Novel Botrytis and Cladosporium Species Associated with Flower Diseases of Macadamia in Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Isolation
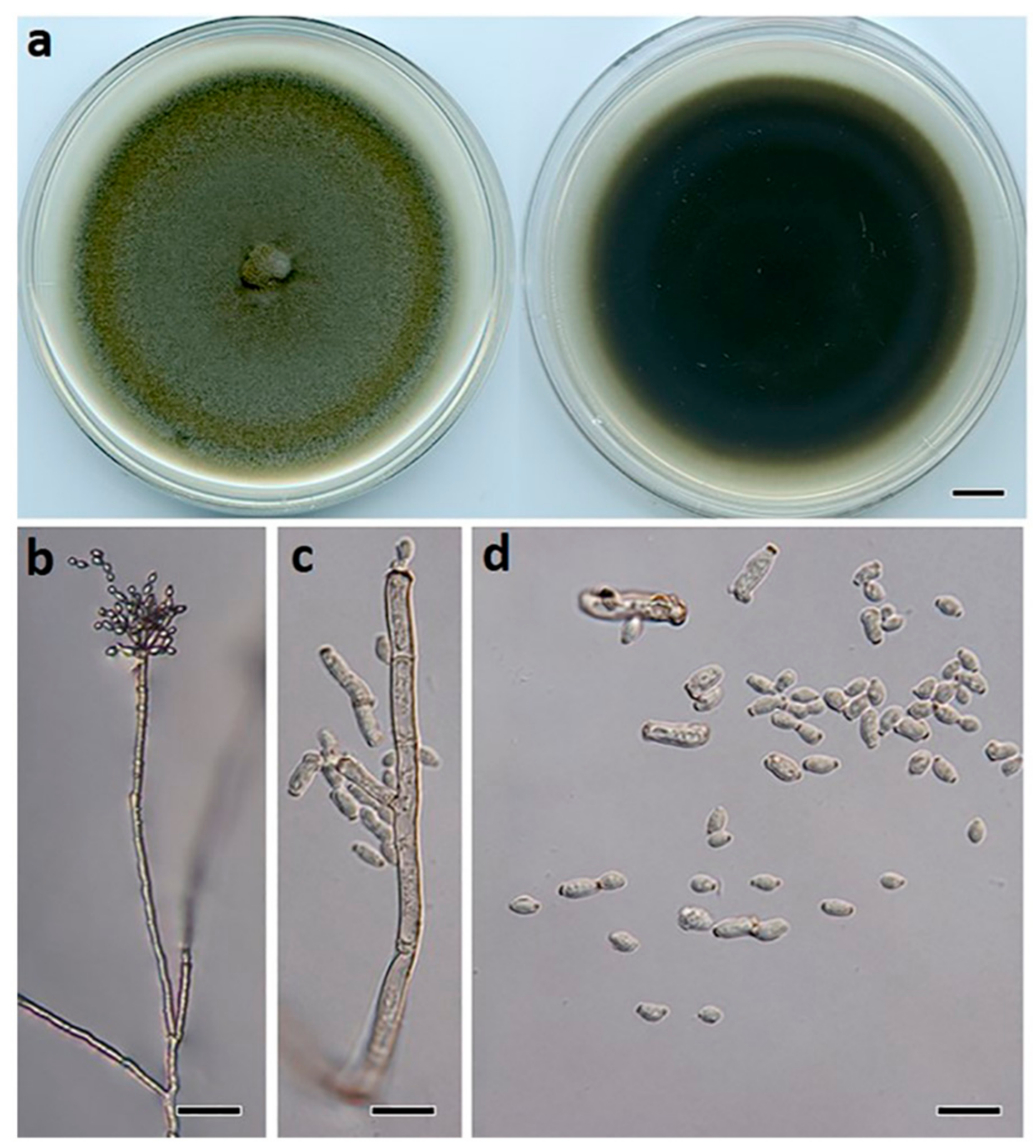
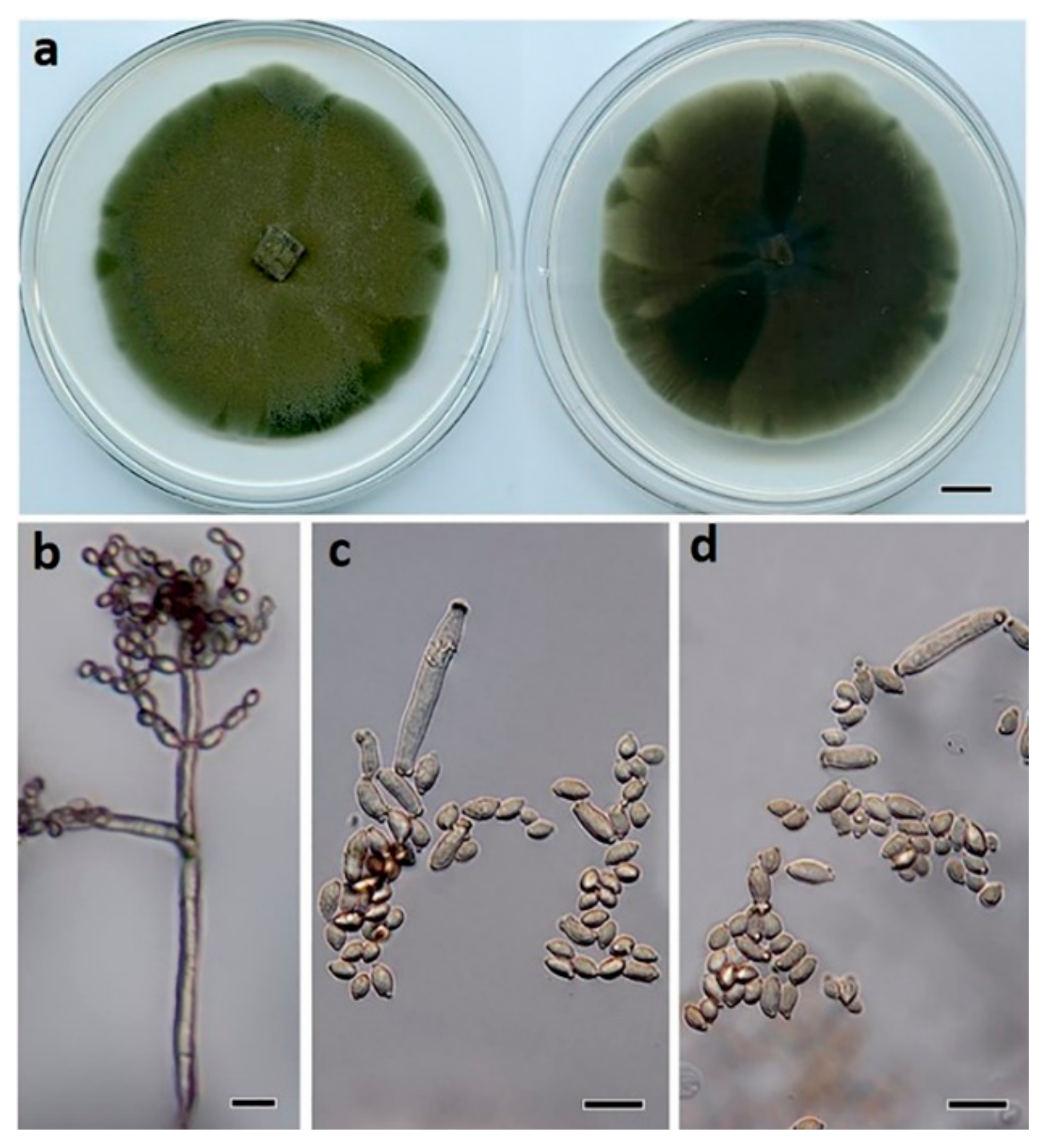
2.2. Macro- and Micro-Morphological Studies
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Phylogenetic Analyses
3. Results
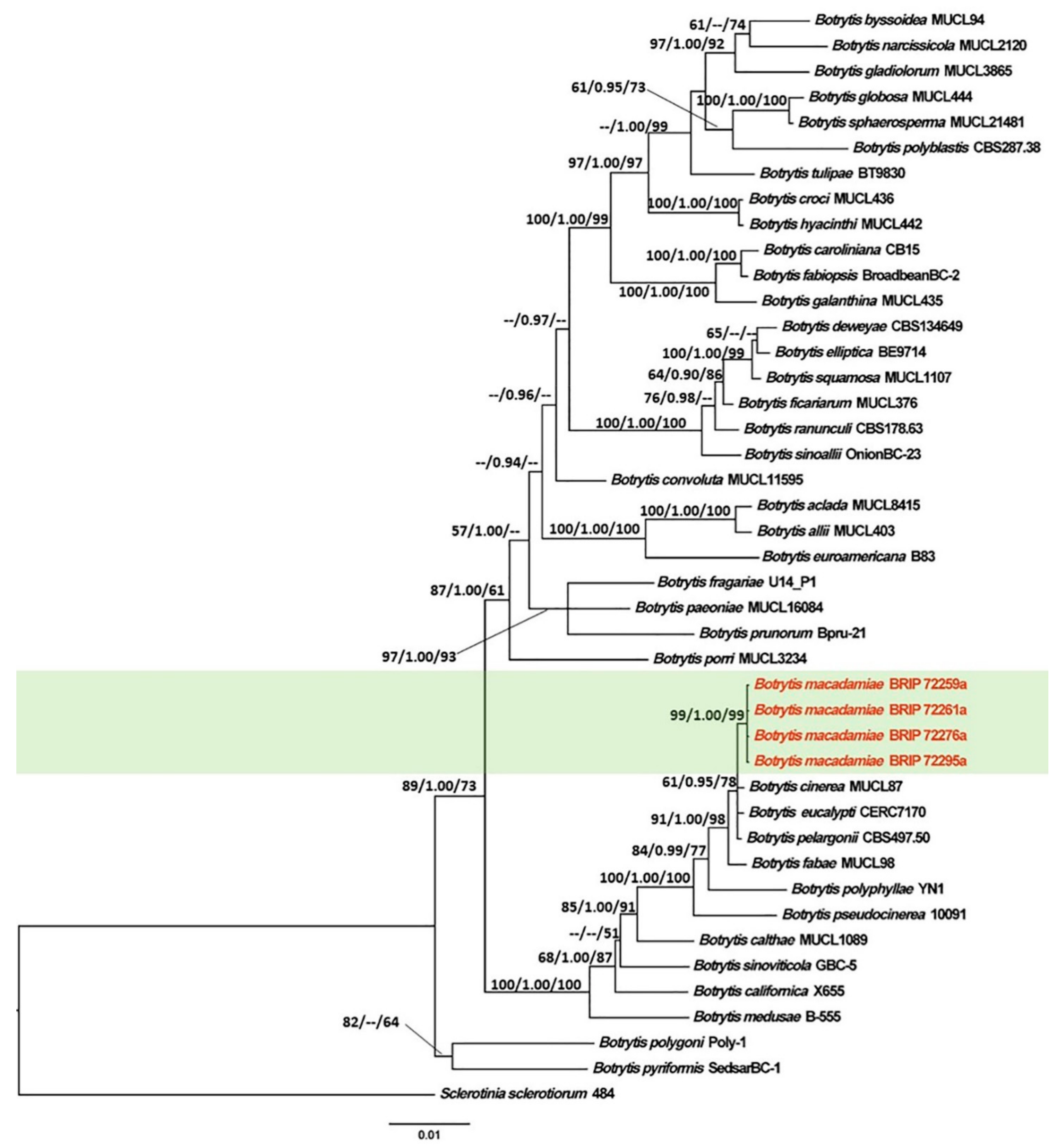
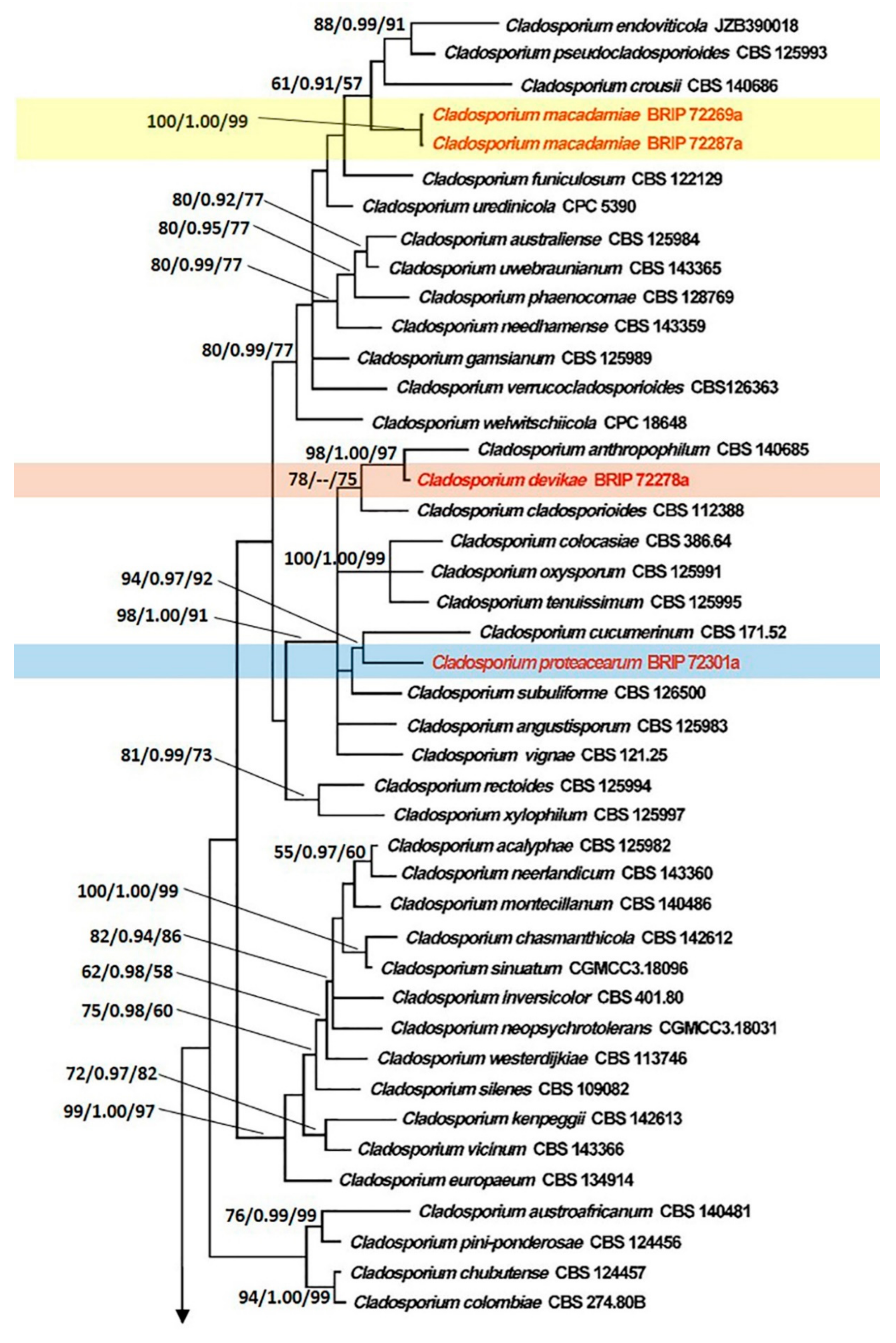
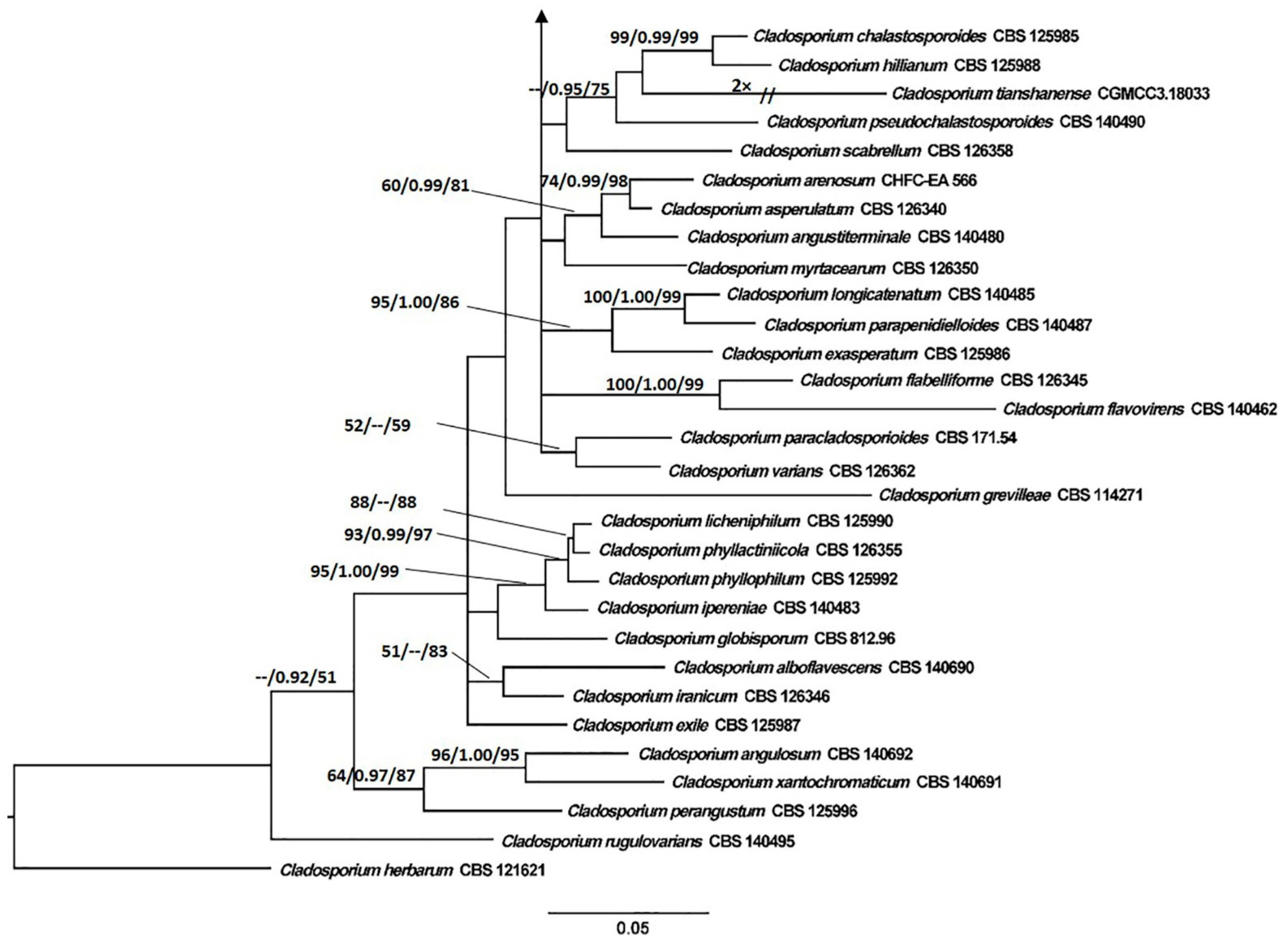
3.1. Phylogenetic Analyses
3.2. Taxonomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardner, C.M.; Peace, C.; Lowe, A.J.; Neal, J.; Pisanu, P.; Powell, M.; Schmidt, A.; Spain, C.; Williams, K. Genetic resources and domestication of macadamia. In Horticultural Reviews; Janick, J., Ed.; John Wiley: New York, NY, USA, 2009; pp. 1–125. [Google Scholar]
- Jeff-Ego, O.S.; Akinsanmi, O.A. Botryosphaeriaceae causing branch dieback and tree death of macadamia in Australia. Australas. Plant Pathol. 2019, 48, 59–64. [Google Scholar] [CrossRef]
- Jeff-Ego, O.S.; Drenth, A.; Topp, B.; Henderson, J.; Akinsanmi, O.A. Prevalence of Phytophthora species in macadamia orchards in Australia and their ability to cause stem canker. Plant Pathol. 2020, 69, 1270–1280. [Google Scholar] [CrossRef]
- Prasannath, K.; Galea, V.J.; Akinsanmi, O.A. Characterisation of leaf spots caused by Neopestalotiopsis clavispora and Colletotrichum siamense in macadamia in Australia. Eur. J. Plant Pathol. 2020, 156, 1219–1225. [Google Scholar] [CrossRef]
