Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats
Abstract
:1. Introduction
2. Material and Methods
2.1. Collection of Wood Decay Fungal Strains
2.2. Molecular Identification
2.3. Choice of Fungal Strains
2.4. Fungal Growth under Different Conditions
- (a)
- Fungal strains growth rate
- (b)
- Method of producing fungal mats and materials made therefrom (patented)
2.5. Thermogravimetric Analysis (TGA) on Mycelium Mats of the 21 Selected Strains Grown on Liquid Culture and Using Mogu’s Patented Method
2.6. Scanning Electron Microscopy (SEM) on Mycelium Mats Grown on Liquid Culture and Using Mogu’s Patented Method
3. Results and Discussion
3.1. Wood Decay Fungal Strains
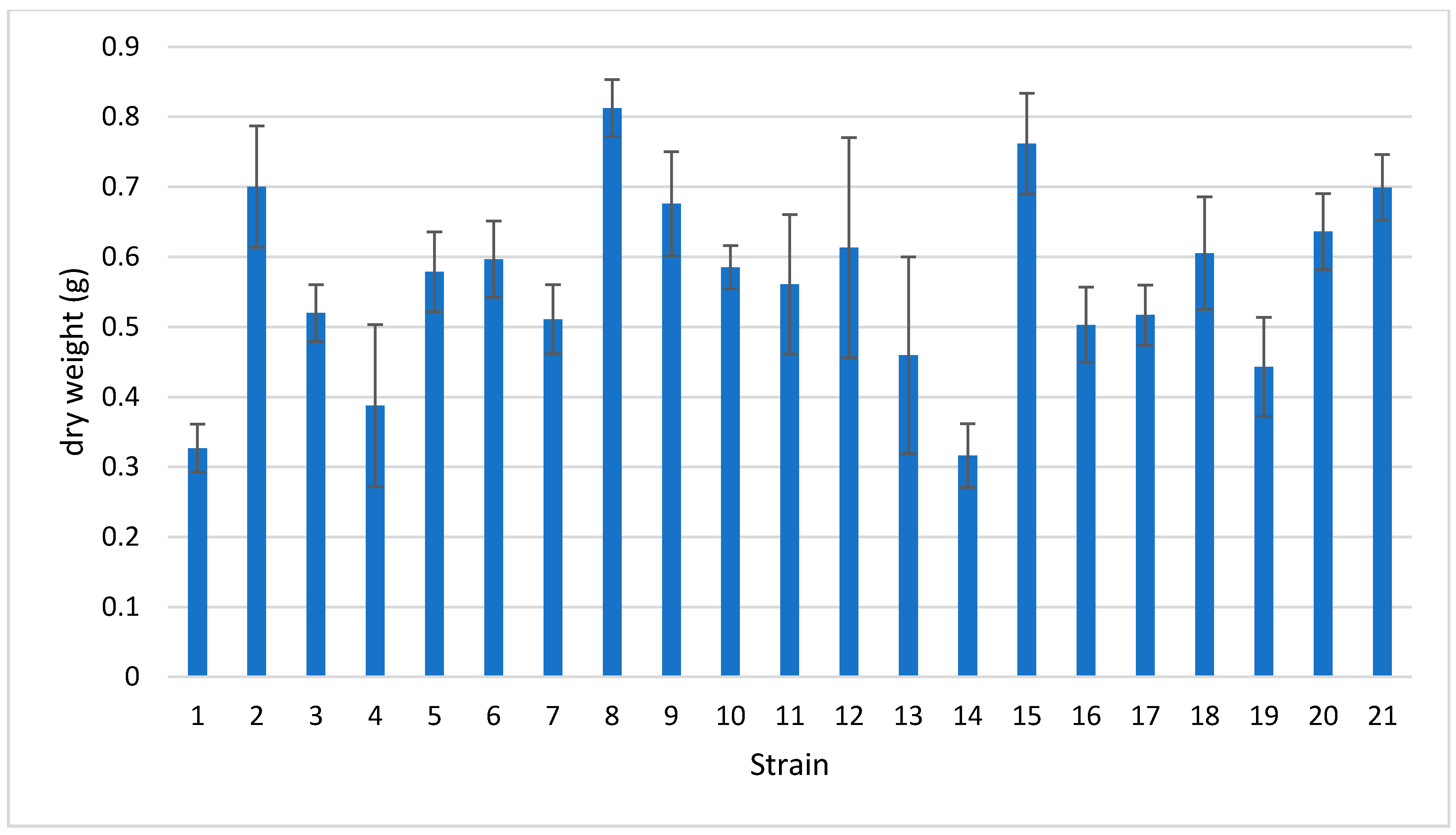
3.2. Fungal Strains Growth Rate
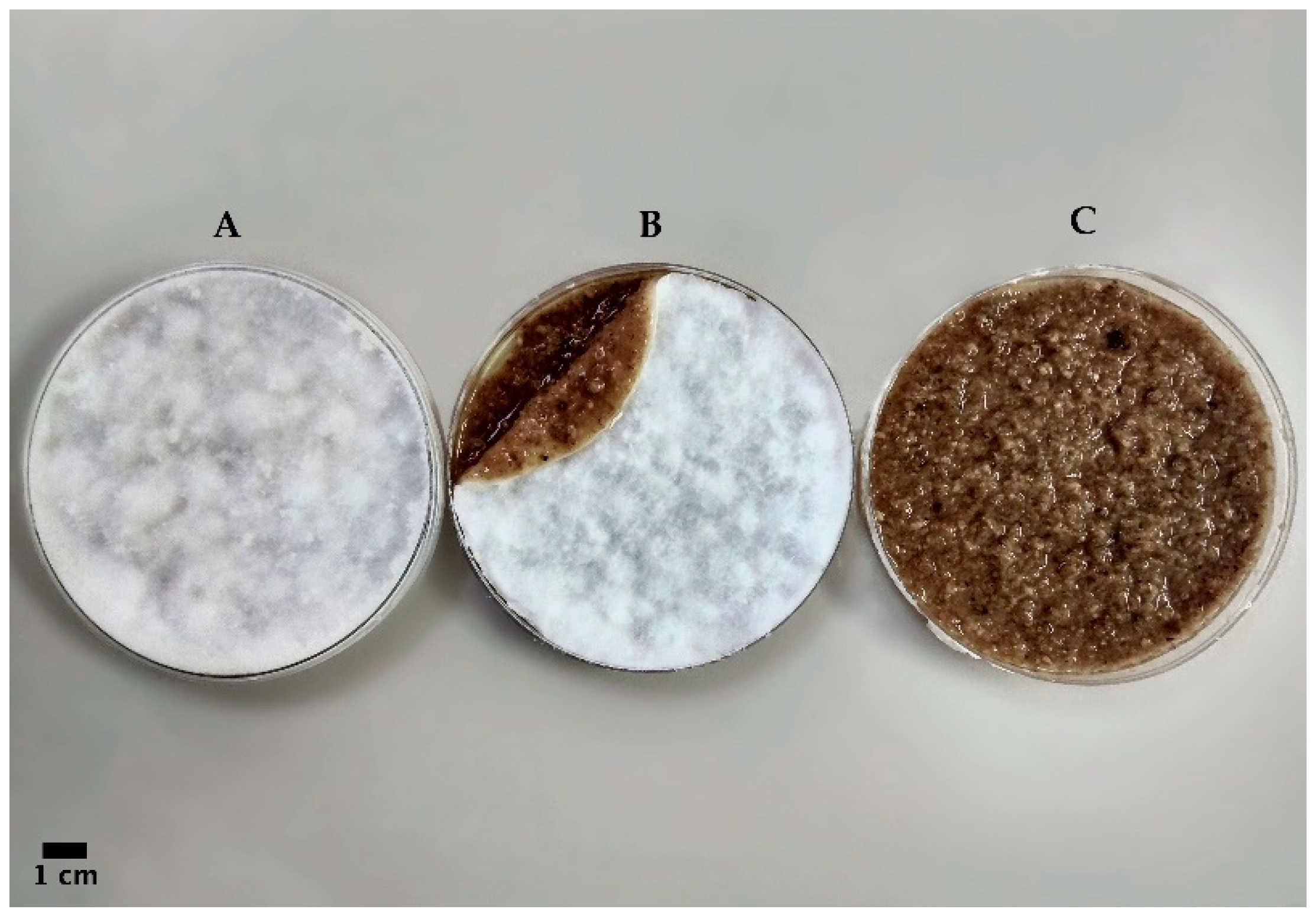
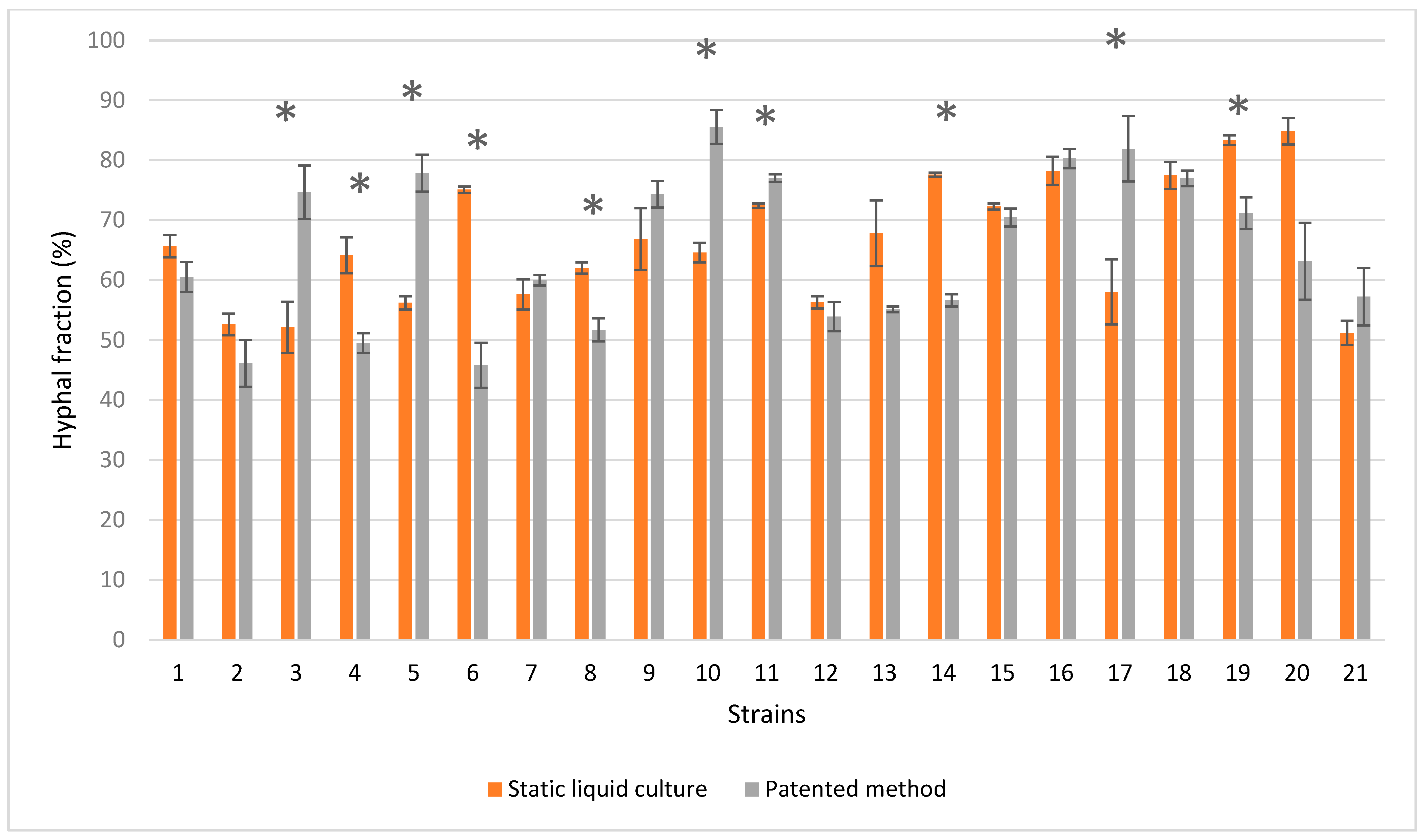
3.3. Strains Cultivation Using Patented Method
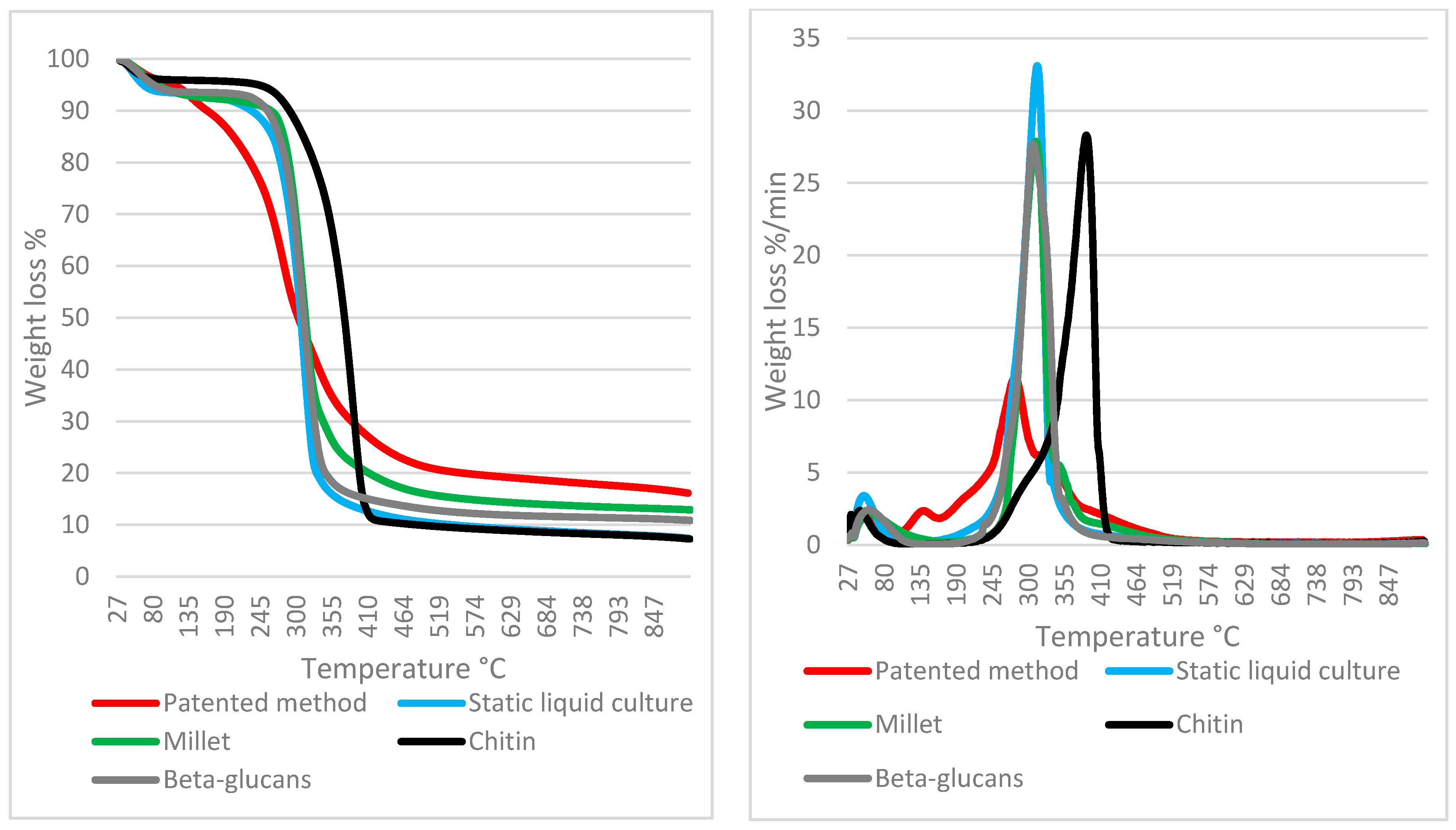
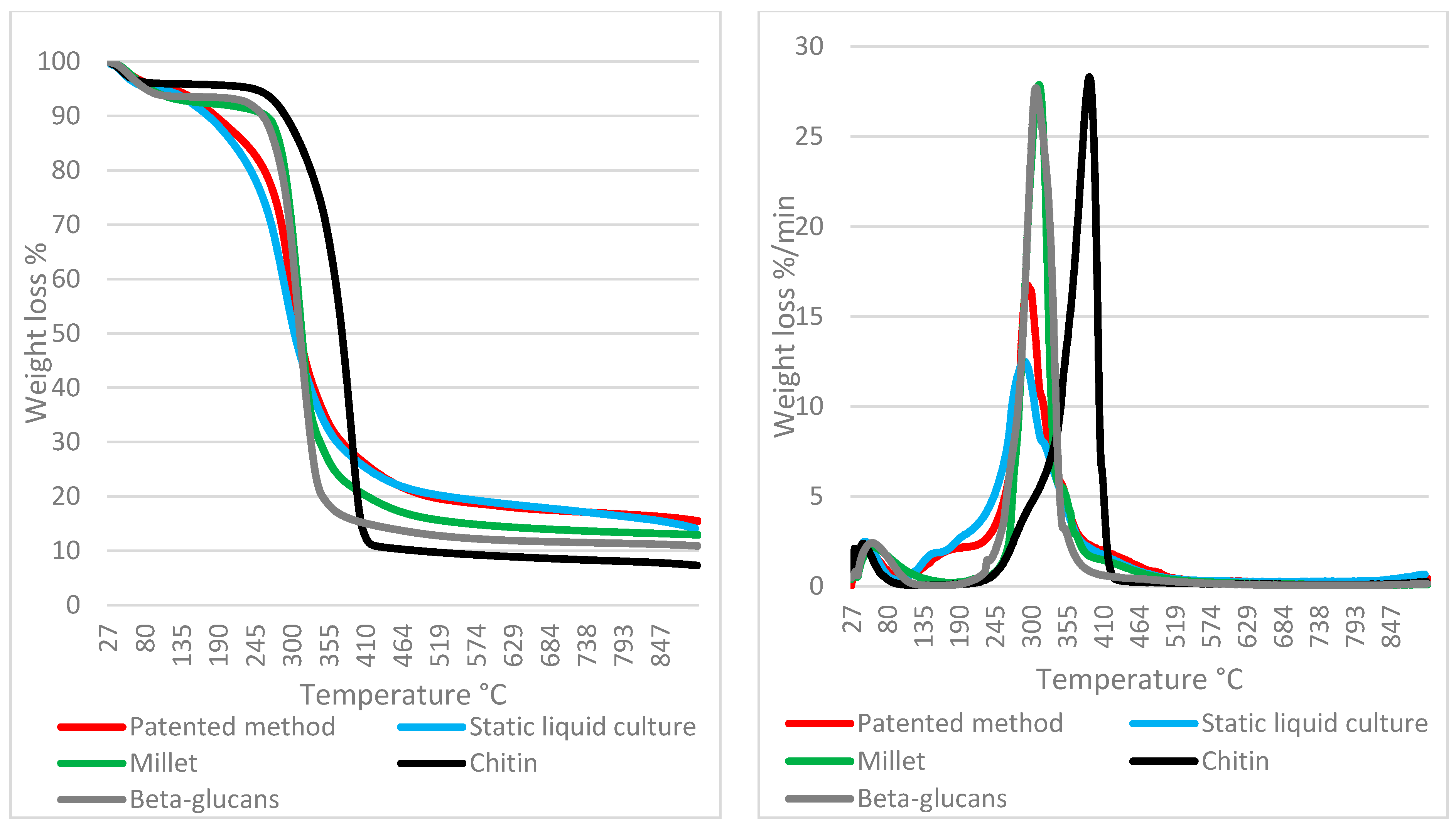
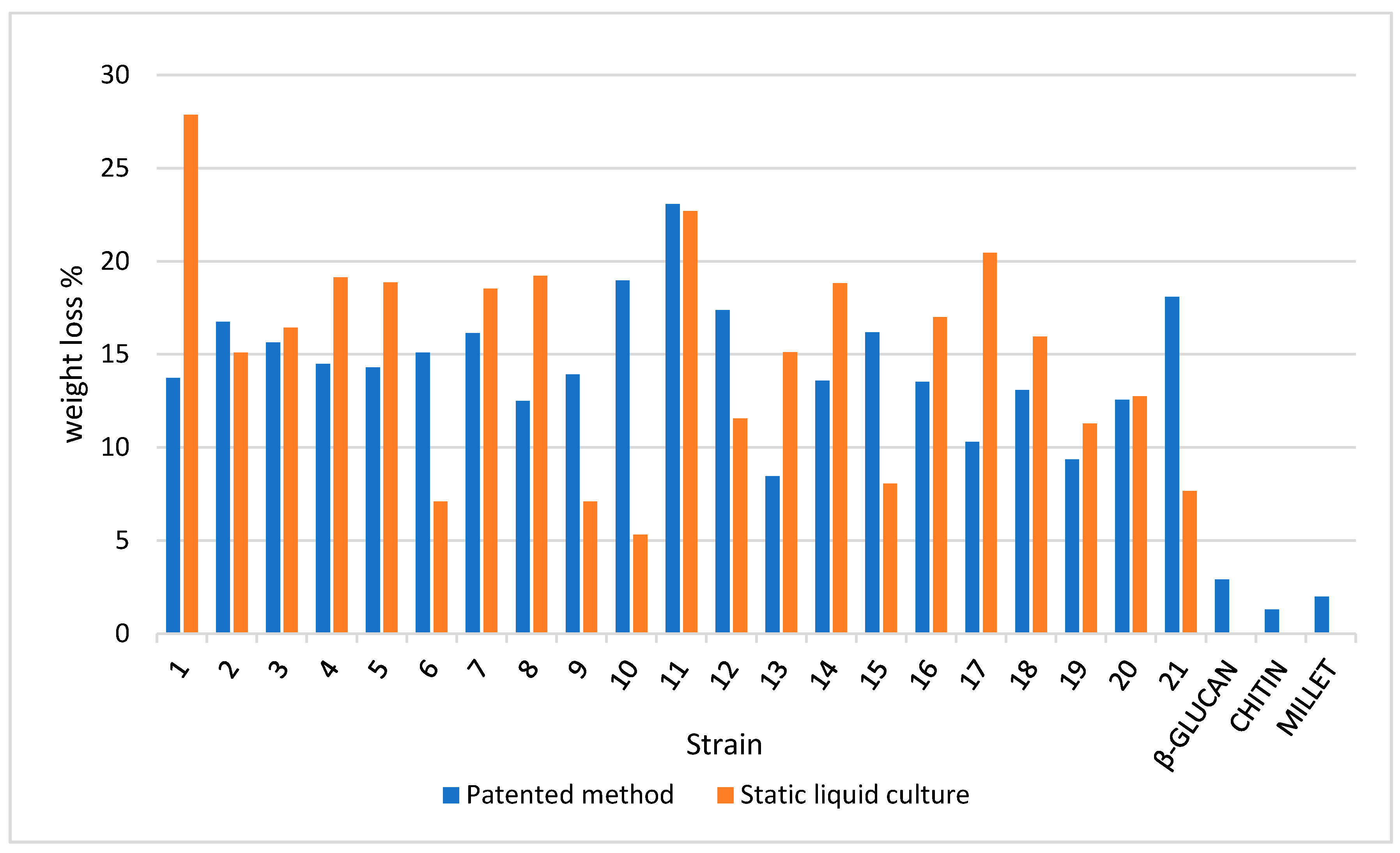
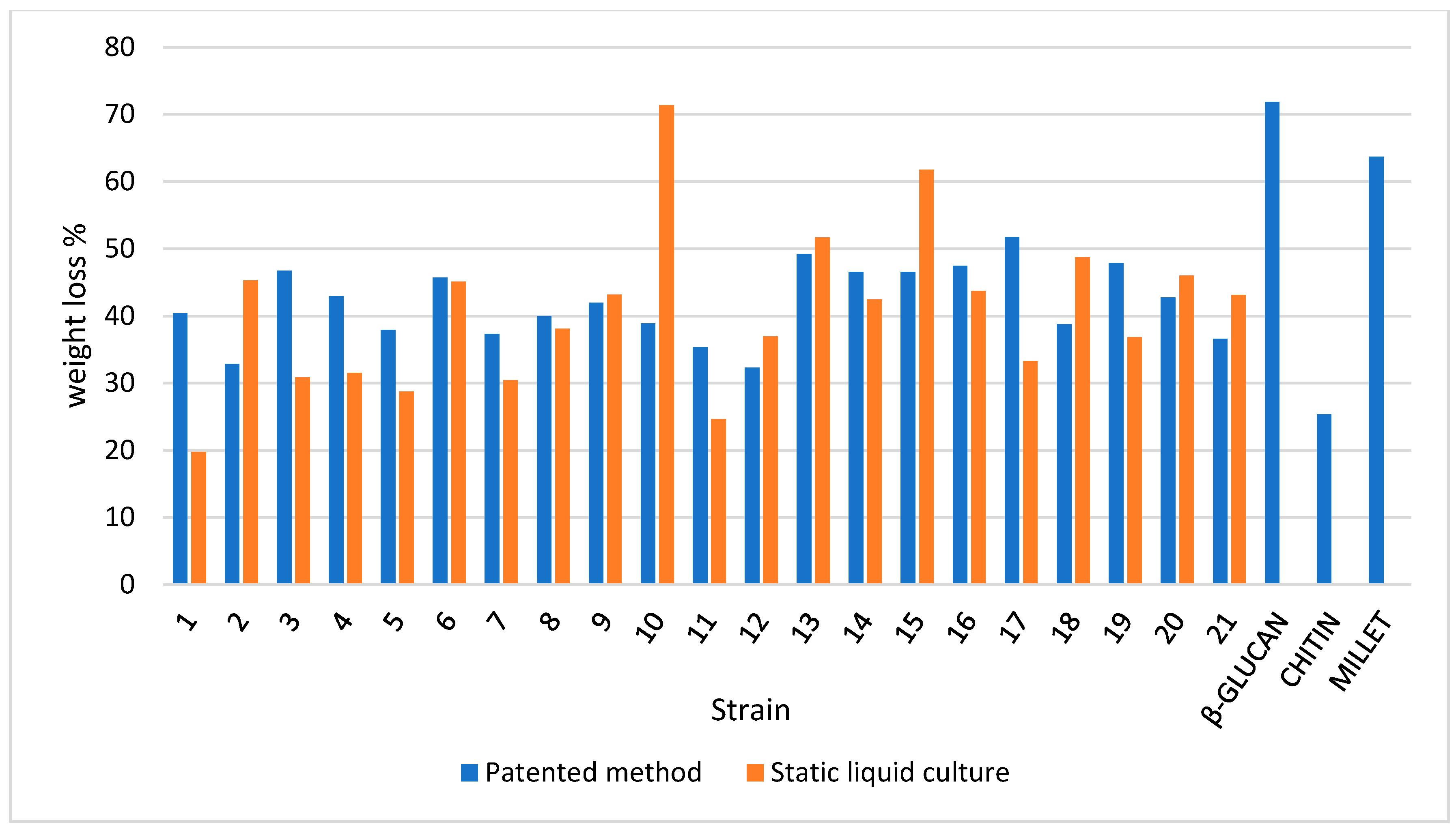
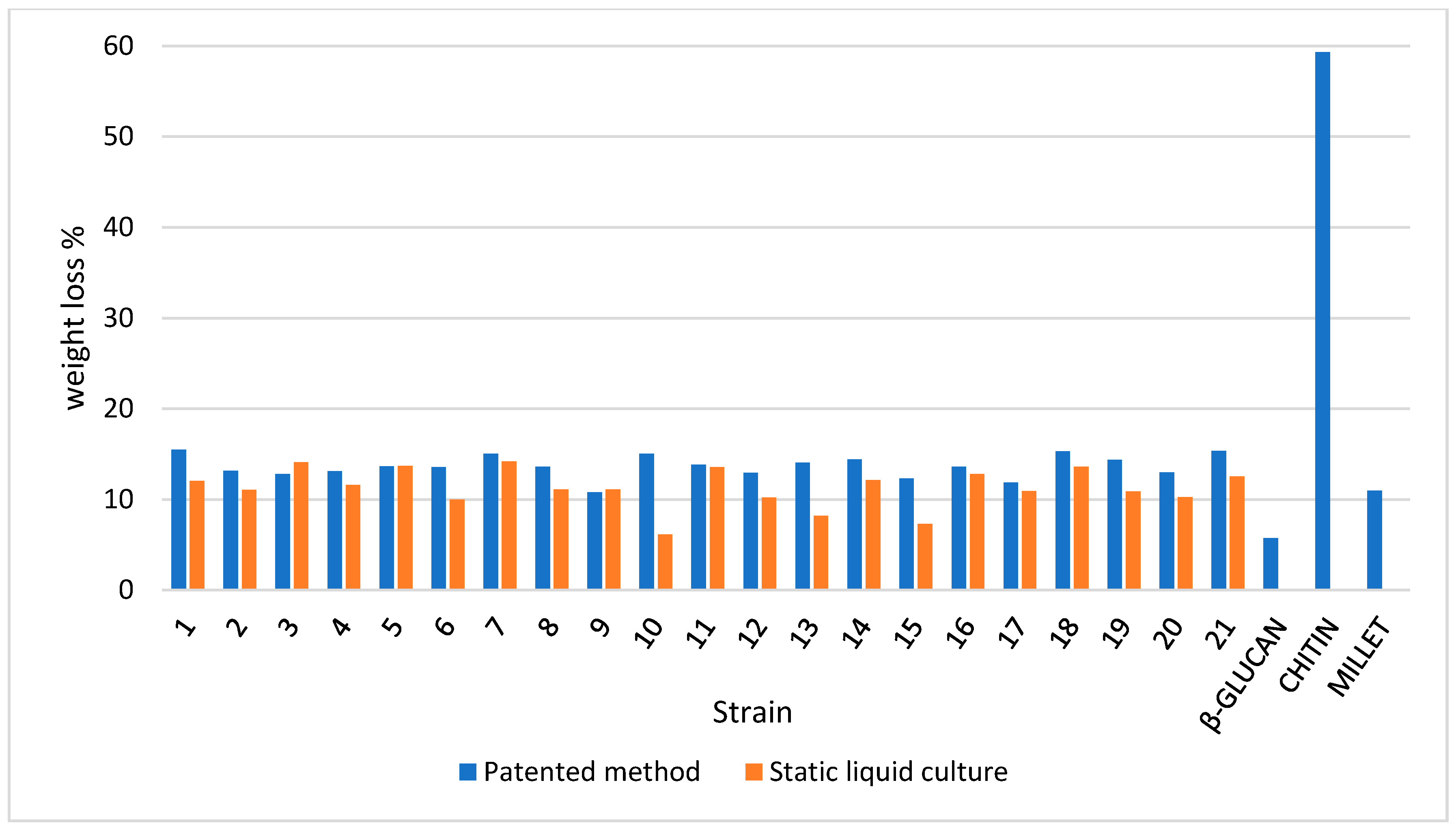
3.4. TGA on Mycelium Mats
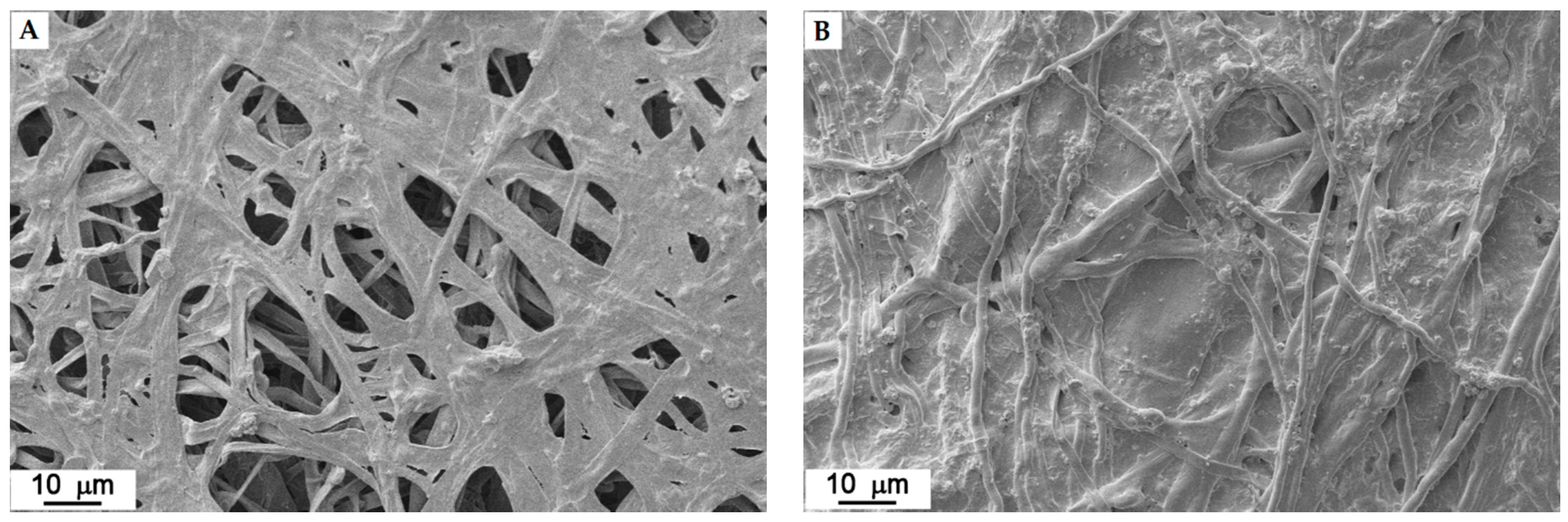
3.5. SEM on Mycelium Mats Grown in Liquid Medium and Following Mogu’s Patented Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mäkelä, M.R.; Donofrio, N.; De