Purified Native and Recombinant Major Alternaria alternata Allergen (Alt a 1) Induces Allergic Asthma in the Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Alternaria alternata Extract and Purification of Native and Recombinant Alt a 1
2.2. SDS-PAGE Immunoblotting
2.3. Endotoxin
2.4. Mice
2.5. Analysis of the Bronchoalveolar Lavages (BALs)
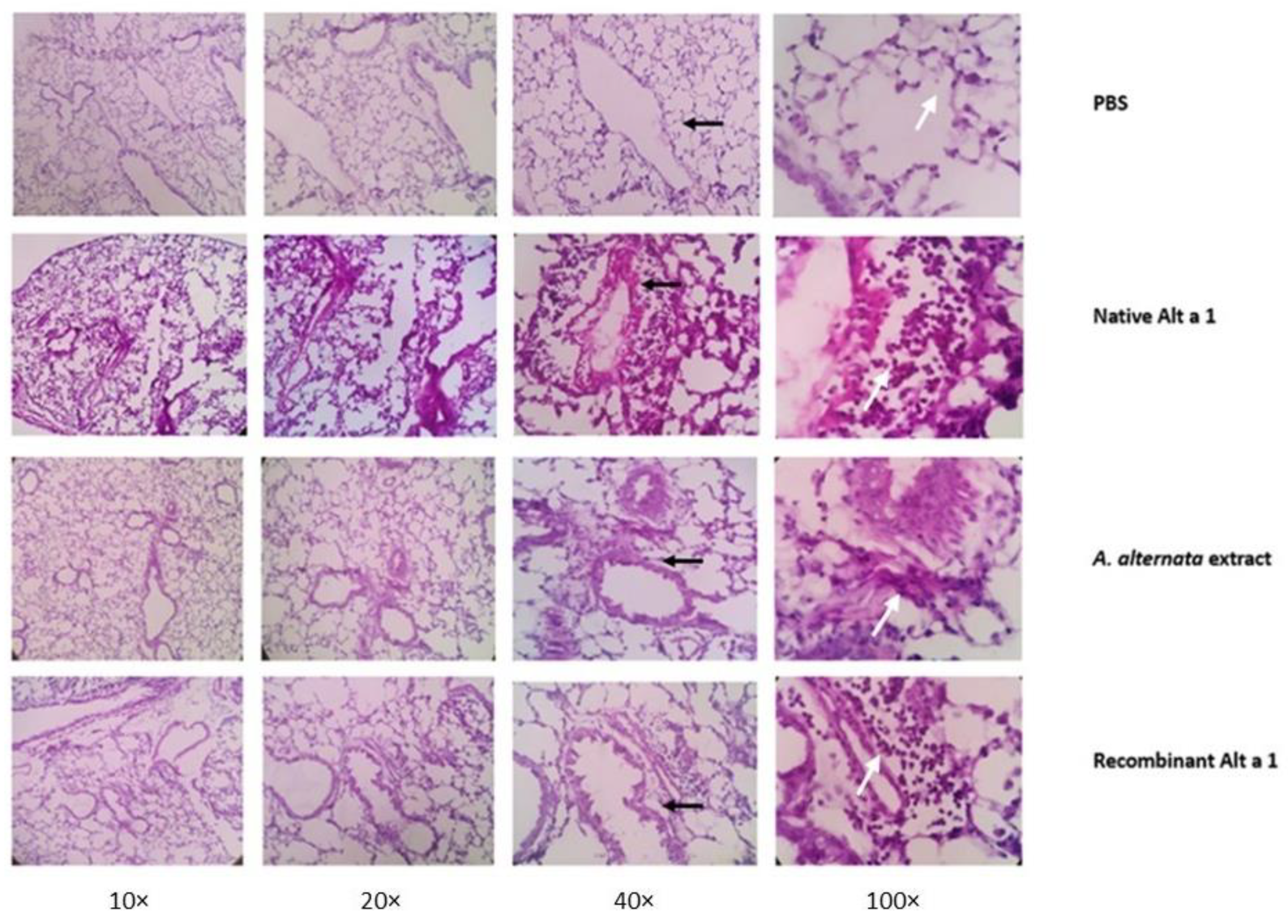
2.6. Histological Analysis
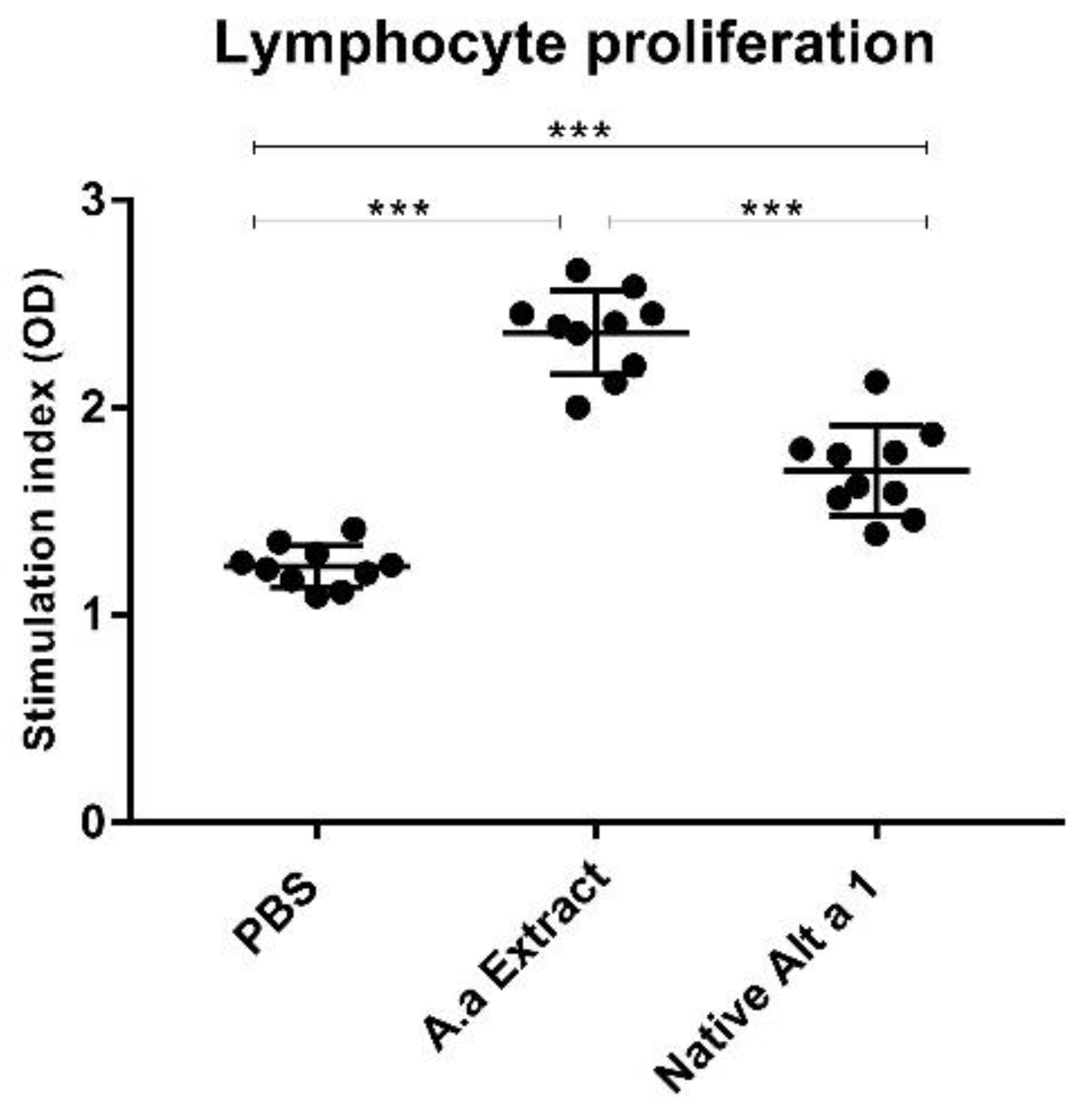
2.7. Proliferation of Lymphocytes
2.8. Cytokines and Total IgE Quantification by ELISA
2.9. Statistical Analysis
3. Results
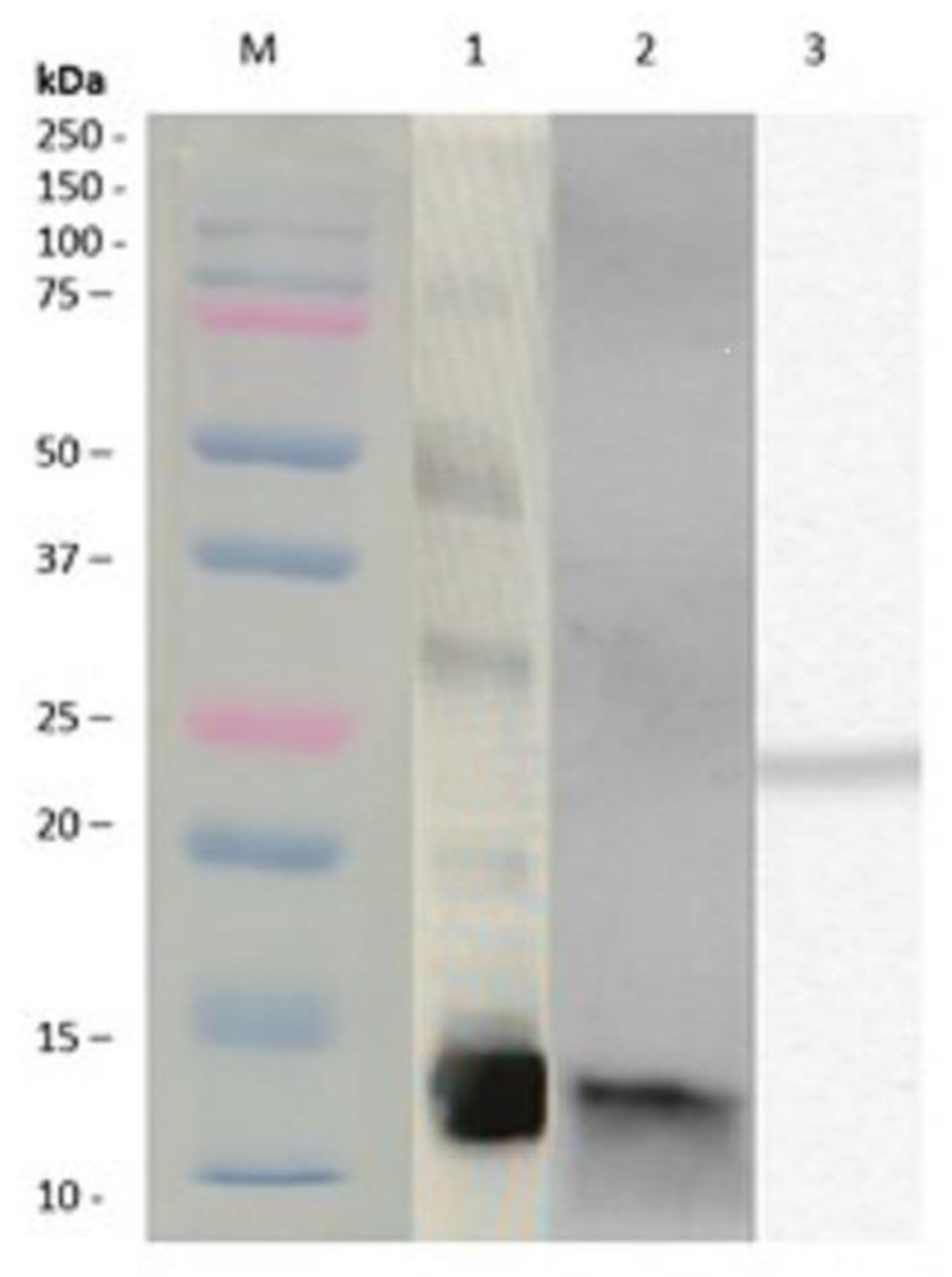
3.1. Alternaria alternata Extracts and Purification of Native and Recombinant Alt a 1
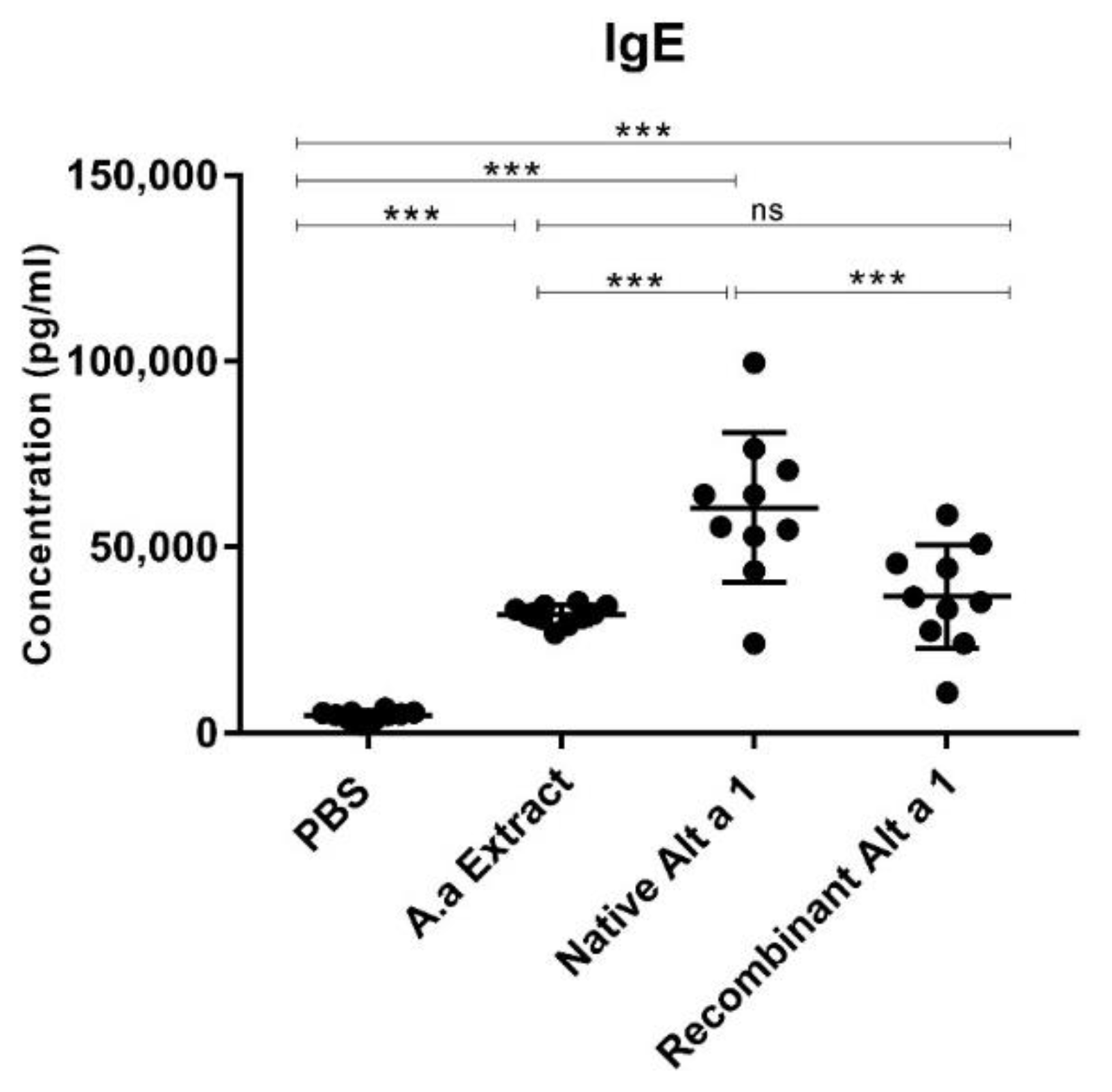
3.2. Quantification of Total IgE in Mouse Serum
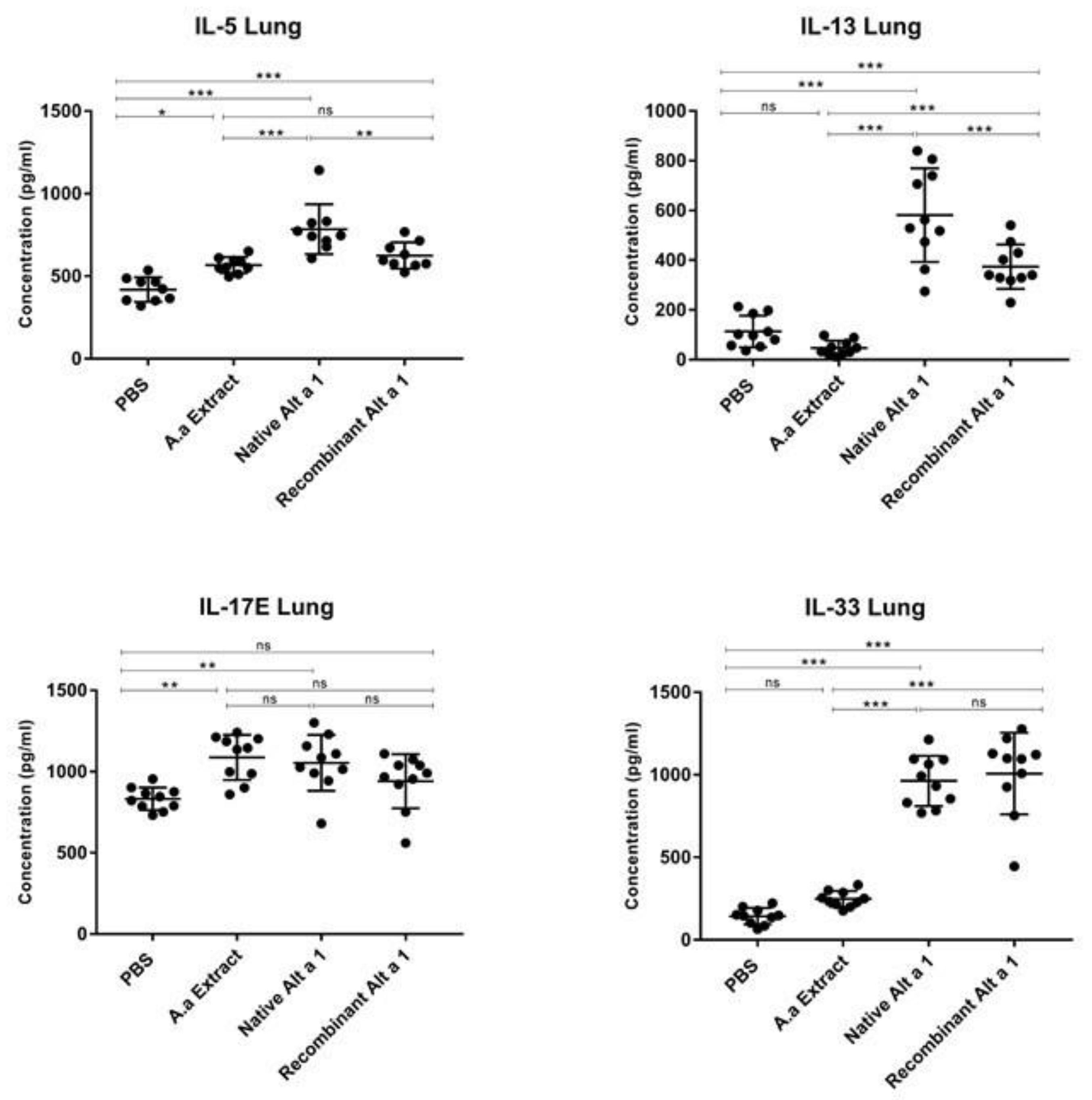
3.3. Cytokine Quantification in Lung Homogenate
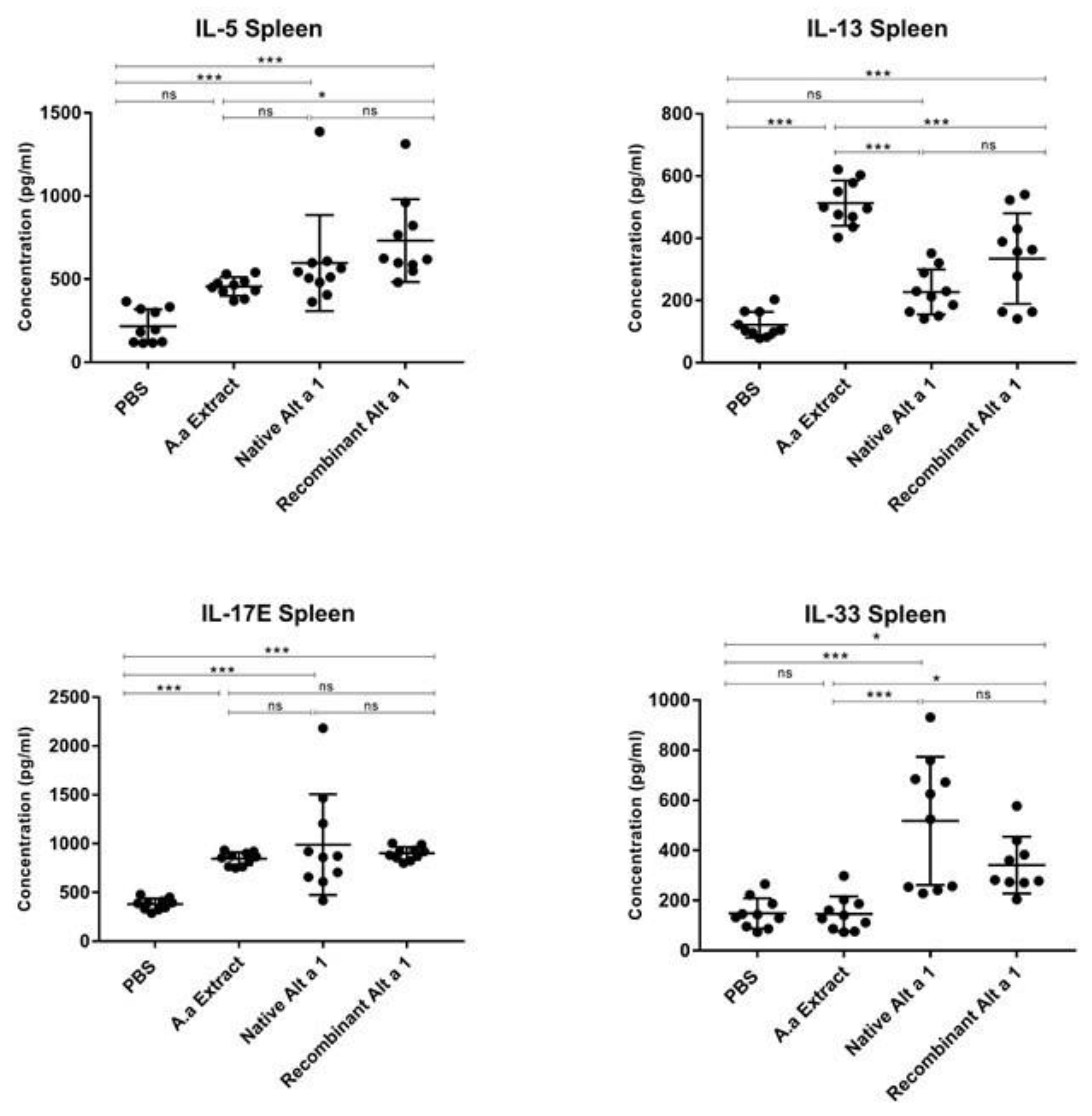
3.4. Cytokine Quantification in Spleen Homogenate
3.5. Analysis of Lymphocyte Proliferation
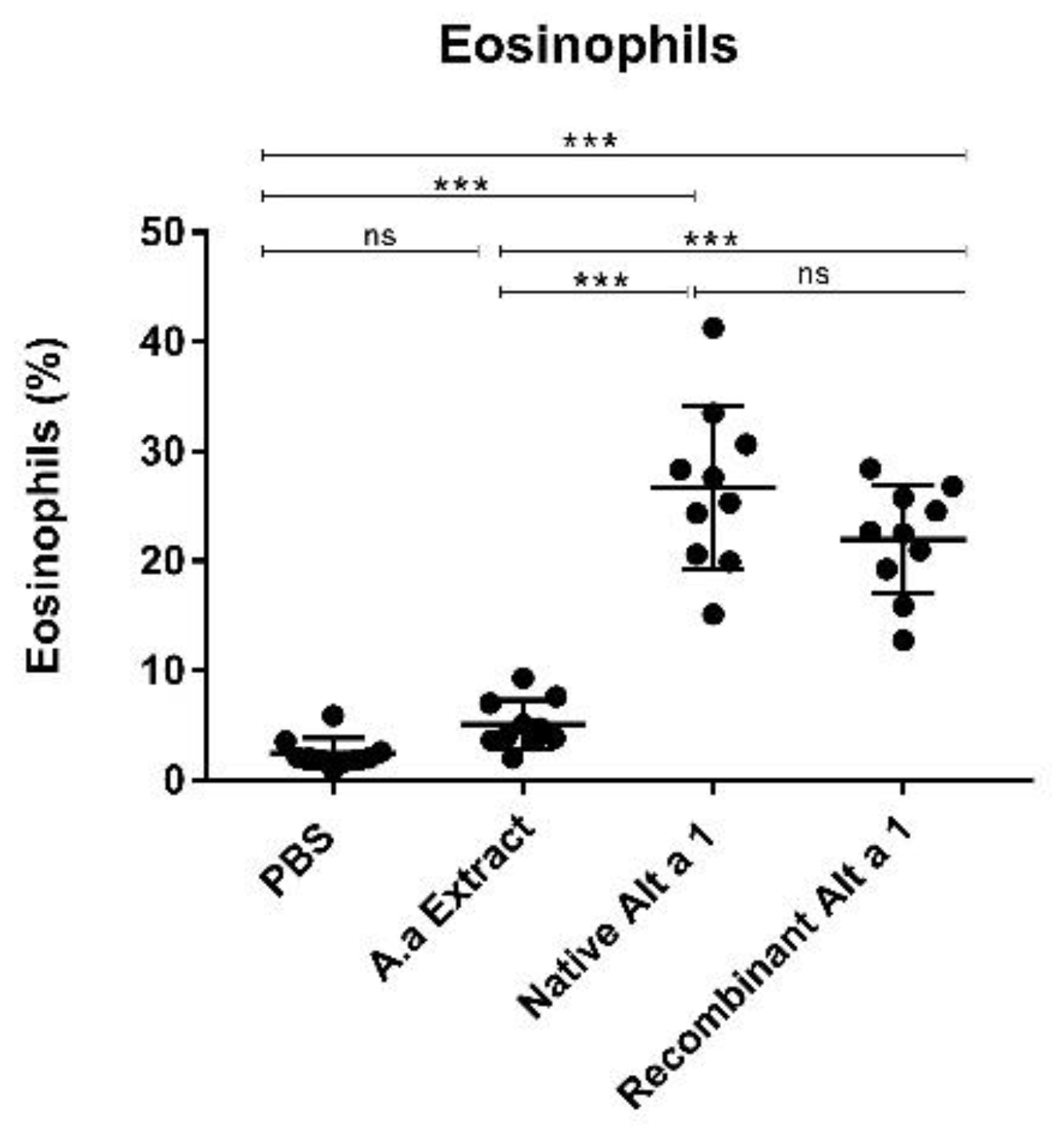
3.6. Eosinophil Count
3.7. Histopathological Analysis of Lung Tissue
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hamilton, D.; Lehman, H. Asthma Phenotypes as a Guide for Current and Future Biologic Therapies. Clin. Rev. Allergy Immunol. 2020, 59, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Voskamp, A.L.; Kormelink, T.G.; van Wijk, R.G.; Hiemstra, P.S.; Taube, C.; de Jong, E.C.; Smits, H.H. Modulating local airway immune responses to treat allergic asthma: Lessons from experimental models and human studies. Semin. Immunopathol. 2020, 42, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.K.; Scadding, G.W. Innate and Adaptive Immunity: ILC2 and Th2 Cells in Upper and Lower Airway Allergic Diseases. J. Allergy Clin. Immunol. Pract. 2021, 9, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, S.; Mindt, B.C.; Fritz, J.H.; Duerr, C.U. Group 2 Innate Lymphoid Cells in Respiratory Allergic Inflammation. Front. Immunol. 2019, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Gurram, R.K.; Zhu, J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell. Mol. Immunol. 2019, 16, 225–235. [Google Scholar] [CrossRef]
- Shin, Y.S.; Takeda, K.; Gelfand, E.W. Understanding Asthma Using Animal Models. Allergy Asthma Immunol. Res. 2009, 1, 10–18. [Google Scholar] [CrossRef]
- Bates, J.H.T.; Rincon, M.; Irvin, C.G. Animal models of asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L401–L410. [Google Scholar] [CrossRef]