- Akinsanmi, O.A.; Drenth, A. Economic returns from fungicide application to control husk spot of macadamia in Australia is influenced by spray efficiency, rates and costs of application. Crop Prot. 2012, 41, 35–41. [Google Scholar] [CrossRef]
- Akinsanmi, O.A.; Drenth, A. Characterisation of husk rot in macadamia. Ann. Appl. Biol. 2017, 170, 104–115. [Google Scholar] [CrossRef]
- Akinsanmi, O.A.; Nisa, S.; Jeff-Ego, O.S.; Shivas, R.G.; Drenth, A. Dry flower disease of macadamia in Australia caused by Neopestalotiopsis macadamiae sp. nov. and Pestalotiopsis macadamiae sp. nov. Plant Dis. 2017, 101, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Trueman, S.J. The reproductive biology of macadamia. Sci. Hortic. 2013, 150, 354–359. [Google Scholar] [CrossRef]
- Trueman, S.J.; Turnbull, C.G.N. Effects of cross-pollination and flower removal on fruit set in macadamia. Ann. Bot. 1994, 73, 23–32. [Google Scholar] [CrossRef]
- Akinsanmi, O. Flower blights of macadamia caused by Botrytis cinerea, Pestalotiopsis macadamiae and Neopestalotiopsis macadamiae in Australia. In Proceedings of the International Congress of Plant Pathology (ICPP) 2018, Boston, MA, USA, 29 July–3 August 2018. [Google Scholar]
- Hunter, J.E.; Kunimoto, R.K. Reduction of macadamia nut set by Botrytis cinerea. Phytopathology 1973, 63, 939–941. [Google Scholar] [CrossRef]
- Van den Berg, N.; Serfontein, S.; Christie, B.; Munro, C. First report of raceme blight caused by Cladosporium cladosporioides on macadamia nuts in South Africa. Plant Dis. 2008, 92, 484. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I.; Prado, A.M.C.; Stewart, A. Plant hosts of Botrytis spp. In Botrytis–The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 413–486. [Google Scholar]
