Effects of Secondary Metabolites from Pea on Fusarium Growth and Mycotoxin Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Infection Studies on Pea Seedlings
2.3. Metabolite Profiling of Pea Cultivars
2.3.1. Sample Preparation
2.3.2. LC-MS Analysis
2.3.3. Data Analysis
2.4. Antifungal Activity Assays Using Pea Derived Metabolites
2.5. Statistical Analysis
2.6. Mycotoxin Analysis
3. Results
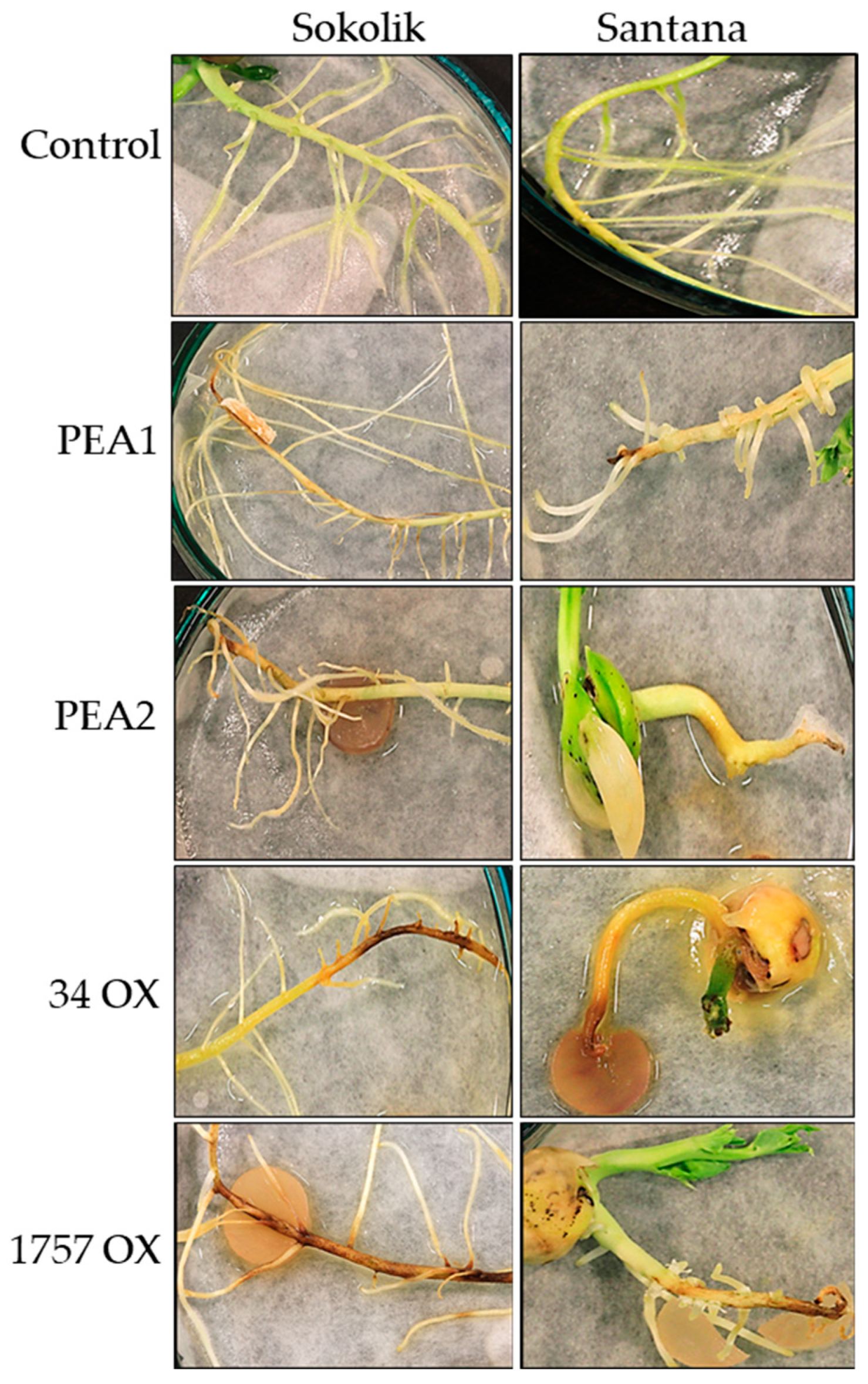
3.1. Infection Studies on Pea Seedlings
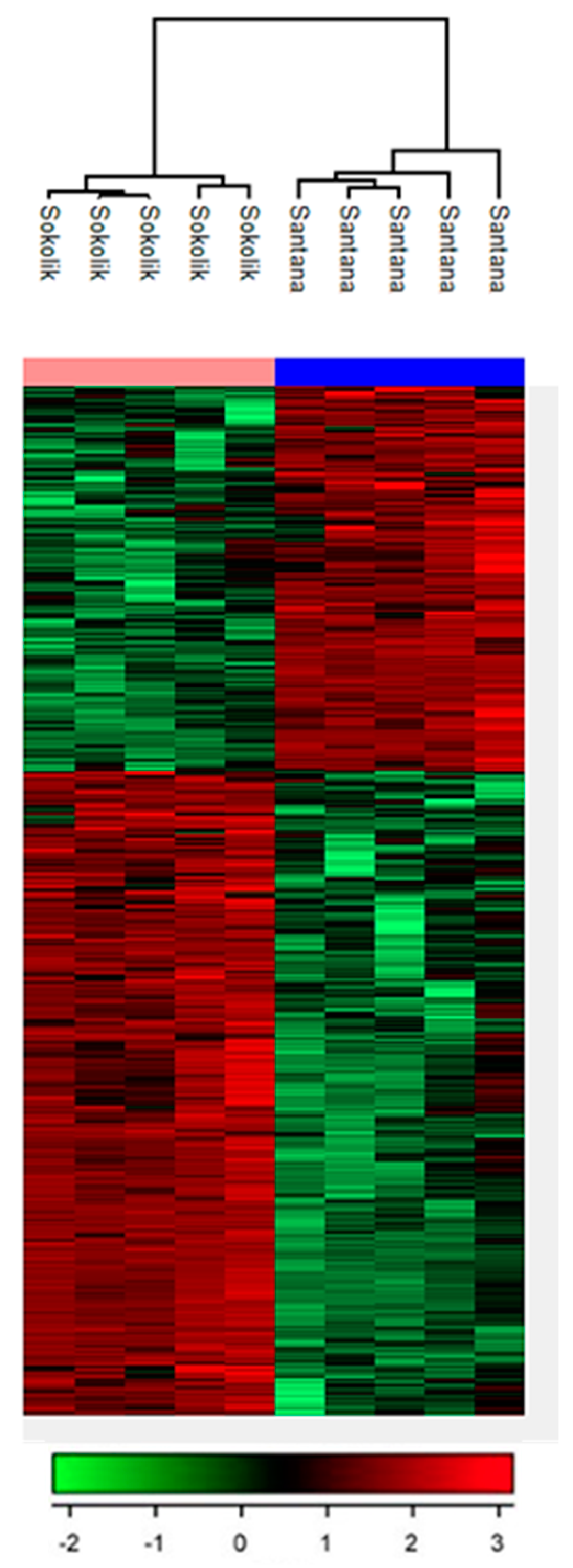
3.2. Metabolite Profiling of Pea Cultivars
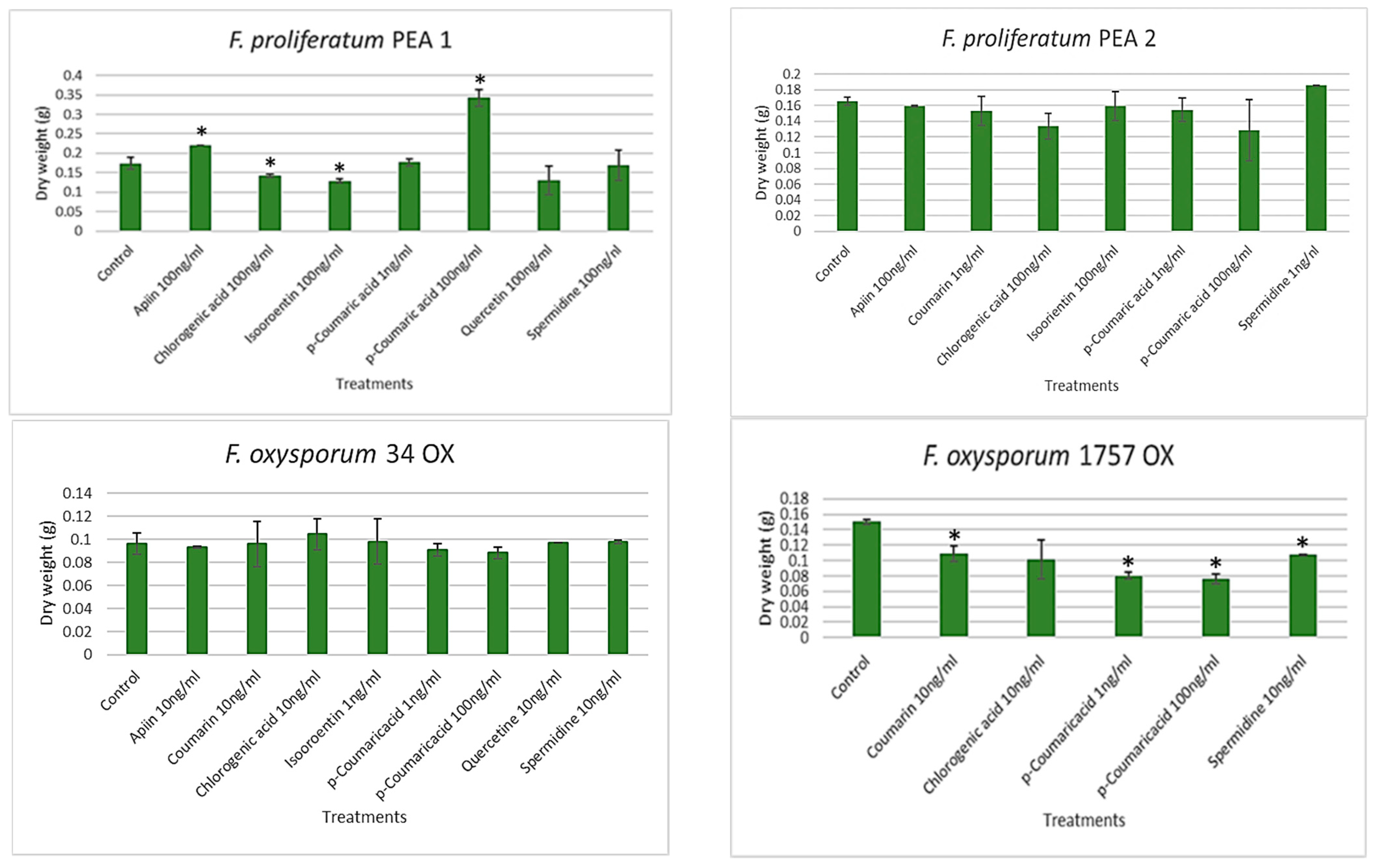
3.3. Antifungal Assay Using Pea-Derived Metabolites
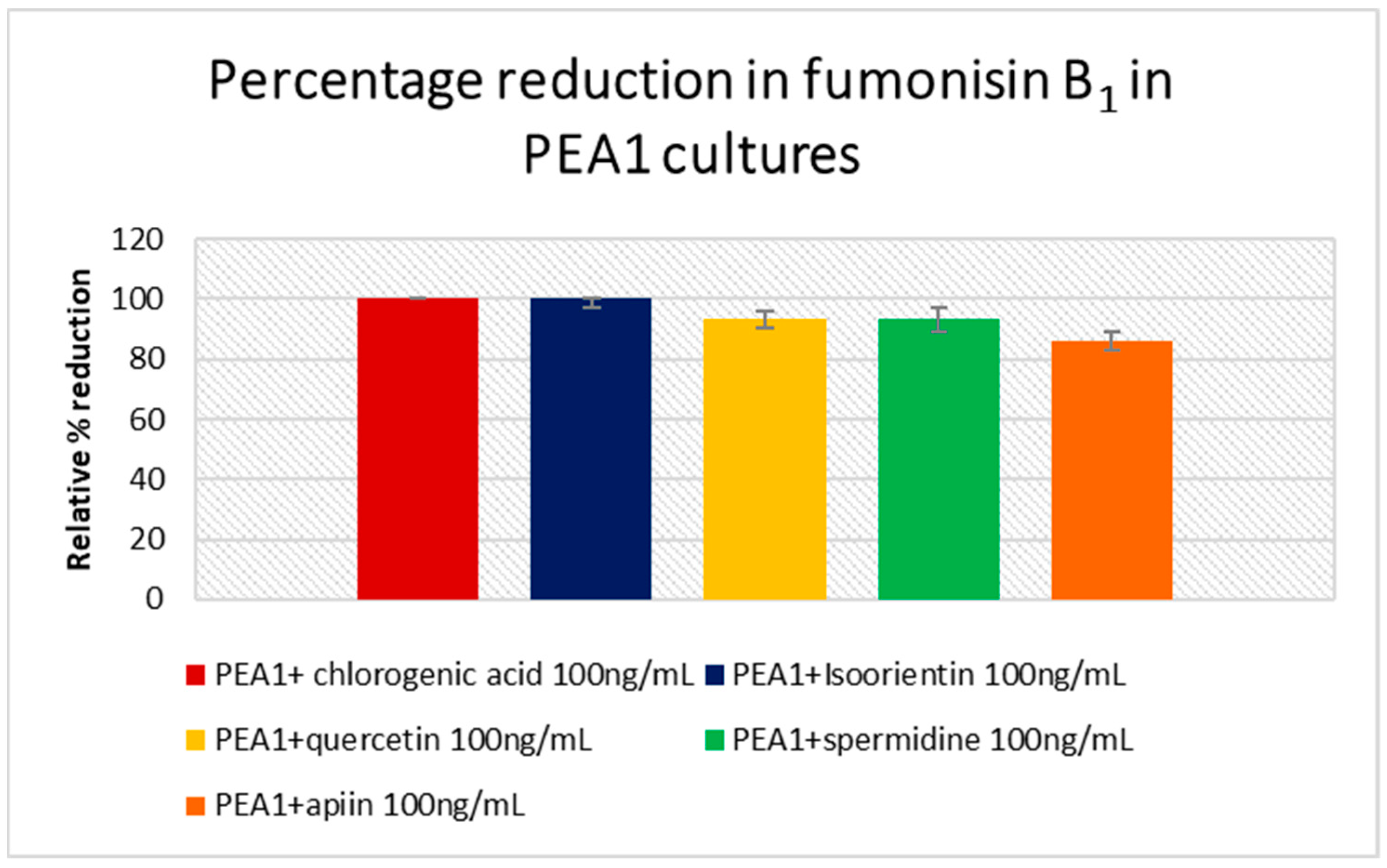
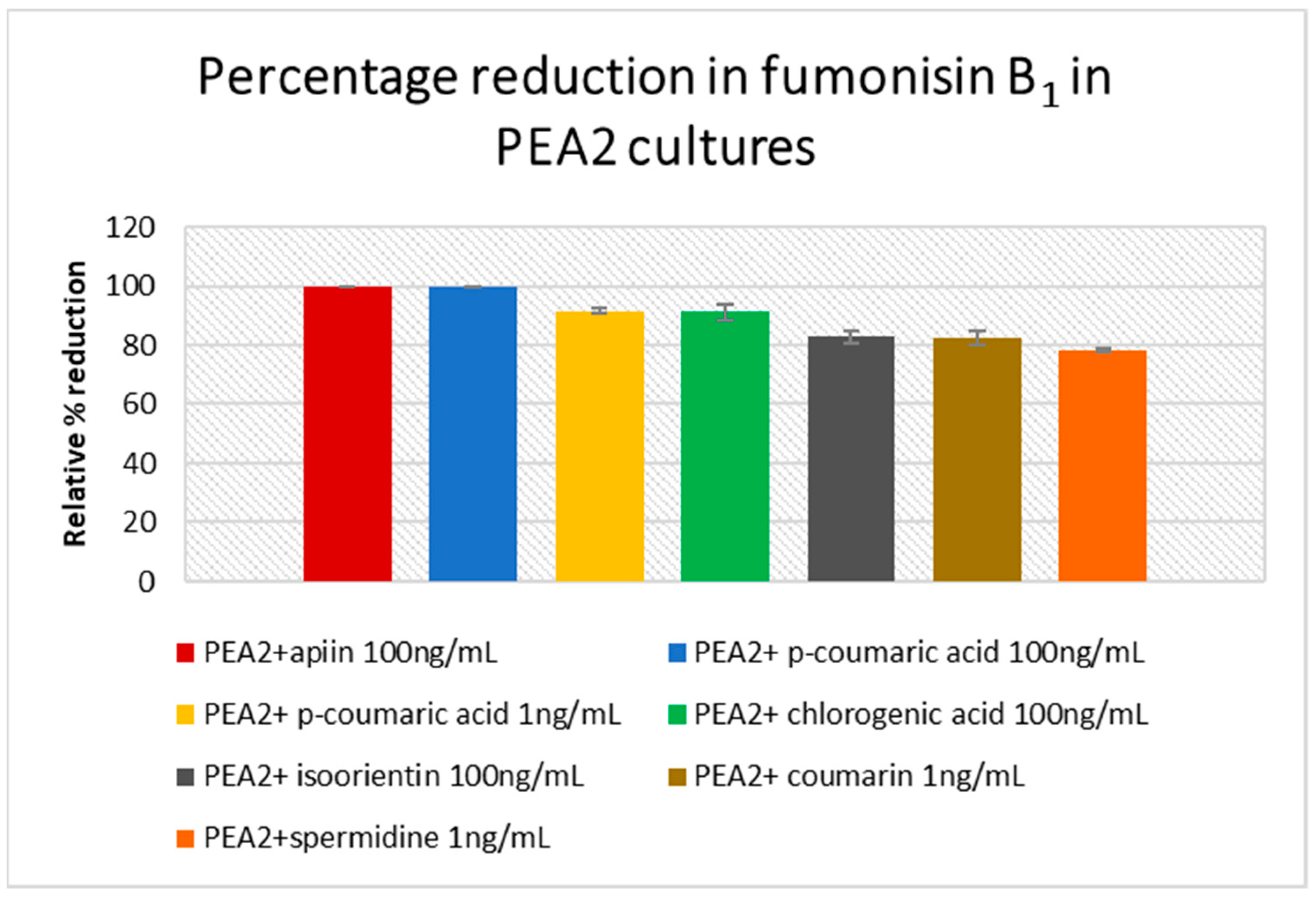
3.4. Mycotoxin Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watson, A.; Liew, E.; Duff, J. Management Options for Fusarium Wilt of Snow Peas. Primefacts 2009, 971, 1–3. [Google Scholar]
- Haglund, W. Fusarium Wilts. In Compendium of Pea Diseases; Hagedorn, D.J., Ed.; American Phytopathological Society Press: St. Paul, MN, USA, 1984; pp. 22–24. [Google Scholar]
- Kraft, J. Fusarium Wilt of Peas (A Review). Agronomie 1994, 14, 561–567. [Google Scholar] [CrossRef]
