No Change of Pneumocystis jirovecii Pneumonia after the COVID-19 Pandemic: Multicenter Time-Series Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Non-Pharmacological Interventions against the COVID-19 Pandemic Imposed by the Korean Government
2.3. Protective Policies for Prevention of Intra-Hospital SARS-CoV-2 Transmission
2.4. P. Jirovecii PCR
2.5. Statistical Analysis
3. Results
3.1. Clinical Information of Total PCP-Confirmed Inpatients
3.2. Trend Analysis and ARIMA Model to Compare the Observed and Forecasted PCP Cases in the Post-COVID-19
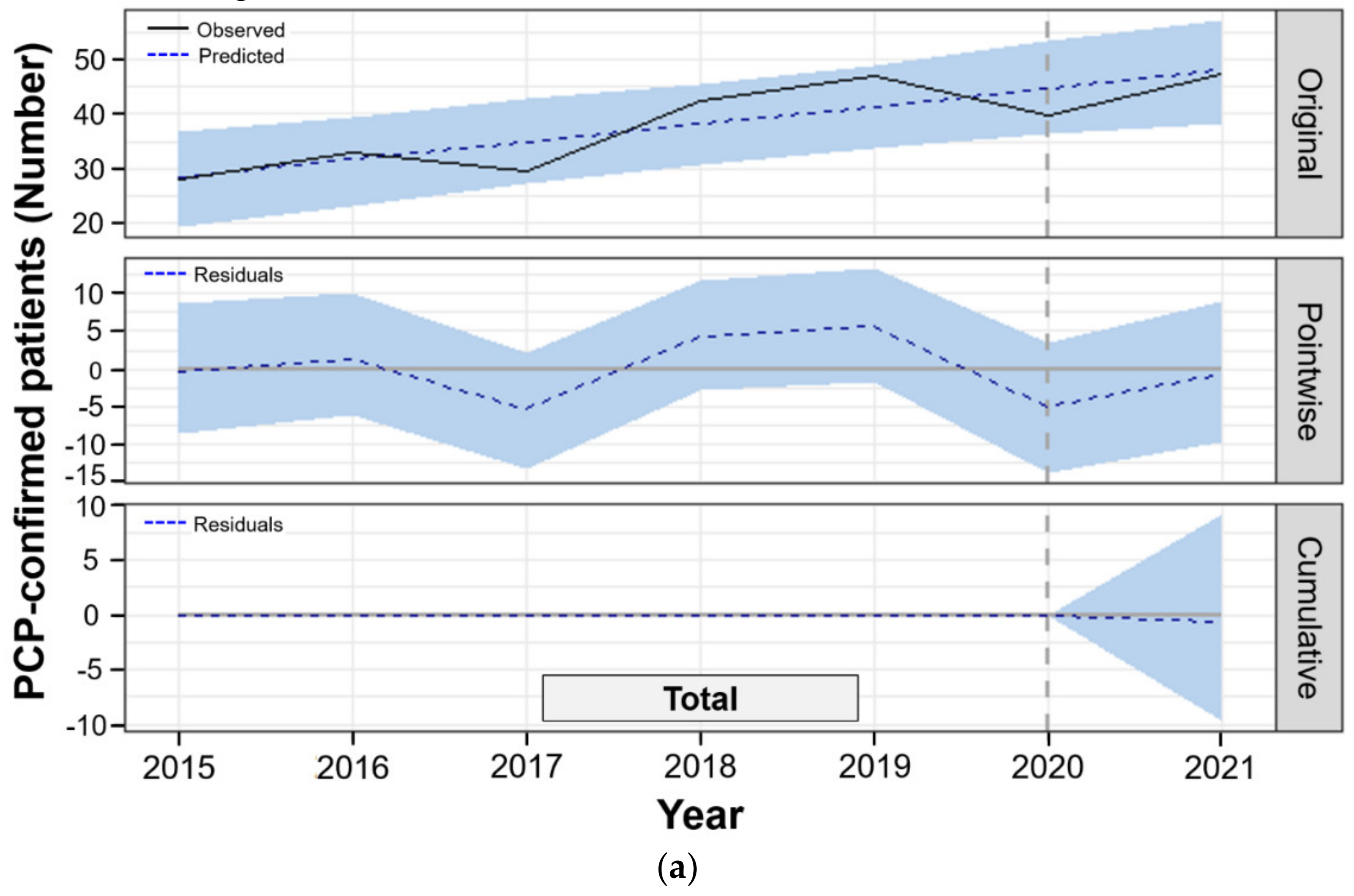
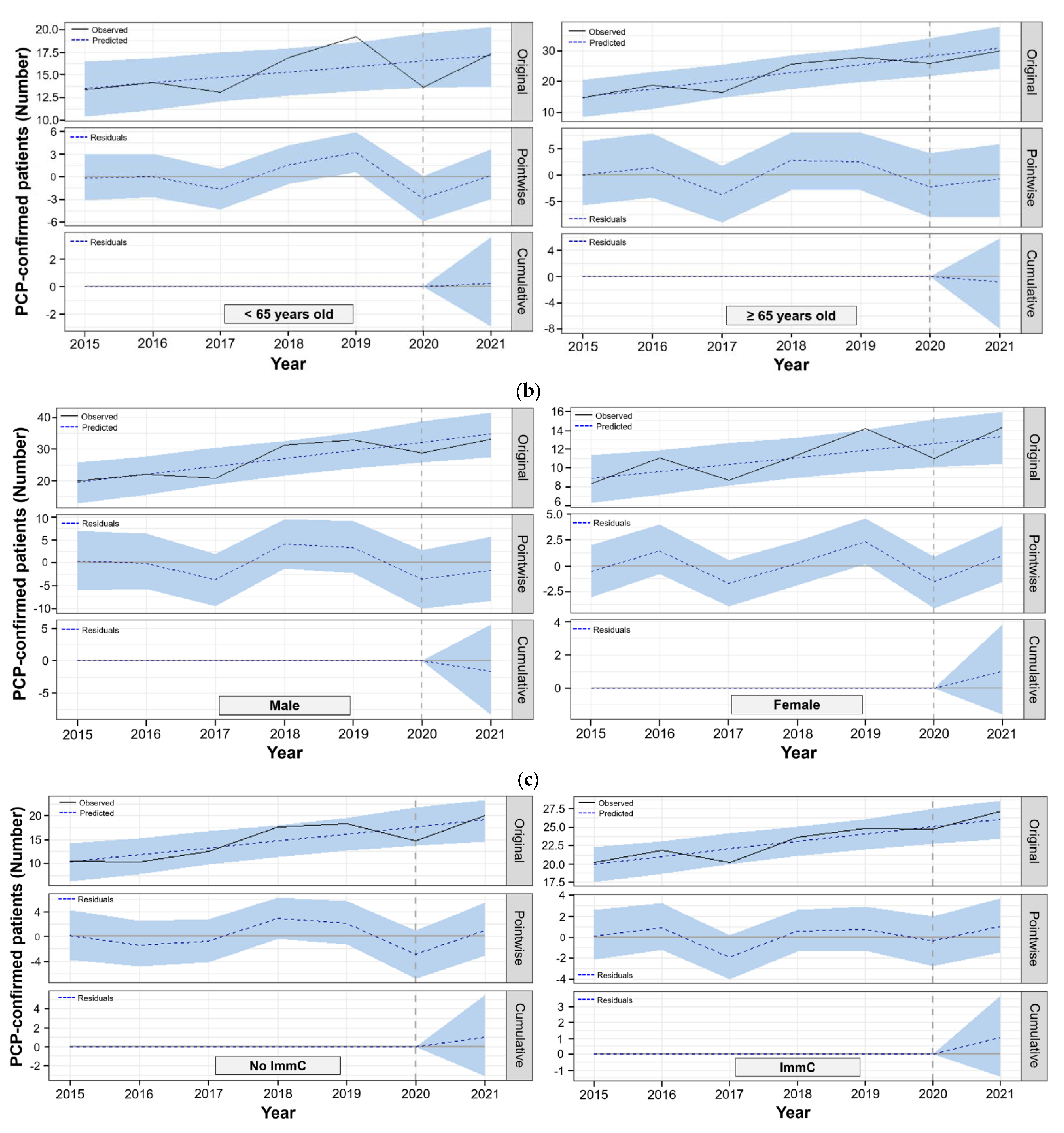
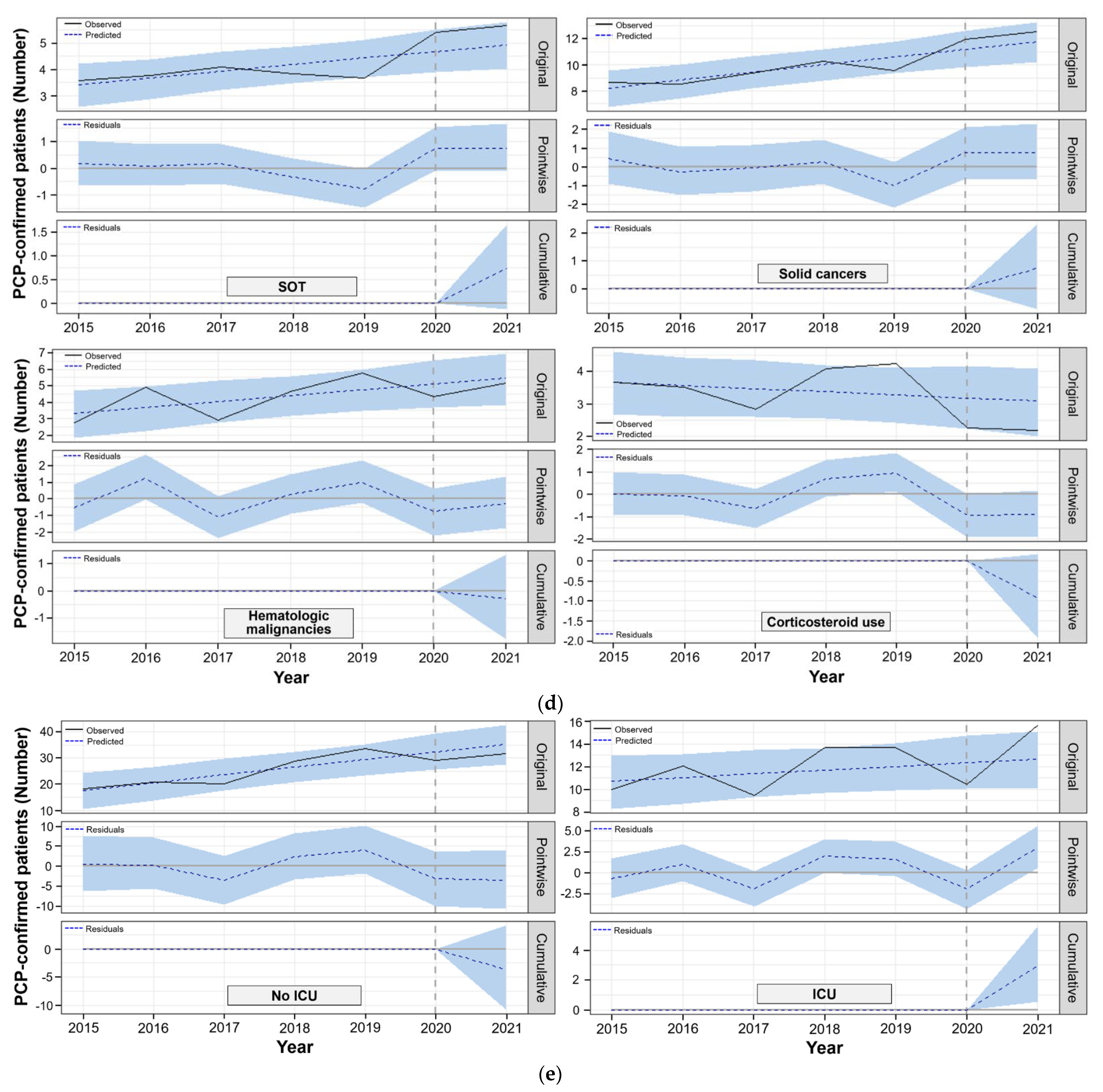
3.3. Bayesian Structural Time-series Model to Compare Observed and Predicted PCP Cases in the Post-COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of sars-cov-2 and Covid-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Thu, T.P.B.; Ngoc, P.N.H.; Hai, N.M.; Tuan, L.A. Effect of the social distancing measures on the spread of Covid-19 in 10 highly infected countries. Sci. Total Env. 2020, 742, 140430. [Google Scholar] [CrossRef]
- Wee, L.E.; Hsieh, J.Y.C.; Phua, G.C.; Tan, Y.; Conceicao, E.P.; Wijaya, L.; Tan, T.T.; Tan, B.H. Respiratory surveillance wards as a strategy to reduce nosocomial transmission of Covid-19 through early detection: The experience of a tertiary-care hospital in singapore. Infect. Control Hosp. Epidemiol. 2020, 41, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, M.S.; Deloney, V.M.; Anderson, D.J.; Cheng, V.C.; Gohil, S.; Kwon, J.H.; Mody, L.; Monsees, E.; Vaughn, V.M.; Wiemken, T.L.; et al. Policies and practices of shea research network hospitals during the Covid-19 pandemic. Infect. Control Hosp. Epidemiol. 2020, 41, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Rivett, L.; Sridhar, S.; Sparkes, D.; Routledge, M.; Jones, N.K.; Forrest, S.; Young, J.; Pereira-Dias, J.; Hamilton, W.L.; Ferris, M.; et al. Screening of healthcare workers for sars-cov-2 highlights the role of asymptomatic carriage in Covid-19 transmission. eLife 2020, 9, e58728. [Google Scholar] [CrossRef] [PubMed]
- McKee, M. Achieving zero covid is not easy, but the alternative is far worse. BMJ 2020, 371, m3859. [Google Scholar] [CrossRef]
- Sulyok, M.; Walker, M. Community movement and covid-19: A global study using google’s community mobility reports. Epidemiol. Infect. 2020, 148, e284. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, A. Personal (non-pharmaceutical) protective measures for reducing transmission of influenza—Ecdc interim recommendations. Euro. Surveill. 2006, 11, 3061. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.M.; Cummings, D.A.; Fraser, C.; Cajka, J.C.; Cooley, P.C.; Burke, D.S. Strategies for mitigating an influenza pandemic. Nature 2006, 442, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.L.; Ferng, Y.H.; Wong-McLoughlin, J.; Wang, S.; Haber, M.; Morse, S.S. Impact of non-pharmaceutical interventions on uris and influenza in crowded, urban households. Public Health Rep. 2010, 125, 178–191. [Google Scholar] [CrossRef]