- Dean, R.; van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, J.K.; Xiao, C.L.; Jurick, W.M., II. Botrytis spp.: A contemporary perspective and synthesis of recent scientific developments of a widespread genus that threatens global food security. Phytopathology 2021, 111, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, A.R. The history of Botrytis taxonomy, the rise of phylogenetics, and implications for species recognition. Phytopathology 2021, 111, 437–454. [Google Scholar] [CrossRef]
- Staats, M.; van Baarlen, P.; van Kan, J.A.L. Molecular phylogeny of the plant pathogenic genus Botrytis and the evolution of host specificity. Mol. Biol. Evol. 2005, 22, 333–346. [Google Scholar] [CrossRef] [Green Version]
- Link, H.F. Observationes in ordines plantarum naturales. Mag. Ges. Nat. Freunde Berlin 1816, 8, 25–45. [Google Scholar]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.W.; Shivas, R.G.; Quaedvlieg, W.; van der Bank, M.; Zhang, Y.; Summerell, B.A.; Guarro, J.; Wingfield, M.J.; Wood, A.R.; Alfenas, A.C.; et al. Fungal planet description sheets: 214–280. Persoonia 2013, 31, 188. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Genã, J.; Sutton, D.A.; Wiederhold, N.P.; Cano-Lira, J.F.; Guarro, J. New species of Cladosporium associated with human and animal infections. Persoonia 2016, 36, 281–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibpromma, S.; Hyde, K.D.; Mckenzie, E.H.C.; Bhat, D.J.; Phillips, A.J.L.; Wanasinghe, D.N.; Samarakoon, M.C.; Jayawardena, R.S.; Dissanayake, A.J.; Tennakoon, D.S.; et al. Fungal diversity notes 840–928: Micro-fungi associated with Pandanaceae. Fungal Divers. 