- Haglund, W.A.; Kraft, J.M. Fusarium Wilt. In Compendium of Pea Diseases and Pests; Kraft, J.M., Pfleger, F.L., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2001; pp. 13–14. [Google Scholar]
- Dong, X.; Xiong, Y.; Ling, N.; Shen, Q.; Guo, S. Fusaric Acid Accelerates the Senescence of Leaf in Banana When Infected by Fusarium. World J. Microbiol. Biotechnol. 2013, 30, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting Mechanisms of Defense against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Neelam Khatkar, A.; Sharma, K.K. Phenylpropanoids and Its Derivatives: Biological Activities and Its Role in Food, Pharmaceutical and Cosmetic Industries. Crit. Rev. Food Sci. Nutr. 2019, 60, 2655–2675. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Paxton, J.D. Phytoalexins—A Working Redefinition. J. Phytopathol. 1981, 101, 106–109. [Google Scholar] [CrossRef]
- Padmavati, M.; Sakthivel, N.; Thara, K.V.; Reddy, A.R. Differential Sensitivity of Rice Pathogens to Growth Inhibition by Flavonoids. Phytochemistry 1997, 46, 499–502. [Google Scholar] [CrossRef]
- Parvez, M.M.; Tomita-Yokotani, K.; Fujii, Y.; Konishi, T.; Iwashina, T. Effects of Quercetin and Its Seven Derivatives on the Growth of Arabidopsis thaliana and Neurospora crassa. Biochem. Syst. Ecol. 2004, 32, 631–635. [Google Scholar] [CrossRef]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can Plant Phenolic Compounds Reduce Fusarium Growth and Mycotoxin Production in Cereals? Food Addit. Contam. Part A 2018, 35, 2455–2470. [Google Scholar] [CrossRef]
- Gauthier, L.; Atanasova-Penichon, V.; Chéreau, S.; Richard-Forget, F. Metabolomics to Decipher the Chemical Defense of Cereals against Fusarium graminearum and Deoxynivalenol Accumulation. Int. J. Mol. Sci. 2015, 16, 24839–24872. [Google Scholar] [CrossRef] [PubMed]
- Wilman, K.; Stępień, Ł.; Fabiańska, I.; Kachlicki, P. Plant-Pathogenic Fungi in Seeds of Different Pea Cultivars in Poland. Arh. Hig. Rada Toksikol. 2014, 65, 329–338. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-Independent MS/MS Deconvolution for Comprehensive Metabolome Analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus Computational Platform for Comprehensive Analysis of (Prote)Omics Data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Urbaniak, M.; Waśkiewicz, A.; Trzebny, A.; Koczyk, G.; Stępień, Ł. Cyclodepsipeptide