Vries, R.P. Plant biomass degradation by fungi. Fungal. Genet. Biol. 2014, 72, 2–9. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R.; Engels, J.; Mattheck, C. Fungal Strategies of Wood Decay in Trees; Springer: Berlin, Germany, 2013; p. 185. [Google Scholar]
- Stalpers, J.A. Identification of wood-inhabiting fungi in pure culture. Stud. Mycol. 1978, 16, 1–248. [Google Scholar]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms, 3rd ed.; Ten Speed Press: Berkeley, CA, USA, 2011. [Google Scholar]
- Hyde, K.D.; Bahkali, A.H.; Moslem, M.A. Fungi—An unusual source for cosmetics. Fungal. Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Gargano, M.L.; van Griensven, L.J.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant. Biosyst. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Kantharaj, P.; Boobalan, B.; Sooriamuthu, S.; Mani, R. Lignocellulose degrading enzymes from fungi and their industrial applications. IJCRR 2017, 9, 1–13. [Google Scholar]
- Daccò, C.; Girometta, C.; Asemoloye, M.D.; Carpani, G.; Picco, A.M.; Tosi, S. Key fungal degradation patterns, enzymes and their applications for the removal of aliphatic hydrocarbons in polluted soils: A review. Int. Biodeterior. Biodegrad. 2020, 147, 10866. [Google Scholar] [CrossRef]
- Roda, E.; De Luca, F.; Di Iorio, C.; Ratto, D.; Siciliani, S.; Ferrari, B.; Cobelli, F.; Borsci, G.; Priori, E.C.; Chinosi, S.; et al. Novel medicinal mushroom blend as a promising supplement in integrative oncology: A multi-tiered study using 4t1 triple-negative mouse breast cancer model. Int. J. Mol. Sci. 2020, 21, 3479. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J. A catchment-scale perspective of plastic pollution. Glob. Chang. Biol. 2019, 25, 1207–1221. [Google Scholar] [CrossRef] [Green Version]
- The European Commission. Available online: https://ec.europa.eu/environment/topics/plastics/single-use-plastics_en (accessed on 13 September 2021).