2018, 72, 1–160. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Q.; Fan, Y.; Wang, Q.; Chen, S.F.; Liu, X.Z.; Cai, L.; Yao, B. Six new soil–inhabiting Cladosporium species from plateaus in China. Mycologia 2017, 109, 244–260. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Braun, U.; Dijksterhuis, J.; de Jesús Yáñez-Morales, M.; Crous, P.W. Common but different: The expanding realm of Cladosporium. Stud. Mycol. 2015, 82, 23–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef] [Green Version]
- Rosado, A.W.C.; Custódio, F.A.; Pinho, D.B.; Ferreira, A.P.S.; Pereira, O.L. Cladosporium species associated with disease symptoms on Passiflora edulis and other crops in Brazil, with descriptions of two new species. Phytotaxa 2019, 409, 239–260. [Google Scholar] [CrossRef]
- Velázquez-Jiménez, Y.; Hernández-Castro, R.; Romero-Romero, L.; Salas-Garrido, C.G.; Martínez-Chavarría, L.C. Feline phaeohyphomycotic cerebellitis caused by Cladosporium cladosporioides-complex: Case report and review of literature. J. Comp. Pathol. 2019, 170, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Sutton, D.A.; Martin-Vicente, A.; Cano-Lira, J.F.; Wiederhold, N.; Guarro, J.; Gené, J. Cladosporium species recovered from clinical samples in the United States. J. Clin. Microbiol. 2015, 53, 2990–3000. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.B.; Chen, P.; Sun, T.T.; Wang, X.J.; Li, D.M. Acne-like subcutaneous phaeohyphomycosis caused by Cladosporium cladosporioides: A rare case report and review of published literatures. Mycopathologia 2016, 181, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Bensch, K.; Groenewald, J.Z.; Meijer, M.; Dijksterhuis, J.; Jurjević, Ž.; Andersen, B.; Houbraken, J.; Crous, P.W.; Samson, R.A. Cladosporium species in indoor environments. Stud. Mycol. 2018, 89, 177–301. [Google Scholar] [CrossRef]