Biosynthesis in Hypocreales Fungi and Sequence Divergence of The Non-Ribosomal Peptide Synthase Genes. Pathogens 2020, 9, 552. [Google Scholar] [CrossRef]
- Witaszak, N.; Lalak-Kańczugowska, J.; Waśkiewicz, A.; Stępień, Ł. The Impacts of Asparagus Extract Fractions on Growth and Fumonisins Biosynthesis in Fusarium proliferatum. Toxins 2020, 12, 95. [Google Scholar] [CrossRef]
- Sideman, E. Fusarium Wilt of Peas—Maine Organic Farmers and Gardeners; Maine Organic Farmers and Gardeners Association: Unity, ME, USA, 2010. [Google Scholar]
- Sukrasno, S. Plant Secondary Metabolites for AntiFusarium and Antiphytophthora. In Fusarium: Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef][Green Version]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary Metabolites in Fungus-Plant Interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef]
- Treutter, D. Significance of Flavonoids in Plant Resistance: A Review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. Phytochem. Adv. Res. 2006, 23–67. [Google Scholar]
- De Oliveira, T.L.C.; de Araújo Soares, R.; Ramos, E.M.; das Graças Cardoso, M.; Alves, E.; Piccoli, R.H. Antimicrobial Activity of Satureja montana, L. Essential Oil against Clostridium perfringens Type A Inoculated in Mortadella-Type Sausages Formulated with Different Levels of Sodium Nitrite. Int. J. Food Microbiol. 2011, 144, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Bharti, S.; Mondin, M.; Raiola, A. Effect of Naturally Occurring Compounds on Fumonisin Production and Fum Gene Expression in Fusarium verticillioides. Agronomy 2021, 11, 1060. [Google Scholar] [CrossRef]
- Stȩpień, Ł.; Jestoi, M.; Chełkowski, J. Cyclic Hexadepsipeptides in Wheat Field Samples and Esyn1 Gene Divergence among Enniatin Producing Fusarium avenaceum Strains. World Mycotoxin J. 2013, 6, 399–409. [Google Scholar] [CrossRef]
- Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium Cyclodepsipeptide Mycotoxins: Chemistry, Biosynthesis, and Occurrence. Toxins 2020, 12, 765. [Google Scholar] [CrossRef]
- Wu, Q.; Patocka, J.; Nepovimova, E.; Kuca, K. A Review on the Synthesis and Bioactivity Aspects of Beauvericin, a Fusarium Mycotoxin. Front. Pharmacol. 2018, 9, 1338. [Google Scholar] [CrossRef] [PubMed]