- Baker, R.E.; Park, S.W.; Yang, W.; Vecchi, G.A.; Metcalf, C.J.E.; Grenfell, B.T. The impact of covid-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc. Natl. Acad. Sci. USA 2020, 117, 30547–30553. [Google Scholar] [CrossRef]
- Cowling, B.J.; Ali, S.T.; Ng, T.W.Y.; Tsang, T.K.; Li, J.C.M.; Fong, M.W.; Liao, Q.; Kwan, M.Y.; Lee, S.L.; Chiu, S.S.; et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: An observational study. Lancet Public Health 2020, 5, e279–e288. [Google Scholar] [CrossRef]
- Sakamoto, H.; Ishikane, M.; Ueda, P. Seasonal influenza activity during the sars-cov-2 outbreak in Japan. JAMA 2020, 323, 1969–1971. [Google Scholar] [CrossRef] [PubMed]
- Soo, R.J.J.; Chiew, C.J.; Ma, S.; Pung, R.; Lee, V. Decreased influenza incidence under covid-19 control measures, singapore. Emerg. Infect Dis. 2020, 26, 1933–1935. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; Azziz-Baumgartner, E.; Budd, A.P.; Brammer, L.; Sullivan, S.; Pineda, R.F.; Cohen, C.; Fry, A.M. Decreased influenza activity during the covid-19 pandemic-united states, australia, chile, and south africa, 2020. Am. J. Transpl. 2020, 20, 3681–3685. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Mook, P.; Lamb, F.; Ferland, L.; Melidou, A.; Amato-Gauci, A.J.; Pebody, R. Very little influenza in the who european region during the 2020/21 season, weeks 40 2020 to 8 2021. Euro. Surveill. 2021, 26, 2100221. [Google Scholar] [CrossRef]
- Yeoh, D.K.; Foley, D.A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Le, H.; Levy, A.; Blyth, C.C.; Moore, H.C. The impact of covid-19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 australian winter. Clin. Infect. Dis. 2020, 72, 2199–2202. [Google Scholar] [CrossRef]
- Eyre, T.A.; Peters, L.; Andersson, M.I.; Peniket, A.; Eyre, D.W. Reduction in incidence of non-covid-19 respiratory virus infection amongst haematology inpatients following uk social distancing measures. Br. J. Haematol. 2021, 195, 194–197. [Google Scholar] [CrossRef]
- Abo, Y.N.; Clifford, V.; Lee, L.Y.; Costa, A.M.; Crawford, N.; Wurzel, D.; Daley, A.J. Covid-19 public health measures and respiratory viruses in children in melbourne. J. Paediatr. Child Health 2021. [Google Scholar] [CrossRef]
- Thomas, C.F., Jr.; Limper, A.H. Pneumocystis pneumonia. N. Engl. J. Med. 2004, 350, 2487–2498. [Google Scholar] [CrossRef]