- De Vooght, V.; Vanoirbeek, J.A.J.; Luyts, K.; Haenen, S.; Nemery, B.; Hoet, P.H.M. Choice of Mouse Strain Influences the Outcome in a Mouse Model of Chemical-Induced Asthma. PLoS ONE 2010, 5, e12581. [Google Scholar] [CrossRef]
- Kumar, R.; Herbert, C.; Foster, P. The “Classical” Ovalbumin Challenge Model of Asthma in Mice. Curr. Drug Targets 2008, 9, 485–494. [Google Scholar] [CrossRef]
- Nials, A.T.; Uddin, S. Mouse models of allergic asthma: Acute and chronic allergen challenge. Dis. Model Mech. 2008, 1, 213–220. [Google Scholar] [CrossRef]
- Ferreira, B.L.; Ferreira, É.R.; de Brito, M.V.; Salu, B.R.; Oliva, M.L.V.; Mortara, R.A.; Orikaza, C.M. BALB/c and C57BL/6. Mice Cytokine Responses to Trypanosoma cruzi Infection Are Independent of Parasite Strain Infectivity. Front. Microbiol. 2018, 26, 553. [Google Scholar] [CrossRef] [PubMed]
- Gueders, M.M.; Paulissen, G.; Crahay, C.; Quesada-Calvo, F.; Hacha, J.; Van Hove, C.; Cataldo, D.D. Mouse models of asthma: A comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm. Res. 2009, 58, 845–854. [Google Scholar] [CrossRef]
- Shinagawa, K.; Kojima, M. Mouse Model of Airway Remodeling. Am. J. Respir. Crit. Care Med. 2003, 15, 959–967. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, G.T.; Daheshia, M.; Daser, A. Genetics of airway hyperresponsiveness. J. Allergy Clin. Immunol. 2001, 108, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Morokata, T.; Ishikawa, J.; Ida, K.; Yamada, T. C57BL/6 mice are more susceptible to antigen-induced pulmonary eosinophilia than BALB/c mice, irrespective of systemic T helper 1/T helper 2 responses. Immunology 1999, 98, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Aun, M.V.; Saraiva-Romanholo, B.M.; de Almeida, F.M.; Brüggemann, T.R.; Kalil, J.; de Martins, M.A.; Giavina-Bianchi, P. Sensitization by subcutaneous route is superior to intraperitoneal route in induction of asthma by house dust mite in a murine mode. Einstein 2015, 13, 560–566. [Google Scholar] [CrossRef]
- Debeuf, N.; Haspeslagh, E.; van Helden, M.; Hammad, H.; Lambrecht, B.N. Mouse Models of Asthma. Curr. Protoc. Mouse Biol. 2016, 1, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Ishida, W.; Fukuda, K.; Sumi, T.; Ebihara, N.; Kajisako, M.; Matsuda, H.; Fukushima, A. Adjuvants determine the contribution of basophils to antigen sensitization in vivo. Immunol. Lett. 2011, 30, 49–54. [Google Scholar] [CrossRef]
- Aun, M.; Bonamichi-Santos, R.; Arantes-Costa, F.M.; Kalil, J.; Giavina-Bianchi, P. Animal models of asthma: Utility and limitations. J. Asthma Allergy 2017, 10, 293–301. [Google Scholar] [CrossRef]