- Antinori, M.E.; Ceseracciu, L.; Mancini, G.; Heredia-Guerrero, J.A.; Athanassiou, A. Fine-tuning of physicochemical properties and growth dynamics of mycelium-based materials. ACS Appl. Bio Mater. 2020, 3, 1044–1051. [Google Scholar] [CrossRef]
- Cerimi, K.; Akkaya, K.C.; Carsten Pohl, C.; Schmidt, B.; Peter Neubauer, P. Fungi as source for new bio-based materials: A patent review. Fungal. Biol. Biotechnol. 2019, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Girometta, C.; Picco, A.M.; Baiguera, R.M.; Dondi, D.; Babbini, S.; Cartabia, M.; Pellegrini, M.; Savino, E. Physico-mechanical and thermodynamic properties of mycelium-based biocomposites: A review. Sustainability 2019, 11, 281. [Google Scholar] [CrossRef] [Green Version]
- Appels, F.V.; van den Brandhof, J.G.; Dijksterhuis, J.; de Kort, G.W.; Wösten, H.A. Fungal mycelium classified in different material families based on glycerol treatment. Commun. Biol. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, 79–95. [Google Scholar]
- Girometta, C.E.; Bernicchia, A.; Baiguera, R.M.; Bracco, F.; Buratti, S.; Cartabia, M.; Picco, A.M.; Savino, E. An Italian research culture collection of wood decay fungi. Diversity 2020, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Bhavana, D.R.; Rakesh, M.G. Mycelium composites: An emerging green building material. Int. Res. J. Eng. Tech. 2018, 5, 3066–3068. [Google Scholar]
- Appels, F.V.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A. Fabrication factors influencing mechanical, moisture-and water-related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Jones, M.; Gandia, A.; John, S.; Bismarck, A. Leather-like material biofabrication using fungi. Nat. Sustain. 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Nam, C.; Lee, Y.A. Multilayered cellulosic material as a leather alternative in the footwear industry. Cloth. Text. Res. J. 2019, 37, 20–34. [Google Scholar] [CrossRef]
- Hashmi, G.J.; Dastageer, G.; Sajid, M.S.; Ali, Z.; Malik, M.F.; Liaqat, I. Leather industry and environment: Pakistan scenario. Int J. Appl. Biol. Forensics 2017, 1, 20–25. [Google Scholar]
- Moktadir, M.A.; Ahmadi, H.B.; Sultana, R.; Liou, J.J.; Rezaei, J. Circular economy practices in the leather industry: A practical step towards sustainable development. J. Clean Prod. 2020, 251, 119737. [Google Scholar] [CrossRef]
- Girometta, C.; Dondi, D.; Baiguera, R.M.; Bracco, F.; Branciforti, D.S.; Buratti, S.; Lazzaroni, S.; Savino, E. Characterization of mycelia from wood-decay species by TGA and IR spectroscopy. Cellulose 2020, 27, 6133–6167. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Coats, A.W.; Redfern, J.P. Thermogravimetric Analysis. A review. Analyst 1963, 88, 906–924. [Google Scholar] [CrossRef]
- López, R.; Fernández, C.; Gómez, X.; Martínez, O.; Sánchez, M.E. Thermogravimetric analysis of lignocellulosic and microalgae biomasses and their blends during combustion. J. Therm. Anal. Calorim. 2013, 114, 295–305. [Google Scholar] [CrossRef]
- AA.VV. Enciclopedia Garzanti Della Chimica, 1st ed.; Garzanti: Milano, Italy, 1998. [Google Scholar]
- Damartzis, T.; Vamvuka, D.; Sfakiotakis, S.; Zabaniotou, A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour. Technol. 2011, 102, 6230–6238. [Google Scholar] [CrossRef]
- Popescu, C.M.; Lisa, G.; Manoliu, A.; Gradinariu, P.; Vasile, C. Thermogravimetric analysis of fungus-degraded lime wood. Carbohydr. Polym. 2010, 80, 78–83. [Google Scholar] [CrossRef]
- Ryvarden, L.; Melo, I. Poroid Fungi of Europe, 2nd ed.; Fungiflora: Oslo, Norway, 2017. [Google Scholar]
- Bernicchia, A.; Gorjon, S.P. Polypores of the Mediterranean Region; Romar: Segrate, Italy, 2020. [Google Scholar]
- Sturini, M.; Girometta, C.; Maraschi, F.; Savino, E.; Profumo, A. A preliminary investigation on Metal Bioaccumulation by Perenniporia fraxinea. Bull. Environ. Contam. Toxicol. 2017, 98, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Rush Wayn, R. Growing Mushrooms—The easy way. In Home Mushroom Cultivation with Hydrogen Peroxide Volume I, 3rd ed.; 2001; Available online: http://mycomasters.com/Updates.html (accessed on 30 May 2021).
- García-García, M.; Rocha-Zavaleta, L.; Valdez-Cruz, N.A.; Trujillo-Roldan, M.A. Conservation of the mycelia of the medicinal mushroom Humphreya coffeata (Berk.) Stey. in sterile distilled water. MethodsX 2014, 1, 19–22. [Google Scholar] [CrossRef]
- Homolka, L. Preservation of live cultures of basidiomycetes—Recent methods. Fungal. Biol. 2014, 118, 107–125. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar]
- Nilsson, R.H.; Hyde, K.D.; Pawlowska, J.; Ryberg, M.; Tedersoo, L.; Aas, A.B.; Alias, S.A.; Alves, A.; Anderson, C.L.; Antonelli, A.; et al. Improving ITS sequence data for identification of plant pathogenic fungi. Fungal Divers. 2014, 67, 11–19. [Google Scholar] [CrossRef]
- Mycobank. Available online: www.mycobank.org (accessed on 13 September 2021).
- NCBI. Available online: https://blast.ncbi.nlm.nih.gov (accessed on 16 November 2021).