- Morris, A.; Beard, C.B.; Huang, L. Update on the epidemiology and transmission of pneumocystis carinii. Microbes Infect. 2002, 4, 95–103. [Google Scholar] [CrossRef]
- Casanova-Cardiel, L.; Leibowitz, M.J. Presence of pneumocystis carinii DNA in pond water. J. Eukaryot. Microbiol. 1997, 44, 28s. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, E.S.; Maiorano, J.N. Survival and infectivity of pneumocystis carinii outside the mammalian host. J. Eukaryot. Microbiol. 1996, 43, 35s. [Google Scholar] [CrossRef] [PubMed]
- Navin, T.R.; Rimland, D.; Lennox, J.L.; Jernigan, J.; Cetron, M.; Hightower, A.; Roberts, J.M.; Kaplan, J.E. Risk factors for community-acquired pneumonia among persons infected with human immunodeficiency virus. J. Infect. Dis. 2000, 181, 158–164. [Google Scholar] [CrossRef]
- Dohn, M.N.; White, M.L.; Vigdorth, E.M.; Ralph Buncher, C.; Hertzberg, V.S.; Baughman, R.P.; George Smulian, A.; Walzer, P.D. Geographic clustering of pneumocystis carinii pneumonia in patients with hiv infection. Am. J. Respir. Crit. Care Med. 2000, 162, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Djawe, K.; Levin, L.; Swartzman, A.; Fong, S.; Roth, B.; Subramanian, A.; Grieco, K.; Jarlsberg, L.; Miller, R.F.; Huang, L.; et al. Environmental risk factors for pneumocystis pneumonia hospitalizations in hiv patients. Clin. Infect. Dis. 2013, 56, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Norris, K.A. Colonization by pneumocystis jirovecii and its role in disease. Clin. Microbiol. Rev. 2012, 25, 297–317. [Google Scholar] [CrossRef]
- Ponce, C.A.; Gallo, M.; Bustamante, R.; Vargas, S.L. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin. Infect. Dis. 2010, 50, 347–353. [Google Scholar] [CrossRef]
- Montes-Cano, M.A.; de la Horra, C.; Dapena, F.J.; Mateos, I.; Friaza, V.; Respaldiza, N.; Muñoz-Lobato, F.; Medrano, F.J.; Calderon, E.J.; Varela, J.M. Dynamic colonisation by different pneumocystis jirovecii genotypes in cystic fibrosis patients. Clin. Microbiol. Infect. 2007, 13, 1008–1011. [Google Scholar] [CrossRef]
- Yiannakis, E.P.; Boswell, T.C. Systematic review of outbreaks of pneumocystis jirovecii pneumonia: Evidence that p. jirovecii is a transmissible organism and the implications for healthcare infection control. J. Hosp. Infect. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- de Boer, M.G.J.; Walzer, P.D.; Mori, S. Healthcare related transmission of pneumocystis pneumonia: From key insights toward comprehensive prevention. Transpl. Infect. Dis. 2018, 20, e12942. [Google Scholar] [CrossRef]