- Xie, J.; Xi, Y.; Zhang, Q.; Chen, G.; Wei, L.; Lai, K.; Zhong, N. An Intratracheal Challenge Murine Model of Asthma: Can Bronchial Inflammation Affect the Nose? Allergy Asthma Immunol. Res. 2015, 7, 76–82. [Google Scholar] [CrossRef]
- Schmit, D.; Le, D.D.; Heck, S.; Bischoff, M.; Tschernig, T.; Herr, C.; Dinh, Q.T. Allergic airway inflammation induces migration of mast cell populations into the mouse airway. Cell Tissue Res. 2017, 369, 331–340. [Google Scholar] [CrossRef]
- Wegmann, M.; Fehrenbach, H.; Fehrenbach, A.; Held, T.; Schramm, C.; Garn, H.; Renz, H. Involvement of distal airways in a chronic model of experimental asthma. Clin. Exp. Allergy 2005, 35, 1263–1271. [Google Scholar] [CrossRef]
- Samarasinghe, A.E.; Hoselton, S.A.; Schuh, J.M. A comparison between intratracheal and inhalation delivery of Aspergillus fumigatus conidia in the development of fungal allergic asthma in C57BL/6 mice. Fungal Biol. 2011, 115, 21–29. [Google Scholar] [CrossRef]
- Tiwary, M.; Samarasinghe, A.E. Initiation and Pathogenesis of Severe Asthma with Fungal Sensitization. Cells 2021, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, J.K.; Liu-Brennan, D.; McKay, K.O.; Green, B.J.; Tovey, E.R. Domestic exposure to fungal allergenic particles determined by halogen immunoassay using subject’s serum versus particles carrying three non-fungal allergens determined by allergen-specific HIA. Indoor Air 2014, 24, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, A.P.; Bush, R.K.; Demain, J.G.; Denning, D.W.; Dixit, A.; Fairs, A.; Wardlaw, A.J. Fungi and allergic lower respiratory tract diseases. J. Allergy Clin. Immunol. 2012, 129, 280–291. [Google Scholar] [CrossRef]
- Denning, D.W. The link between fungi and severe asthma: A summary of the evidence. Eur. Respir. J. 2006, 27, 615–626. [Google Scholar] [CrossRef]
- Denning, D.W.; Pashley, C.; Hartl, D.; Wardlaw, A.; Godet, C.; Del Giacco, S.; Sergejeva, S. Fungal allergy in asthma–state of the art and research needs. Clin. Transl. Allergy 2014, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Forkel, S.; Beutner, C.; Schröder, S.S.; Bader, O.; Gupta, S.; Fuchs, T.; Schön, M.P.; Geier, J.; Buhl, T. Sensitization against Fungi in Patients with Airway Allergies over 20 Years in Germany. Int. Arch. Allergy Immunol. 2021, 182, 515–523. [Google Scholar] [CrossRef]
- Salo, P.; Arbesjr, S.; Sever, M.; Jaramillo, R.; Cohn, R.; London, S.; Zeldin, D. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J. Allergy Clin. Immunol. 2006, 118, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Pulimood, T.B.; Corden, J.M.; Bryden, C.; Sharples, L.; Nasser, S.M. Epidemic asthma and the role of the fungal mold Alternaria alternata. Allergy Clin. Immunol. 2007, 120, 610–617. [Google Scholar] [CrossRef]