| PEA1 | PEA2 | 34 OX | 1757 OX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc ng/mL | 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 |
| Apiin | √ | √ | √ | |||||||||
| Coumarin | √ | √ | √ | |||||||||
| Chlorogenic acid | √ | √ | √ | √ | ||||||||
| Isoorientin | √ | √ | √ | |||||||||
| p-coumaric acid | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| Quercetin | √ | √ | ||||||||||
| Spermidine | √ | √ | √ | √ | ||||||||
| RT [min] | Metabolite Name | Ionization | Molecular Formula | Mass Calculated | Mass Measured | Δ [ppm] | Fragment Ions | PubChem ID | Santana | Sokolik |
|---|---|---|---|---|---|---|---|---|---|---|
| 5.87 | Apiin (Apigenin 7-O-diglucoside) | [M−H]− | C26H28O14 | 563.1406 | 563.1412 | 1.0774 | 473, 563, 284 | 280746 | ||
| 7.05 | Coumarin | [M+H]+ | C9H6O2 | 147.044 | 147.0441 | −0.5453 | 103 | 323 | ||
| 5.05 | Chlorogenic Acid | [M−H]− | C16H18O9 | 353.0878 | 353.0878 | −0.2770 | 191 | 1794427 | ||
| 6.59 | Isoorientin | [M+H]+ | C21H20O11 | 449.1078 | 449.1075 | −0.6544 | 449, 287 | 114776 | ||
| 3.67 | p-coumaric Acid | [M+H]+ | C9H8O3 | 165.0545 | 165.0546 | −0.5070 | 147 | 637542 | ||
| 6.28 | Quercetin | [M+H]+ | C15H10O7 | 303.0499 | 303.0499 | −0.2092 | 229, 153 | 5280343 | ||
