbura, S.S.N.; Ryberg, M.; Liu, Z.-Y.; Zhao, Q. Ranking higer taxa using divergence times: A case study in Dothideomycetes. Fungal Divers. 2017, 84, 75–99. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Mortimer, P.E.; Senwanna, C.; Cheewangkoon, R. Saprobic Dothideomycetes in Thailand: Phaeoseptum hydei sp. nov., a new terrestrial ascomycete in Phaeoseptaceae. Phytotaxa 2020, 449, 149–163. [Google Scholar] [CrossRef]
- Mapook, A.; Hyde, K.D.; McKenzie, E.H.C.; Jones, E.G.B.; Bhat, D.J.; Jeewon, R.; Stadler, M.; Samarakoon, M.C.; Malaithong, M.; Tanunchai, B.; et al. Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (Siam weed). Fungal Divers. 2020, 101, 1–175. [Google Scholar] [CrossRef]
- Magaña-Dueñas, V.; Stchigel, A.M.; Cano-Lira, J.F. New Coelomycetous fungi from freshwater in Spain. J. Fungi 2021, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Dueñas, V.; Stchigel, A.M.; Cano-Lira, J.F. New Taxa of the Family Amniculicolaceae (Pleosporales, Dothiceomycetes, Ascomycota) from Freshwater Habitats in Spain. Miroorganisms 2020, 8, 1355. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0. for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic. Acids. Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic. Acids. Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: Enabling High-Impact science for phylogenetics researchers with limited resources. In Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the Extreme to the Campus and Beyond, Chicago, IL, USA, 16–20 July 2012; Association for Computing Machinery: New York, NY, USA, 2012; pp. 1–8. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van Der, M.P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Posada, D. JModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Hespanhol, L.; Vallio, C.S.; Costa, L.M.; Saragiotto, B.T. Understanding and interpreting confidence and credible intervals around effect estimates. Braz. J. Phys. Ther. 2019, 23, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hou, L.W.; Duan, W.J.; Crous, P.W.; Cai, L. Didymellaceae revisited. Stud. Mycol. 2017, 87, 105–159. [Google Scholar] [CrossRef]
- Valenzuela-Lopez, N.; Cano-Lira, J.F.; Guarro, J.; Sutton, D.A.; Wiederhold, N.; Crous, P.W.; Schigel, A.M. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud. Mycol. 2018, 90, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Wanasinghe, D.N.; Jeewon, R.; Person, D.; Jones, E.B.G.; Camporesi, E.; Bulgakov, T.S.; Gafforov, Y.S.; Hyde, K.D. Taxonomic circumscription and phylogenetics of novel didymellaceous taxa with Brown muriform spores. Stud. Fungi 2018, 3, 152–175. [Google Scholar] [CrossRef]
- De Gruyter, J.; Noordeloos, M.E. Contributions towards a monograph of Phoma (Coelomycetes)-I.1. Section Phoma: Taxa with very small conidia in vitro. Persoonia 1992, 15, 71–92. [Google Scholar]
- Pookamsak, R.; Liu, J.-K.; McKenzie, E.H.C.; Manamgoda, D.S.; Ariyawansa, H.; Thambugala, K.M.; Dai, D.-Q.; Camporesi, E.; Chukeatirote, E.; Wijayawardene, N.N.; et al. Revision of Phaeosphaereceae. Fungal Divers. 2014, 68, 159–238. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.W.; Hernández-Restrepo, M.; Groenewald, J.Z.; Crous, P.W. Citizen science project reveals high diversity in Didymellaceae (Pleosporales, Dothideomycetes). MycoKeys 2020, 65, 49–99. [Google Scholar] [CrossRef]
- Hou, L.W.; Groenewald, J.Z.; Pfenning, L.H.; Yarden, O.; Crous, P.W.; Cai, L. The phoma-like dilemma. Stud. Mycol. 2020, 96, 309–396. [Google Scholar] [CrossRef]
- Jiang, J.-R.; Chen, Q.; Cai, L. Polyphasic characterisation of three novel species of Paraboeremia. Mycol. Prog. 2016, 16, 285–295. [Google Scholar] [CrossRef]
- Saccardo, P.A. Conspectus generum fungorum Italiae inferiorum, nempe ad Sphaerosideas, Melanconieas et Hyphomyceteas pertinentium, systemate sporologico dispositum. Michelia 1880, 2, 33. [Google Scholar]