- Zimowska, B.; Becchimanzi, A.; Krol, E.D.; Furmanczyk, A.; Bensch, K.; Nicoletti, R. New Cladosporium species from normal and galled flowers of Lamiaceae. Pathogens 2021, 10, 369. [Google Scholar] [CrossRef]
- Akinsanmi, O.A.; Mitter, V.; Simpfendorfer, S.; Backhouse, D.; Chakraborty, S. Identity and pathogenicity of Fusarium spp. isolated from wheat fields in Queensland and northern New South Wales. Aust. J. Agric. Res. 2004, 55, 97–107. [Google Scholar] [CrossRef]
- Crous, P.W.; Gams, W.; Stalpers, J.A.; Robert, V.; Stegehuis, G. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004, 50, 19–22. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Rehner, S. Primers for Elongation Factor 1-α (EF1-α); Insect Biocontrol Laboratory USDA, ARS, PSI: Beltsville, MD, USA, 2001; p. 4. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; Claverie, J.M.; Gascuel, O. Phylogeny. fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree: Tree Figure Drawing Tool, Version 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Walker, A.-S. Diversity within and between species of Botrytis. In Botrytis–The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 91–125. [Google Scholar]
- He, S.Q.; Wen, Z.H.; Bai, B.; Jing, Z.Q.; Wang, X.W. Botrytis polygoni, a new species of the genus Botrytis infecting Polygonaceae in Gansu, China. Mycologia 2021, 113, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Kozhar, O.; Peever, T.L. How does Botrytis cinerea infect red raspberry? Phytopathology 2018, 108, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Plesken, C.; Weber, R.W.S.; Rupp, S.; Leroch, M.; Hahn, M. Botrytis pseudocinerea is a significant pathogen of several crop plants but susceptible to displacement by fungicide-resistant B. cinerea strains. Appl. Environ. Microbiol. 2015, 81, 7048–7056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steentjes, M.B.F.; Scholten, O.E.; van Kan, J.A.L. Peeling the onion: Towards a better understanding of Botrytis diseases of onion. Phytopathology 2021, 111, 164–473. [Google Scholar] [CrossRef]
- Veloso, J.; van Kan, J.A. Many shades of grey in Botrytis–host plant interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef]
- Fradkin, A.; Tarlo, S.M.; Tobin, R.S.; Tucic-Porretta, M.; Malloch, D. Species identification of airborne molds and its significance for the detection of indoor pollution. Air Repair 1987, 37, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Bullerman, L.B. Spoilage fungi in food–An overview. Encycl. Food Sci. Nutr. 2003, 1, 5511–5522. [Google Scholar]
- Horner, W.E.; Worthan, A.G.; Morey, P.R. Air-and dustborne mycoflora in houses free of water damage and fungal growth. Appl. Environ. Microbiol. 2004, 70, 6394–6400. [Google Scholar] [CrossRef] [Green Version]
- El-Morsy, E.M. Fungi isolated from the endorhizosphere of halophytic plants from the Red Sea Coast of Egypt. Fungal Divers. 2000, 5, 43–54. [Google Scholar]
- Levetin, E.; Dorseys, K. Contribution to leaf surface fungi to the air spora. Aerobiologia 2006, 22, 2–12. [Google Scholar] [CrossRef]