- Bush, R.K.; Prochnau, J.J. Alternaria-induced asthma. J. Allergy Clin. Immunol. 2004, 113, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.F.; Postigo, I.; Tomaz, C.T.; Martínez, J. Alternaria alternata allergens: Markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ. Int. 2016, 89, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rodríguez, A.; Postigo, I.; Guisantes, J.A.; Suñén, E.; Martínez, J. Identification of allergens homologous to Alt a 1 from Stemphylium botryosum and Ulocladium botrytis. Med. Mycol. 2011, 49, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Asturias, J.A.; Ibarrola, I.; Ferrer, A.; Andreu, C.; López-Pascual, E.; Quiralte, J.; Martínez, A. Diagnosis of Alternaria alternata sensitization with natural and recombinant Alt a 1 allergens. J. Allergy Clin. Immunol. 2005, 115, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- De Vouge, M.W.; Thaker, A.J.; Zhang, L.; Muradia, G.; Rode, H.; Vijay, H.M. Molecular cloning of IgE-binding fragments of Alternaria alternata allergens. Int. Arch. Allergy Immunol. 1998, 116, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Leino, M.S.; Loxham, M.; Blume, C.; Swindle, E.J.; Jayasekera, N.P.; Dennison, P.W.; Davies, D.E. Barrier Disrupting Effects of Alternaria Alternata Extract on Bronchial Epithelium from Asthmatic Donors. PLoS ONE 2013, 8, e71278. [Google Scholar] [CrossRef]
- Causton, B.; Pardo-Saganta, A.; Gillis, J.; Discipio, K.; Kooistra, T.; Rajagopal, J.; Medoff, B.D. CARMA3 Mediates Allergic Lung Inflammation in Response to Alternaria alternata. Am. J. Respir. Cell Mol. Biol. 2018, 59, 684–694. [Google Scholar] [CrossRef]
- Balenga, N.A.; Klichinsky, M.; Xie, Z.; Chan, E.C.; Zhao, M.; Jude, J.; Laviolette, M.; Panettieri, R.A.; Druey, K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015, 13, 6763. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Arandia, M.; Tome-Amat, J.; Pazos-Castro, D.; Esteban, V.; Escribese, M.M.; Hernández-Ramírez, G.; Díaz-Perales, A. Interaction of Alt a 1 with SLC 22A17 in the airway mucosa. Allergy 2019, 74, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Casado, C.; Murua-García, A.; Garrido-Arandia, M.; González-Melendi, P.; Sánchez-Monge, R.; Barber, D.; Pacios, L.F.; Díaz-Perales, A. Alt a 1 from Alternaria interacts with PR5 thaumatin-like proteins. FEBS Lett. 2014, 588, 1501–1508. [Google Scholar] [CrossRef]