- Nobles, M.K. Studies in forest pathology. VI. Identification of cultures of wood-rotting fungi. Can. J. Micol. 1948, 26, 281–431. [Google Scholar] [CrossRef] [PubMed]
- Petre, C.V.; Tanase, C. Culture characteristics of 20 lignicolous basidiomycetes species that synthesize volatile organic compounds. Analele Stiintifice ale Universitatii “Al. I. Cuza” din Iasi 2013, 59, 37. [Google Scholar]
- Fischer, M. A new wood-decaying basidiomycete species associated with esca of grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycol. Prog. 2002, 1, 315–324. [Google Scholar] [CrossRef]
- Balaeş, T.; Tănase, C. Description of in vitro cultures for some spontaneous lignicolous basidiomycetes species. Analele Ştiinţifice ale Universităţii “Al.I.Cuza” Iaşi s.II a Biologie Vegetală 2012, 58, 19–29. [Google Scholar]
- Dresch, P.; Rosam, K.; Grienke, U.; Rollinger, J.M.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the European medicinal polypores Fomes fomentarius, Fomitopsis pinicola and Piptoporus betulinus. AMB Express 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandia, A.; Montalti, M.; Babbini, S.; MOGU srl. Metodo di Produzione di Feltri Fungini e dei Materiali da essi Derivati. Ministero per lo Sviluppo Economico—Direzione Generale per la Lotta alla Contraffazione—Ufficio Italiano Brevetti e Marchi, 06/06/2020. Domada di Invenzione numero 102018000010869. Available online: https://patents.google.com/patent/WO2020115690A1/en?oq=IT201800010869A1 (accessed on 17 September 2021).
- Kapur, J.N.; Sahoo, P.K.; Wong, A.K.C. A new method for gray-level picture thresholding using the entropy of the histogram. Comput. Vis. Graph. Image Process 1985, 29, 273–285. [Google Scholar] [CrossRef]
- Pilotti, M.; Tizzani, L.; Brunetti, A.; Gervasi, F.; Di Lernia, G.; Lumia, V. Molecular identification of Fomitiporia mediterranea on declining and decayed hazelnut. J. Plant. Pathol. 2010, 92, 115–129. [Google Scholar]
- Koukol, O.; Kotlaba, F.; Pouzar, Z. Taxonomic evaluation of the polypore Daedaleopsis tricolor based on morphology and molecular data. Czech. Mycol. 2014, 66, 107–119. [Google Scholar] [CrossRef]
- Mentrida, S.; Krisai-Greilhuber, I.R.M.G.A.R.D.; Voglmayr, H. Molecular evaluation of species delimitation and barcoding of Daedaleopsis confragosa specimens in Austria. Österr. Z. Pilzk 2015, 24, 173–179. [Google Scholar]
- Harris, D.C. Quantitative Chemical Analysis, 7th ed.; W.H.Freeman and Company: New York, NY, USA, 2007. [Google Scholar]
- Doria, E.; Altobelli, E.; Girometta, C.; Nielsen, E.; Zhang, T.; Savino, E. Evaluation of lignocellulolytic activities of ten fungal species able to degrade poplar wood. Int. Biodeterior. Biodegrad. 2014, 94, 160–166. [Google Scholar] [CrossRef]
- Marković, M.; Orlović, S.; Pap, P.; Galović, V.; Pekeč, S.; Galić, Z. Influence of temperature on the micelium growth of Daedaleopsis confragosa (Bolt.: Fr.) J. Schröt. Topola 2013, 191, 31–41. [Google Scholar]
- Elena, K.; Dimou, D.M.; Dimou, D.; Fischer, M. “Fomitiporia mediterranea” Infecting Citrus Trees in Greece. Phytopathol. Mediterr. 2006, 45, 35–39. [Google Scholar]






| Strain Code | Fungal Species | Mycelium Characteristics |
|---|---|---|
| 1 | Abortiporus biennis (Bull.) Singer | Colony white to cream, some felty parts; aerial hyphae often resembling skeletal one, submerged hyphae up to 7.5 µm wide. Primordia of sporophores (none of which reaches maturity) are easily formed [3]. |
| 2 | Bjerkandera adusta (Willd.) P. Karst. | Mat white; at first silky becoming cottony-woolly, woolly floccose; advancing zone raised, reaching the lid of the Petri dish [3,43]. |
| 3 | Coriolopsis gallica (Fr.) Ryvarden | Mat white mat at the beginning of its growth, then becomes olive-brownish and thin and crusty after 4–5 days. Aereal hyphae pigmented. Reverse creamed. Appressed to the margin, farinaceous to felty [3]. |
| 4 | Coriolopsis gallica (Fr.) Ryvarden | |
| 5 | Coriolopsis trogii (Berk.) Domanski | Colony white to cream; marginal hyphae appressed, mat downy to felty; thin and almost transparent. It becomes fluffy and inconsistent [3]. |
| 6 | Daedaleopsis confragosa (Bolton) J. Schröt. | Colony white darkening with age, presence of crustose areas hazel, “pinkish cinnamon” or “snuff brown”; mat downy to fine woolly, becoming felty; reverse darkened [3,43,44]. |
| 7 | Daedaleopsis tricolor (Bull.) Bondartsev & Singer | Colony white, dense, downy—felty, homogeneous when young. After a few weeks of incubation, several primordia of sporophores (none of which reaches maturity) are formed [44]. |
| 8 | Fomes fomentarius (L.) Fr. | Colony wthite to cream to chamois, reverse darker. Advancing zone raised with aerial mycelium uniform. Mat downy to cottony or woolly, then appressed and felty, relatively homogeneous. When it reaches maturity, the mycelium forms brown crusty leathery areas [3,43,44] |
| 9 | Fomitiporia mediterranea M. Fisch. | Mycelial cultures are cottony to woolly, with aerial hyphae that are yellowish to brownish; mat characterized by a sparse development of aerial hyphae that easily reach the lid of the Petri dish [45]. |
| 10 | Fomitopsis iberica Melo & Ryvarden | References not available; see results 3.1 |
| 11 | Fomitopsis pinicola (Sw.) P. Karst. | Mat white, heterogeneous, at first raised, cottony and woolly; usually uniform in appearance, sometimes forming scattered dots of more compact mycelium [3,43,44]. |
| 12 | Ganoderma carnosum Pat. | References not available; see results 3.1 |
| 13 | Ganoderma lucidum (Curtis) P. Karst. | Mat white with darker (brownish) zones, concentrically arranged, appressed, and powdery near the point of inoculation. Distal zone is ± homogeneous and felty, with small hyphal clusters [46] |
| 14 | Irpex lacteus (Fr.) Fr. | Mat white, downy to cottony and woolly floccose. Reverse bleached. Advanced zone raised. Some aerial hyphae with thickened walls [3]. |