- Heuchert, B.; Braun, U.; Schubert, K. Morphotaxonomic revision of fungicolous Cladosporium species (Hyphomycetes). Schlechtendalia 2005, 13, 1–78. [Google Scholar]
- Khan, M.I.H.; Sohrab, M.H.; Rony, S.R.; Tareq, F.S.; Hasan, C.M.; Mazid, M.A. Cytotoxic and antibacterial naphthoquinones from an endophytic fungus, Cladosporium sp. Toxicol. Rep. 2016, 3, 861–865. [Google Scholar] [CrossRef]
- Adorisio, S.; Fierabracci, A.; Muscari, I.; Liberati, A.M.; Cannarile, L.; Thuy, T.T.; Sung, T.V.; Sohrab, H.; Hasan, C.M.; Ayroldi, E.; et al. Fusarubin and Anhydrofusarubin isolated from a Cladosporium species inhibit cell growth in human cancer cell lines. Toxins 2019, 11, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogórek, R.; Lejman, A.; Pusz, W.; Miłuch, A.; Miodyńska, P. Characteristics and taxonomy of Cladosporium fungi. Mikol. Lek. 2012, 19, 80–85. [Google Scholar]
- Medina, R.; López, S.M.Y.; Franco, M.E.E.; Rollan, C.; Ronco, B.L.; Saparrat, M.C.N.; De Wit, P.J.G.M.; Balatti, P.A. A survey on occurrence of Cladosporium fulvum identifies race 0 and race 2 in tomato-growing areas of Argentina. Plant Dis. 2015, 99, 1732–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Young, K.; Kang, H.; Ahn, K.; Min, K.; Cha, B. Cucumber scab caused by Cladosporium cucumerinum in Korea. Korean J. Plant Pathol. 1997, 13, 288–294. [Google Scholar]
- Kwon, J.-H.; Kang, S.-W.; Kim, J.-S.; Park, C.-S. Occurrence of strawberry scab caused by Cladosporium herbarum in Korea. Mycobiology 2001, 29, 110–112. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Kang, S.-W.; Kim, J.-S.; Park, C.-S. Scab of tea (Thea sinensis) caused by Cladosporium herbarum in Korea. Plant Pathol. J. 2001, 17, 350–353. [Google Scholar]