- Gabriel, M.F.; Uriel, N.; Teifoori, F.; Postigo, I.; Suñén, E.; Martínez, J. The major Alternaria alternata allergen, Alt a 1: A reliable and specific marker of fungal contamination in citrus fruits. Int. J. Food Microbiol. 2017, 18, 26–30. [Google Scholar] [CrossRef]
- Sáenz-de-Santamaría, M.; Guisantes, J.A.; Martínez, J. Enzymatic activities of Alternaria alternata allergenic extracts and its major allergen (Alt a 1). Mycoses 2006, 49, 288–292. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Fischer, S.G.; Kirschner, M.W.; Laemmli, U.K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J. Biol. Chem. 1977, 10, 1102–1106. [Google Scholar] [CrossRef]
- Saenz-de-Santamaria, M.; Postigo, I.; Gutierrez-Rodriguez, A.; Cardona, G.; Guisantes, J.A.; Asturias, J.; Martinez, J. The major allergen of Alternaria alternata (Alt a 1) is expressed in other members of the Pleosporaceae family. Mycoses 2006, 49, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Boraschi, D. Endotoxin contamination: A key element in the interpretation of nanosafety studies. Nanomedicine 2016, 11, 269–287. [Google Scholar] [CrossRef]
- Li-Ping Thio, C.; Chuan-Ying Lai, A.; Chi, P.Y.; Webster, G.; Chang, Y.J. TLR9-dependent interferon production prevents group 2 innate lymphoid cell-driven airway hyperreactivity. J. Allergy Clin. Immunol. 2019, 144, 682–697. [Google Scholar] [CrossRef]
- Stevens, W.W.; Kim, T.S.; Pujanauski, L.M.; Hao, X.; Braciale, T.J. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods 2007, 31, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Skuljec, J.; Chmielewski, M.; Happle, C.; Habener, A.; Busse, M.; Abken, H.; Hansen, G. Chimeric Antigen Receptor-Redirected Regulatory T Cells Suppress Experimental Allergic Airway Inflammation, a Model of Asthma. Front. Immunol. 2017, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, H.; Meng, X.; Hao, L.; Fu, Y.; Fang, L.; Shen, D.; Yu, X.; Li, J. Immunomodulatory Effects of Cinobufagin on Murine Lymphocytes and Macrophages. Evid.-Based Comp. Alter. Med. 2015, 2015, 835263. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Haczku, A.; Lee, J.J.; Irvin, C.G.; Gelfand, E.W. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L394–L402. [Google Scholar] [CrossRef]
- Fulton, R.B.; Weiss, K.A.; Pewe, L.L.; Harty, J.T.; Varga, S.M. Aged mice exhibit a severely diminished CD8 T cell response following respiratory syncytial virus infection. J. Virol. 2013, 87, 12694–12700. [Google Scholar] [CrossRef]