- Valade, S.; Azoulay, E.; Damiani, C.; Derouin, F.; Totet, A.; Menotti, J. Pneumocystis jirovecii airborne transmission between critically ill patients and health care workers. Intensive Care Med. 2015, 41, 1716–1718. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, S.; Damiani, C.; Rouillé, A.; Grall, A.; Tréguer, L.; Virmaux, M.; Moalic, E.; Quinio, D.; Moal, M.C.; Berthou, C.; et al. A cluster of pneumocystis infections among renal transplant recipients: Molecular evidence of colonized patients as potential infectious sources of pneumocystis jirovecii. Clin. Infect. Dis. 2012, 54, e62–e71. [Google Scholar] [CrossRef] [PubMed]
- Fréalle, E.; Valade, S.; Guigue, N.; Hamane, S.; Chabé, M.; Le Gal, S.; Damiani, C.; Totet, A.; Aliouat, E.M.; Nevez, G.; et al. Diffusion of pneumocystis jirovecii in the surrounding air of patients with pneumocystis colonization: Frequency and putative risk factors. Med. Mycol. 2017, 55, 568–572. [Google Scholar] [CrossRef]
- Choukri, F.; Aliouat el, M.; Menotti, J.; Totet, A.; Gantois, N.; Garin, Y.J.; Bergeron, V.; Dei-Cas, E.; Derouin, F. Dynamics of pneumocystis carinii air shedding during experimental pneumocystosis. J. Infect. Dis. 2011, 203, 1333–1336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pougnet, L.; Grall, A.; Moal, M.C.; Pougnet, R.; Le Govic, Y.; Négri, S.; Nevez, G.; Le Gal, S. Pneumocystis jirovecii exhalation in the course of pneumocystis pneumonia treatment. Infect. Control Hosp. Epidemiol. 2018, 39, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Ebara, M.; Hasebe, S.; Yakushijin, Y. A study on the colonization of pneumocystis jirovecii among outpatients during cancer chemotherapy and among healthy smokers. J. Infect. Chemother. 2017, 23, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, A.E.; Stewart, T.J.; Moxon, E.R.; Marsh, K.; Hopkin, J.M. Infection with pneumocystis carinii is prevalent in healthy gambian children. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 800–802. [Google Scholar] [CrossRef]
- Vargas, S.L.; Hughes, W.T.; Santolaya, M.E.; Ulloa, A.V.; Ponce, C.A.; Cabrera, C.E.; Cumsille, F.; Gigliotti, F. Search for primary infection by pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 2001, 32, 855–861. [Google Scholar] [CrossRef]
- Respaldiza, N.; Medrano, F.J.; Medrano, A.C.; Varela, J.M.; de la Horra, C.; Montes-Cano, M.; Ferrer, S.; Wichmann, I.; Gargallo-Viola, D.; Calderon, E.J. High seroprevalence of pneumocystis infection in spanish children. Clin. Microbiol. Infect. 2004, 10, 1029–1031. [Google Scholar] [CrossRef]
- Le Gal, S.; Pougnet, L.; Damiani, C.; Fréalle, E.; Guéguen, P.; Virmaux, M.; Ansart, S.; Jaffuel, S.; Couturaud, F.; Delluc, A.; et al. Pneumocystis jirovecii in the air surrounding patients with pneumocystis pulmonary colonization. Diagn. Microbiol. Infect. Dis. 2015, 82, 137–142. [Google Scholar] [CrossRef]