| 5.08 | Spermidine | [M+H]+ | C7H19N3 | 146.1652 | 146.1651 | −0.5070 | 129, 112, 172 | 1102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perincherry, L.; Witaszak, N.; Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł. Effects of Secondary Metabolites from Pea on Fusarium Growth and Mycotoxin Biosynthesis. J. Fungi 2021, 7, 1004. https://doi.org/10.3390/jof7121004
Perincherry L, Witaszak N, Urbaniak M, Waśkiewicz A, Stępień Ł. Effects of Secondary Metabolites from Pea on Fusarium Growth and Mycotoxin Biosynthesis. Journal of Fungi. 2021; 7(12):1004. https://doi.org/10.3390/jof7121004
Chicago/Turabian StylePerincherry, Lakshmipriya, Natalia Witaszak, Monika Urbaniak, Agnieszka Waśkiewicz, and Łukasz Stępień. 2021. "Effects of Secondary Metabolites from Pea on Fusarium Growth and Mycotoxin Biosynthesis" Journal of Fungi 7, no. 12: 1004. https://doi.org/10.3390/jof7121004
APA StylePerincherry, L., Witaszak, N., Urbaniak, M., Waśkiewicz, A., & Stępień, Ł. (2021). Effects of Secondary Metabolites from Pea on Fusarium Growth and Mycotoxin Biosynthesis. Journal of Fungi, 7(12), 1004. https://doi.org/10.3390/jof7121004