- Mosquera, R.A.; Stark, J.M.; Atkins, C.L.; Colasurdo, G.N.; Chevalier, J.; Samuels, C.L. Functional and immune response to respiratory syncytial virus infection in aged BALB/c mice: A search for genes determining disease severity. Exp. Lung Res. 2014, 40, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Melgert, B.N.; Postma, D.S.; Kuipers, I.; Geerlings, M.; Luinge, M.A.; Strate, B.W.A.; Hylkema, M.N. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin. Exp. Allergy 2005, 35, 1496–1503. [Google Scholar] [CrossRef]
- Takeda, M.; Tanabe, M.; Ito, W.; Ueki, S.; Konnno, Y.; Chihara, M.; Chihara, J. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirology 2013, 18, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Song, M.K.; Lee, K. Comparison of asthma phenotypes in OVA-induced mice challenged via inhaled and intranasal routes. BMC Pul. Med. 2019, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.K.; Popova, A.P.; Bozyk, P.D.; Linn, M.J.; Baek, A.E.; Lei, J.; Hershenson, M.B. Ovalbumin sensitization and challenge increases the number of lung cells possessing a mesenchymal stromal cell phenotype. Respir. Res. 2010, 21, 127. [Google Scholar] [CrossRef]
- Sakai, K.; Yokoyama, A.; Kohno, N.; Hamada, H.; Hiwada, K. Prolonged Antigen Exposure Ameliorates Airway Inflammation but Not Remodeling in a Mouse Model of Bronchial Asthma. Int. Arch. Allergy Immunol. 2001, 126, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, C.L.; Maes, T.; Joos, G.F.; Tournoy, K.G. Prolonged Inhaled Allergen Exposure Can Induce Persistent Tolerance. Am. J. Respir. Cell Mol. Biol. 2007, 36, 573–584. [Google Scholar] [CrossRef]
- Gabriele, L.; Schiavoni, G.; Mattei, F.; Sanchez, M.; Sestili, P.; Butteroni, C.; Afferni, C. Novel allergic asthma model demonstrates ST2-dependent dendritic cell targeting by cypress pollen. J. Allergy Clin. Immunol. 2013, 132, 686–695.e7. [Google Scholar] [CrossRef] [PubMed]
- Simeone-Penney, M.C.; Severgnini, M.; Tu, P.; Homer, R.J.; Mariani, T.J.; Cohn, L.; Simon, A.R. Airway Epithelial STAT3 Is Required for Allergic Inflammation in a Murine Model of Asthma. J. Immunol. 2007, 15, 6191–6199. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.H.; Park, Y.H.; Shin, S.H. Mouse model of Aspergillus and Alternaria induced rhinosinusitis. Auris Nasus Larynx 2009, 36, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Cates, E.C.; Gajewska, B.U.; Goncharova, S.; Alvarez, D.; Fattouh, R.; Coyle, A.J.; Jordana, M. Effect of GM-CSF on immune, inflammatory, and clinical responses to ragweed in a novel mouse model of mucosal sensitization. J. Allergy Clin. Immunol. 2003, 111, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cheng, D.; Zhang, K.; Huo, X.; Mo, Y.; Shi, H.; Zhen, G. Intelectin contributes to allergen-induced IL-25, IL-33, and TSLP expression and type 2 response in asthma and atopic dermatitis. Mucosal Immunol. 2017, 10, 1491–1503. [Google Scholar] [CrossRef]