- Pougnet, L.; Hoffmann, C.V.; Pougnet, R.; Le Ny, F.; Gaitan, L.; Cros, P.; Artus, M.; Le Gal, S.; Nevez, G. Pneumocystis exhalation by infants developing pneumocystis primary infection: Putative infectious sources in hospitals and the community. J. Hosp. Infect. 2021, 113, 10–13. [Google Scholar] [CrossRef]
- Choukri, F.; Menotti, J.; Sarfati, C.; Lucet, J.C.; Nevez, G.; Garin, Y.J.; Derouin, F.; Totet, A. Quantification and spread of pneumocystis jirovecii in the surrounding air of patients with pneumocystis pneumonia. Clin. Infect. Dis. 2010, 51, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Cissé, O.H.; Ma, L.; Jiang, C.; Snyder, M.; Kovacs, J.A. Humans are selectively exposed to pneumocystis jirovecii. Mbio 2020, 11, e03138-19. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A.; Gans, H. Pneumocystis jiroveci in solid organ transplantation: Guidelines from the american society of transplantation infectious diseases community of practice. Clin. Transpl. 2019, 33, e13587. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A.; Hauser, P.M.; Lagrou, K.; Melchers, W.J.; Helweg-Larsen, J.; Matos, O.; Cesaro, S.; Maschmeyer, G.; Einsele, H.; Donnelly, J.P.; et al. Ecil guidelines for the diagnosis of pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J. Antimicrob. Chemother. 2016, 71, 2386–2396. [Google Scholar] [CrossRef] [PubMed]
- Avino, L.J.; Naylor, S.M.; Roecker, A.M. Pneumocystis jirovecii pneumonia in the non-hiv-infected population. Ann. Pharm. 2016, 50, 673–679. [Google Scholar] [CrossRef] [PubMed]
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—Recommendations of the advisory committee on immunization practices (acip). MMWR Recomm. Rep. 2011, 60, 1–64. [Google Scholar]
- WHO COVID-19 Dashboard. Available online: https://covid19.who.int/region/wpro/country/kr (accessed on 13 September 2021).
- Korean Central Disease Control Headquarters. Coronavirus (COVID-19), Republic of Korea. Available online: http://ncov.mohw.go.kr/en/ (accessed on 13 September 2021).
- Huh, K.; Jung, J.; Hong, J.; Kim, M.; Ahn, J.G.; Kim, J.H.; Kang, J.M. Impact of nonpharmaceutical interventions on the incidence of respiratory infections during the coronavirus disease 2019 (covid-19) outbreak in korea: A nationwide surveillance study. Clin. Infect. Dis. 2021, 72, e184–e191. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. Enhanced Social Distancing Campaign. Available online: http://ncov.mohw.go.kr/searchBoardView.do?brdId=3&brdGubun=32&dataGubun=321&ncvContSeq=1497 (accessed on 13 September 2021).
- Central Quarantine and Countermeasure Headquarters. The Covid-19 Guidelines of the Republic of Korea for Distancing in Daily Life. Available online: http://ncov.mohw.go.kr/en/guidelineView.do?brdId=18&brdGubun=181&dataGubun=&ncvContSeq=2763&contSeq=2763&board_id=&gubun=# (accessed on 13 September 2021).