- Aveskamp, M.M.; De Gruyter, J.; Crous, P.W. Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Divers. 2008, 31, 1–18. [Google Scholar]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Carnegie, A.J.; Hardy, G.E.S.; Smith, D.; Summerell, B.A.; Cano-Lira, J.F.; Guarro, J.; Hobraken, J.; et al. Fungal Planet description sheets: 625–715. Persoonia 2017, 39, 270–467. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.S.; Gené, J.; Guarro, J.; Baseia, I.G.; García, D.; Gusmao, L.F.P.; Souza-Motta, C.M.; et al. Fungal Planet description sheets: 716–784. Peersonia 2018, 40, 239–392. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Dong, Y.; Phookamsak, R.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Liu, N.-G.; Abeywickrama, P.D.; Mapook, A.; Wei, D.; et al. Fungal diversity notes 1151–1276: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2020, 100, 5–277. [Google Scholar] [CrossRef] [Green Version]
- Miyake, I. Studies on the parasitic fungi of rice in Japan. Bot. Mag. Tokyo 1909, 23, 85–97. [Google Scholar]
- Eriksson, O.E. On graminicolous pyrenomycetes from Fennoscandia. I, II, III. Ark. Bot. 1967, 26, 339–466. [Google Scholar]
- Barr, M.E. A classification of Loculoascomycetes. Mycologia 1979, 71, 935–957. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Jeewon, R.; Gentekaki, E.; Kuo, C.H.; Hyde, K.D. Multi-gene phylogeny and morphotaxonomy of Phaeosphaeria ampeli sp. nov. from Ficus ampelas and a new record of P. musae from Roystonea regia. Phytotaxa 2019, 406, 111–128. [Google Scholar] [CrossRef]
- Morgan-Jones, G.; White, J.F. Studies on the genus Phoma. III. Paraphoma, a new genus to accommodate Phoma radicina. Mycotaxon 1983, 18, 57–65. [Google Scholar]
- Boerema, G.H. Contributions towards a monograph of Phoma (Coelomycetes)—V. Subdivision of the genus in sections. Mycotaxon 1997, 64, 321–333. [Google Scholar]
- De Gruyter, J.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Systematic reappaisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 2010, 102, 1066–1081. [Google Scholar] [CrossRef]
- Boerema, G.H.; de Gruyer, J.; Noordeloos, M.E.; Hamers, M. Phoma Identification Manual: Differentiation of Specific and Infra-Specific Taxa in Culture, 1st ed.; CABI Publishing: Wallingford, UK, 2004; 448p. [Google Scholar]
- Lawrey, J.L.; Diederich, P.; Nelsen, M.N.; Freebury, C.; Van den Broceck, D.; Sikaroodi, M.; Ertz, D. Phylogenetic placement of lochenicolous Phoma species in the Phaeosphaeriaceae (Pleosporales Dothideomycetes). Fungal Divers. 2012, 55, 195–213. [Google Scholar] [CrossRef]
- Trakunyingcharoen, T.; Lombard, L.; Groenewald, J.Z.; Cheewangkoon, R.; Toanun, C.; Alfenas, A.C.; Crous, P.W. Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus 2014, 5, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Shearer, C.A. The freshwater Ascomycetes. Nova Hedwigia 1993, 56, 1–33. [Google Scholar]
- Wanasinghe, D.N.; Gareth Jones, E.B.; Camporesi, E.; Mortimer, P.E.; Xu, J.; Bahkali, A.H.; Hyde, K.D. The Genus Murispora. Cryptogam. Mycol. 2015, 36, 419–448. [Google Scholar] [CrossRef]
- Raja, H.A.; Paguigan, N.D.; Fournier, J.; Oberlies, N.H. Additions to Lindgomyces (Lindgomycetaceae, Pleosporales, Dothideomycetes), Including two new species occurring on submerged wood from North Carolina, USA, with notes on secondary metabolite profiles. Mycol. Prog. 2017, 16, 535–552. [Google Scholar] [CrossRef]
- Abdel-Aziz, F.A. The genus Lolia from freshwater habitats in Egypt with one new species. Phytotaxa 2016, 267, 279–288. [Google Scholar] [CrossRef]
- Zhang, H.; Hyde, K.D.; Mckenzie, E.H.; Bahkali, A.H.; Zhou, D. Sequence data reveals phylogenetic affinities of Acromalia aquatica sp. nov., Aquasubmersa mircensis gen. et sp. nov. and Clohesyomuces aquaticus. (freshwater Coelomycetes). Cryptogam. Mycol. 2012, 33, 333–346. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaña-Dueñas, V.; Cano-Lira, J.F.; Stchigel, A.M. New Dothideomycetes from Freshwater Habitats in Spain. J. Fungi 2021, 7, 1102. https://doi.org/10.3390/jof7121102
Magaña-Dueñas V, Cano-Lira JF, Stchigel AM. New Dothideomycetes from Freshwater Habitats in Spain. Journal of Fungi. 2021; 7(12):1102. https://doi.org/10.3390/jof7121102
Chicago/Turabian StyleMagaña-Dueñas, Viridiana, José Francisco Cano-Lira, and Alberto Miguel Stchigel. 2021. "New Dothideomycetes from Freshwater Habitats in Spain" Journal of Fungi 7, no. 12: 1102. https://doi.org/10.3390/jof7121102
APA StyleMagaña-Dueñas, V., Cano-Lira, J. F., & Stchigel, A. M. (2021). New Dothideomycetes from Freshwater Habitats in Spain. Journal of Fungi, 7(12), 1102. https://doi.org/10.3390/jof7121102