- Salter, B.M.; Aw, M.; Sehmi, R. The role of type 2 innate lymphoid cells in eosinophilic asthma. J. Leukoc. Biol. 2019, 106, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.A.; Caniga, M.; Woodhouse, J.D.; Eckman, J.; Lee, H.H.; Salmon, M.; Cicmil, M. Anti-inflammatory actions of Chemoattractant Receptor-homologous molecule expressed on Th2 by the antagonist MK-7246 in a novel rat model of Alternaria alternata elicited pulmonary inflammation. Eur. J. Pharmacol. 2014, 15, 106–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oeser, K.; Maxeiner, J.; Symowski, C.; Stassen, M.; Voehringer, D. T cells are the critical source of IL-4/IL-13 in a mouse model of allergic asthma. Allergy 2015, 70, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 16, 975–991. [Google Scholar] [CrossRef]
- Jacobsen, E.A.; Doyle, A.D.; Colbert, D.C.; Zellner, K.R.; Protheroe, C.A.; LeSuer, W.E.; Lee, J.J. Differential activation of airway eosinophils induces IL-13-mediated allergic Th2 pulmonary responses in mice. Allergy 2015, 70, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Grunig, G.; Devries, J.; Malefyt, R. The Cytokine Handbook Volumen I. Interleukin-13, 4th ed.; Elsevier: London, UK, 2003; pp. 409–429. ISBN 0-12-689663-1. [Google Scholar] [CrossRef]
- Crevel, R. Lymphocyte Proliferation. In Encyclopedic Reference of Immunotoxicology; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Bhakta, N.R.; Woodruff, P.G. Human asthma phenotypes: From the clinic, to cytokines, and back again. Immunol. Rev. 2011, 242, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Possa, S.S.; Leick, E.A.; Prado, C.M.; Martins, M.A.; Tibério, I.F.L.C. Eosinophilic Inflammation in Allergic Asthma. Front. Pharmacol. 2013, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Nakagome, K.; Nagata, M. Involvement and Possible Role of Eosinophils in Asthma Exacerbation. Front. Immunol. 2018, 28, 2220. [Google Scholar] [CrossRef] [PubMed]