- Central Quarantine and Countermeasure Headquarters. Overview of Social Distancing System overview of Social Distancing System. Available online: http://ncov.mohw.go.kr/en/socdisBoardView.do?brdId=19&brdGubun=191&dataGubun=191&ncvContSeq=&contSeq=&board_id=&gubun= (accessed on 13 September 2021).
- Chang, M.C.; Hur, J.; Park, D. Strategies for the prevention of the intra-hospital transmission of Covid-19: A retrospective cohort study. Healthcare 2020, 8, 195. [Google Scholar] [CrossRef]
- Sasso, M.; Chastang-Dumas, E.; Bastide, S.; Alonso, S.; Lechiche, C.; Bourgeois, N.; Lachaud, L. Performances of four real-time pcr assays for diagnosis of pneumocystis jirovecii pneumonia. J. Clin. Microbiol. 2016, 54, 625–630. [Google Scholar] [CrossRef]
- Tia, T.; Putaporntip, C.; Kosuwin, R.; Kongpolprom, N.; Kawkitinarong, K.; Jongwutiwes, S. A highly sensitive novel pcr assay for detection of pneumocystis jirovecii DNA in bronchoalveloar lavage specimens from immunocompromised patients. Clin. Microbiol. Infect. 2012, 18, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, D.A.; Muhajarine, N. Time series prediction of under-five mortality rates for nigeria: Comparative analysis of artificial neural networks, holt-winters exponential smoothing and autoregressive integrated moving average models. BMC Med. Res. Methodol. 2020, 20, 292. [Google Scholar] [CrossRef] [PubMed]
- Perone, G. Comparison of arima, ets, nnar, tbats and hybrid models to forecast the second wave of covid-19 hospitalizations in italy. Eur. J. Health Econ. 2021. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, K.H.; Gallusser, F.; Koehler, J.; Remy, N.; Scott, S.L. Inferring causal impact using bayesian structural time-series models. Ann. Appl. Stat. 2015, 9, 247–274. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing (version 4.1.0). Available online: http://www.r-project.org (accessed on 10 September 2021).
- CausalImpact: An R package for causal inference in time series. Available online: http://google.github.io/CausalImpact/ (accessed on 10 September 2021).
- McQuire, C.; Tilling, K.; Hickman, M.; de Vocht, F. Forecasting the 2021 local burden of population alcohol-related harms using bayesian structural time-series. Addiction 2019, 114, 994–1003. [Google Scholar] [CrossRef]
- de Vocht, F. Inferring the 1985-2014 impact of mobile phone use on selected brain cancer subtypes using bayesian structural time series and synthetic controls. Env. Int. 2016, 97, 100–107. [Google Scholar] [CrossRef]
- Kurz, C.F.; Rehm, M.; Holle, R.; Teuner, C.; Laxy, M.; Schwarzkopf, L. The effect of bariatric surgery on health care costs: A synthetic control approach using bayesian structural time series. Health Econ. 2019, 28, 1293–1307. [Google Scholar] [CrossRef]
- Wang, X.; Zou, C.; Wang, L. Analysis on the temporal distribution characteristics of air pollution and its impact on human health under the noticeable variation of residents’ travel behavior: A case of guangzhou, china. Int. J. Env. Res. Public Health 2020, 17, 4947. [Google Scholar] [CrossRef] [PubMed]
- Alim, M.; Ye, G.H.; Guan, P.; Huang, D.S.; Zhou, B.S.; Wu, W. Comparison of arima model and xgboost model for prediction of human brucellosis in mainland china: A time-series study. BMJ Open 2020, 10, e039676. [Google Scholar] [CrossRef] [PubMed]
- Thiruchelvam, L.; Dass, S.C.; Asirvadam, V.S.; Daud, H.; Gill, B.S. Determine neighboring region spatial effect on dengue cases using ensemble arima models. Sci. Rep. 2021, 11, 5873. [Google Scholar] [CrossRef]
- Maini, R.; Henderson, K.L.; Sheridan, E.A.; Lamagni, T.; Nichols, G.; Delpech, V.; Phin, N. Increasing pneumocystis pneumonia, england, uk, 2000–2010. Emerg. Infect. Dis. 2013, 19, 386–392. [Google Scholar] [CrossRef]
- Pereira-Díaz, E.; Moreno-Verdejo, F.; de la Horra, C.; Guerrero, J.A.; Calderón, E.J.; Medrano, F.J. Changing trends in the epidemiology and risk factors of pneumocystis pneumonia in spain. Front. Public Health 2019, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Grønseth, S.; Rogne, T.; Hannula, R.; Åsvold, B.O.; Afset, J.E.; Damås, J.K. Epidemiological and clinical characteristics of immunocompromised patients infected with pneumocystis jirovecii in a twelve-year retrospective study from norway. BMC Infect. Dis. 2021, 21, 659. [Google Scholar] [CrossRef]