| 15 | Irpiciporus pachyodon (Pers.) Kotl. & Pouzar | Mat white, downy to cottony to felty with sometimes scattered dots of more compact mycelium. Aerial hyphae easily reaching the lid of the Petri dish [3]. |
| 16 | Lenzites betulinus (L.) Pilát | Mat at first floccose and woolly, beoming patchy, with some areas raised, felty-woolly;intervening areas appressed, thin felty [47] |
| 17 | Neofavolus alveolaris (DC.) Sotome & T. Hatt. | Colony white to cream. Mat thick, dense and homogeneous at the beginning, then fluffy and woolly; also develops on the edges of the plate. Skeletal hyphae [3,44] |
| 18 | Stereum hirsutum (Willd.) Pers. | Cream to yellow to light brown with dark brown areas; mycelium heterogeneous, downy to felty areas alternating with translucent ones. The advancing zone is irregular, sinuous [3,44] |
| 19 | Terana caerulea (Schrad. ex Lam.) Kuntze | References not available; see results 3.1 |
| 20 | Trametes hirsuta (Wulfen) Lloyd | Mat cottony to cottony and woolly and becoming (sub)felty and sometimes farinaceous, white to cream. Advancing zone appressed to raised. Reverse bleached. Most of skeletal hyphae are branched [3]. |
| 21 | Trametes suaveolens (L.) Fr. | Mat white at first, later cream with orange tinges. Downy floccose, becoming woolly to subfelty. Advancing zone appressed to slightly raised. Odor strong, sweet [3,43]. |
| Code | Species | Basidiomes Sample Sites (Italy) | Date of Basidiomes Collection | Host Species | Basidiomes Legit and Determinavit | MFSC Code |
|---|---|---|---|---|---|---|
| 1 | Abortiporus biennis | Varese (VA) | 24 November 2018 | Tilia cordata | M. Cartabia | 064-2018 |
| 2 | Bjerkandera adusta | Albizzate (VA) | 29 May 2019 | Populus alba | M. Cartabia | 101-2019 |
| 3 | Coriolopsis gallica | Varese (VA) | 30 March 2019 | Fraxinus excelsior | M. Cartabia | 086-2019 |
| 4 | Coriolopsis gallica | Varese (VA) | 23 June 2019 | Fagus sylvatica | M. Cartabia | 121-2019 |
| 5 | Coriolopsis trogii | Cazzago Brabbia (VA) | 12 June 2018 | Populus tremula | M. Cartabia | 027-2018 |
| 6 | Daedaleopsis confragosa | Inarzo (VA) | 29 August 2019 | Fraxinus excelsior | M. Cartabia | 155-2019 |
| 7 | Daedaleopsis tricolor | Baceno (VB) | 3 August 2019 | Prunus avium | M. Cartabia | 148-2019 |
| 8 | Fomes fomentarius | Viterbo (VT) | 2 December 2019 | Fagus sylvatica | M. Cartabia | 179-2019 |
| 9 | Fomitiporia mediterranea | Varese (VA) | 17 December 2018 | Fagus sylvatica | M. Cartabia | 079-2018 |
| 10 | Fomitopsis iberica | Varese (VA) | 8 June 2019 | Cedrus deodara | M. Cartabia | 104-2019 |
| 11 | Fomitopsis pinicola | Valganna (VA) | 22 June 2019 | Picea abies | M. Cartabia | 117-2019 |
| 12 | Ganoderma carnosum | Varese (VA) | 6 September 2019 | Picea abies | M. Cartabia | 161-2019 |
| 13 | Ganoderma lucidum | Varese, (VA) | 16 July 2019 | Quercus pubescens | M. Cartabia | 137-2019 |
| 14 | Irpex lacteus | Cazzago Brabbia (VA) | 7 December 2018 | Populus tremula | M. Cartabia | 076-2018 |
| 15 | Irpiciporus pachyodon | Imperia (IM) | 9 November 2019 | Quercus pubescens | M. Cartabia | 175-2019 |
| 16 | Lenzites betulinus | Cittiglio (VA) | 8 April 2019 | Betula pendula | M. Cartabia | 088-2019 |
| 17 | Neofavolus alveolaris | Varese (VA) | 3 May 2019 | Populus alba | M. Cartabia | 096-2019 |
| 18 | Stereum hirsutum | Inarzo (VA) | 5 December 2018 | Corylus avellana | M. Cartabia | 073-2018 |
| 19 | Terana caerulea | Varese (VA) | 2 November 2019 | Ostrya carpinifolia | M. Cartabia | 177-2019 |
| 20 | Trametes hirsuta | Varese (VA) | 25 November 2018 | Fagus sylvatica | M. Cartabia | 067-2018 |
| 21 | Trametes suaveolens | Cazzago Brabbia (VA) | 4 December 2018 | Salix alba | M. Cartabia | 070-2018 |
| Code | Species | MFSC Code | Average Growth Rate (mm day−1) |
|---|---|---|---|
| 1 | Abortiporus biennis | 064-2018 | 9 |
| 2 | Bjerkandera adusta | 101-2019 | 11 |
| 3 | Coriolopsis gallica | 086-2019 | 8 |
| 4 | Coriolopsis gallica | 121-2019 | 8 |
| 5 | Coriolopsis trogii | 027-2018 | 6 |
| 6 | Daedaleopsis confragosa | 155-2019 | 4 |
| 7 | Daedaleopsis tricolor | 148-2019 | 7 |
| 8 | Fomes fomentarius | 179-2019 | 7 |
| 9 | Fomitiporia mediterranea | 079-2018 | 5 |
| 10 | Fomitopsis iberica | 104-2019 | 7 |
| 11 | Fomitopsis pinicola | 117-2019 | 6 |
| 12 | Ganoderma carnosum | 161-2019 | 6 |
| 13 | Ganoderma lucidum | 137-2019 | 2 |
| 14 | Irpex lacteus | 076-2018 | 10 |
| 15 | Irpiciporus pachyodon | 175-2019 | 5 |
| 16 | Lenzites betulinus | 088-2019 | 7 |
| 17 | Neofavolus alveolaris | 096-2019 | 7 |
| 18 | Stereum hirsutum | 073-2018 | 11 |
| 19 | Terana caerulea | 177-2019 | 4 |
| 20 | Trametes hirsuta | 067-2018 | 6 |
| 21 | Trametes suaveolens | 070-2018 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartabia, M.; Girometta, C.E.; Milanese, C.; Baiguera, R.M.; Buratti, S.; Branciforti, D.S.; Vadivel, D.; Girella, A.; Babbini, S.; Savino, E.; et al. Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats. J. Fungi 2021, 7, 1008. https://doi.org/10.3390/jof7121008
Cartabia M, Girometta CE, Milanese C, Baiguera RM, Buratti S, Branciforti DS, Vadivel D, Girella A, Babbini S, Savino E, et al. Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats. Journal of Fungi. 2021; 7(12):1008. https://doi.org/10.3390/jof7121008
Chicago/Turabian StyleCartabia, Marco, Carolina Elena Girometta, Chiara Milanese, Rebecca Michela Baiguera, Simone Buratti, Diego Savio Branciforti, Dhanalakshmi Vadivel, Alessandro Girella, Stefano Babbini, Elena Savino, and et al. 2021. "Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats" Journal of Fungi 7, no. 12: 1008. https://doi.org/10.3390/jof7121008
APA StyleCartabia, M., Girometta, C. E., Milanese, C., Baiguera, R. M., Buratti, S., Branciforti, D. S., Vadivel, D., Girella, A., Babbini, S., Savino, E., & Dondi, D. (2021). Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats. Journal of Fungi, 7(12), 1008. https://doi.org/10.3390/jof7121008