- Kouzaki, H.; Iijima, K.; Kobayashi, T.; O’Grady, S.M.; Kita, H. The Danger Signal, Extracellular ATP, Is a Sensor for an Airborne Allergen and Triggers IL-33 Release and Innate Th2-Type Responses. J. Immunol. 2011, 1, 4375–4387. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Kewin, P.; Murphy, G.; Russo, R.C.; Stolarski, B.; Garcia, C.C.; Xu, D. IL-33 Induces Antigen-Specific IL-5+ T Cells and Promotes Allergic-Induced Airway Inflammation Independent of IL-4. J. Immunol. 2008, 1, 4780–4790. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Makhija, S.; Stahr, N.; Sandey, M.; Suryawanshi, A.; Mishra, A. Pyruvate kinase M2 in lung APCs regulates Alternaria-induced airway inflammation. Immunobiology 2020, 225, 151956. [Google Scholar] [CrossRef]
- Johnston, L.K.; Hsu, C.L.; Krier-Burris, R.A.; Chhiba, K.D.; Chien, K.B.; McKenzie, A.; Berdnikovs, S.; Bryce, P.J. IL-33 Precedes IL-5 in Regulating Eosinophil Commitment and Is Required for Eosinophil Homeostasis. J. Immunol. 2016, 1, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Camelo, A.; Rosignoli, G.; Ohne, Y.; Stewart, R.A.; Overed-Sayer, C.; Sleeman, M.A.; May, R.D. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv. 2017, 30, 577–589. [Google Scholar] [CrossRef]
- Terrier, B.; Bieche, I.; Maisonobe, T.; Laurendeau, I.; Rosenzwajg, M.; Kahn, J.-E.; Saadoun, D. Interleukin-25: A cytokine linking eosinophils and adaptive immunity in Churg-Strauss syndrome. Blood 2010, 25, 4523–4531. [Google Scholar] [CrossRef]
- Clarke, A.H.; Thomas, W.R.; Rolland, J.M.; Dow, C.; O’Brien, R.M. Murine Allergic Respiratory Responses to the Major House Dust Mite Allergen Der p 1. Int. Arch. Allergy Immunol. 1999, 120, 126–134. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez-del-Burgo, A.; Sánchez, P.; Suñen, E.; Martínez, J.; Postigo, I. Purified Native and Recombinant Major Alternaria alternata Allergen (Alt a 1) Induces Allergic Asthma in the Murine Model. J. Fungi 2021, 7, 896. https://doi.org/10.3390/jof7110896
Vélez-del-Burgo A, Sánchez P, Suñen E, Martínez J, Postigo I. Purified Native and Recombinant Major Alternaria alternata Allergen (Alt a 1) Induces Allergic Asthma in the Murine Model. Journal of Fungi. 2021; 7(11):896. https://doi.org/10.3390/jof7110896
Chicago/Turabian StyleVélez-del-Burgo, Ainara, Patricia Sánchez, Ester Suñen, Jorge Martínez, and Idoia Postigo. 2021. "Purified Native and Recombinant Major Alternaria alternata Allergen (Alt a 1) Induces Allergic Asthma in the Murine Model" Journal of Fungi 7, no. 11: 896. https://doi.org/10.3390/jof7110896
APA StyleVélez-del-Burgo, A., Sánchez, P., Suñen, E., Martínez, J., & Postigo, I. (2021). Purified Native and Recombinant Major Alternaria alternata Allergen (Alt a 1) Induces Allergic Asthma in the Murine Model. Journal of Fungi, 7(11), 896. https://doi.org/10.3390/jof7110896