- Park, S.W.; Bolker, B.M.; Champredon, D.; Earn, D.J.D.; Li, M.; Weitz, J.S.; Grenfell, B.T.; Dushoff, J. Reconciling early-outbreak estimates of the basic reproductive number and its uncertainty: Framework and applications to the novel coronavirus (sars-cov-2) outbreak. J. R. Soc. Interface 2020, 17, 20200144. [Google Scholar] [CrossRef]
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The basic reproduction number (r(0)) of measles: A systematic review. Lancet Infect. Dis. 2017, 17, e420–e428. [Google Scholar] [CrossRef]
- White, L.F.; Pagano, M. Transmissibility of the influenza virus in the 1918 pandemic. PLoS ONE 2008, 3, e1498. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.O.; Davoudi, B.; Riley, S.; Pourbohloul, B. Early real-time estimation of the basic reproduction number of emerging or reemerging infectious diseases in a community with heterogeneous contact pattern: Using data from hong kong 2009 h1n1 pandemic influenza as an illustrative example. PLoS ONE 2015, 10, e0137959. [Google Scholar]
- Reis, J.; Shaman, J. Retrospective parameter estimation and forecast of respiratory syncytial virus in the united states. PLoS Comput. Biol. 2016, 12, e1005133. [Google Scholar] [CrossRef] [PubMed]
- Rivero, L.; de la Horra, C.; Montes-Cano, M.A.; Rodríguez-Herrera, A.; Respaldiza, N.; Friaza, V.; Morilla, R.; Gutiérrez, S.; Varela, J.M.; Medrano, F.J.; et al. Pneumocystis jirovecii transmission from immunocompetent carriers to infant. Emerg. Infect. Dis. 2008, 14, 1116–1118. [Google Scholar] [CrossRef]
- Morilla, R.; Medrano, F.J.; Calzada, A.; Quintana, E.; Campano, E.; Friaza, V.; Calderón, E.J.; de la Horra, C. Pneumocystis jirovecii among patients with cystic fibrosis and their household members. Med. Mycol. 2021, 59, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Guerrisi, C.; Turbelin, C.; Souty, C.; Poletto, C.; Blanchon, T.; Hanslik, T.; Bonmarin, I.; Levy-Bruhl, D.; Colizza, V. The potential value of crowdsourced surveillance systems in supplementing sentinel influenza networks: The case of france. Euro. Surveill. 2018, 23, 1700337. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Hughes, G.; Fenton, K.A. Surveillance systems for sexually transmitted infections: A global review. Curr. Opin. Infect. Dis. 2016, 29, 64–69. [Google Scholar] [CrossRef]
- Babu Rajendran, N.; Mutters, N.T.; Marasca, G.; Conti, M.; Sifakis, F.; Vuong, C.; Voss, A.; Baño, J.R.; Tacconelli, E. Mandatory surveillance and outbreaks reporting of the who priority pathogens for research & discovery of new antibiotics in european countries. Clin. Microbiol. Infect. 2020, 26, e941–e943. [Google Scholar]
- Hsieh, Y.H.; Kuo, M.J.; Hsieh, T.C.; Lee, H.C. Underreporting and underestimation of gonorrhea cases in the taiwan national gonorrhea notifiable disease system in the tainan region: Evaluation by a pilot physician-based sentinel surveillance on neisseria gonorrhoeae infection. Int. J. Infect. Dis. 2009, 13, e413–e419. [Google Scholar] [CrossRef][Green Version]
- Korean Central Disease Control Headquarters. Coronavirus (COVID-19), Republic of Korea. Available online: http://ncov.mohw.go.kr/en/bdBoardList.do?brdId=16&brdGubun=161&dataGubun=&ncvContSeq=&contSeq=&board_id= (accessed on 13 September 2021).

| Total Duration (N = 2922) | Pre-COVID-19 (N = 2163) | Post-COVID-19 (N = 759) | p-Value a | |
|---|---|---|---|---|
| Age, years | 65.7 ± 13.3 | 65.0 ± 13.4 | 67.5 ± 12.9 | <0.001 |
| <65 | 1189 (40.7) | 921 (42.6) | 268 (35.3) | |
| ≥65 | 1733 (59.3) | 1242 (57.4) | 491 (64.7) | |
| Sex, male | 2061 (70.5) | 1520 (70.3) | 541 (71.3) | 0.257 |
| ICU care at admission | 926 (31.7) | 707 (32.7) | 219 (28.9) | <0.001 |
| All-cause death at admission | 1044 (35.7) | 780 (36.1) | 264 (34.8) | 0.128 |
| Hospital stay, days | 19 (10–36) | 18 (10-36) | 19 (11–37) | 0.753 |
| PCP diagnosis | ||||
| PCR | ||||
| Sputum | 2492 (85.3) | 1884 (87.1) | 608 (80.1) | <0.001 |
| Bronchial washing | 136 (4.7) | 66 (3.0) | 70 (9.2) | 0.176 |
| BAL fluid | 214 (7.3) | 166 (7.7) | 48 (6.4) | 0.859 |
| Cytology | 59 (2.0) | 30 (1.4) | 29 (3.8) | 0.357 |
| Histopathology | 21 (0.7) | 17 (0.8) | 4 (0.5) | 0.795 |
| Quantitative cycles in real-time PCR tests, Ct values | 29.2 ± 11.2 | 30.9 ± 9.4 | 29.1 ± 10.5 | 0.503 |
| Positive b | 2688 (92.0) | 1987 (91.9) | 701 (92.4) | 0.718 |
| Weak positive c | 234 (8.0) | 176 (8.1) | 58 (7.6) | 0.652 |
| Immunocompromised conditions | 1789 (61.2) | 1329 (61.4) | 460 (60.6) | 0.935 |
| SOT recipients | 260 (8.9) | 220 (10.2) | 40 (5.3) | 0.012 |
| HSCT recipients | 35 (1.2) | 25 (1.2) | 10 (1.3) | 0.912 |
| Solid cancers | 738 (25.3) | 526 (24.3) | 216 (28.5) | <0.001 |
| Hematologic malignancies | 335 (11.5) | 252 (11.7) | 83 (10.9) | 0.752 |
| HIV-1 infection | 62 (2.1) | 52 (2.4) | 10 (1.3) | 0.376 |
| Chronic lung diseases | 53 (1.8) | 47 (2.2) | 6 (0.8) | 0.121 |
| High-dose and long-term corticosteroid therapy | 306 (10.5) | 207 (9.6) | 95 (12.5) | 0.002 |
| Characteristics | Estimate (SE) (%) | p-Value |
|---|---|---|
| Total PCP-confirmed inpatients | 7.42 (22.64) | 0.765 |
| Age (years old) | ||
| <65 | 0.18 (7.07) | 0.981 |
| ≥65 | 7.27 (15.7) | 0.675 |
| Sex | ||
| Male | 5.38 (6.44) | 0.465 |
| Female | 2.03 (6.64) | 0.780 |
| Immunocompromised conditions | ||
| No | 4.04 (7.13) | 0.610 |
| Yes | 3.79 (6.73) | 0.613 |
| SOT recipients | −1.25 (0.96) | 0.282 |
| Solid cancers | 2.92 (2.65) | 0.351 |
| Hematologic malignancies | 0.61 (2.99) | 0.851 |
| High-dose and long-term corticosteroid therapy | 1.70 (1.53) | 0.346 |
| ICU care at admission | ||
| No | 6.13 (6.60) | 0.421 |
| Yes | 1.28 (4.21) | 0.782 |
| Characteristics | Pre- COVID-19 | Bayesian Structural Time-Series Model | ||||
|---|---|---|---|---|---|---|
| Post-COVID-19 | ||||||
| Observed PCP Cases (No.) a | Observed PCP Cases (No.) a | Predicted PCP Cases (No.) a | Absolute Average Effect (%) (95% CI) | Relative Average Effect (%) (95% CI) | p -Value | |
| Total PCP-confirmed inpatients | 36.1 | 47.3 | 48.0 | −0.6 (−9.6~9.1) | −1.3 (−20.0~19.0) | 0.450 |
| Age (years old) | ||||||
| <65 | 15.4 | 17.3 | 17.1 | 0.2 (−2.9~3.6) | 1.0 (−17.0~21.0) | 0.448 |
| ≥65 | 20.7 | 30.0 | 30.8 | −0.8 (−7.9~5.8) | −2.7 (−26.0~19.0) | 0.392 |
| Sex | ||||||
| Male | 25.3 | 33.0 | 34.6 | −1.6 (−8.3~5.6) | −4.7 (−24.0~16.0) | 0.319 |
| Female | 10.7 | 14.3 | 13.3 | 1.0 (−1.6~3.9) | 7.7 (−12.0~29.0) | 0.221 |
| Immunocompromised conditions | ||||||
| No | 13.9 | 20.2 | 19.1 | 1.0 (−3.1~5.5) | 5.4 (−16.0~29.0) | 0.308 |
| Yes | 22.2 | 27.2 | 26.1 | 1.1 (−1.4~3.7) | 4.0 (−5.4~14.0) | 0.202 |
| SOT recipients | 3.8 | 5.7 | 4.9 | 0.7 (−0.1~1.7) | 15.0 (−2.0~33.0) | 0.072 |
| Solid cancers | 9.3 | 12.5 | 11.8 | 0.7 (−0.7~2.3) | 6.0 (−6.0~20.0) | 0.166 |
| Hematologic malignancies | 4.2 | 5.2 | 5.5 | −0.3 (−1.8~1.3) | −5.0 (−32.0~24.0) | 0.347 |
| High-dose and long-term corticosteroid therapy | 3.7 | 2.2 | 3.1 | −0.9 (−1.9~0.2) | −30.0 (−63.0~5.0) | 0.057 |
| ICU care at admission | ||||||
| No | 24.3 | 31.7 | 35.3 | −3.6 (−10.7~4.1) | −10.0 (−30.0~12.0) | 0.170 |
| Yes | 11.8 | 15.7 | 12.7 | 3.0 (0.6~5.6) | 24.0 (4.5~44.0) | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, S.B.; Jeon, S.; Kim, S.; Lee, K.H.; Lee, H.S.; Han, S.H. No Change of Pneumocystis jirovecii Pneumonia after the COVID-19 Pandemic: Multicenter Time-Series Analyses. J. Fungi 2021, 7, 990. https://doi.org/10.3390/jof7110990
Kim D, Kim SB, Jeon S, Kim S, Lee KH, Lee HS, Han SH. No Change of Pneumocystis jirovecii Pneumonia after the COVID-19 Pandemic: Multicenter Time-Series Analyses. Journal of Fungi. 2021; 7(11):990. https://doi.org/10.3390/jof7110990
Chicago/Turabian StyleKim, Dayeong, Sun Bean Kim, Soyoung Jeon, Subin Kim, Kyoung Hwa Lee, Hye Sun Lee, and Sang Hoon Han. 2021. "No Change of Pneumocystis jirovecii Pneumonia after the COVID-19 Pandemic: Multicenter Time-Series Analyses" Journal of Fungi 7, no. 11: 990. https://doi.org/10.3390/jof7110990
APA StyleKim, D., Kim, S. B., Jeon, S., Kim, S., Lee, K. H., Lee, H. S., & Han, S. H. (2021). No Change of Pneumocystis jirovecii Pneumonia after the COVID-19 Pandemic: Multicenter Time-Series Analyses. Journal of Fungi, 7(11), 990. https://doi.org/10.3390/jof7110990