- Chen, R.S.; Wang, W.L.; Li, J.C.; Wang, Y.Y.; Tsay, J.G. First report of papaya scab caused by Cladosporium cladosporioides in Taiwan. Plant Dis. 2009, 93, 426. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Park, C.-S. Sooty mold of persimmon (Diospyros kaki) caused by Cladosporium cladosporioides. Plant Pathol. J. 2003, 19, 266–268. [Google Scholar] [CrossRef]
- Nam, M.H.; Park, M.S.; Kim, H.S.; Kim, T.I.; Kim, H.G. Cladosporium cladosporioides and C. tenuissimum cause blossom blight in strawberry in Korea. Mycobiology 2015, 43, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Robles-Yerena, L.; Ayala-Escobar, V.; Leyva-Mir, S.G.; Lima, N.B.; Camacho-Tapia, M.; Tovar-Pedraza, J.M. First report of Cladosporium cladosporioides causing leaf spot on tomato in Mexico. J. Plant Pathol. 2019, 101, 759. [Google Scholar] [CrossRef] [Green Version]
- Iturrieta-González, I.; García, D.; Gené, J. Novel species of Cladosporium from environmental sources in Spain. MycoKeys 2021, 77, 1–25. [Google Scholar] [CrossRef]



| Isolate 1 | Species | Cultivar | Flower Growth Stage | Location 2 |
|---|---|---|---|---|
| BRIP 72259a | Botrytis macadamiae | HAES 246 | 3 | Alstonville, NSW |
| BRIP 72261a | B. macadamiae | HAES 246 | 3 | Alstonville, NSW |
| BRIP 72276a | B. macadamiae | HAES 344 | 3 | Fernleigh, NSW |
| BRIP 72295a | B. macadamiae | A16 | 3 | Knockrow, NSW |
| BRIP 72278a | Cladosporium devikae | HAES 344 | 1 | Fernleigh, NSW |
| BRIP 72269a | C. macadamiae | HAES 792 | 4 | Nambour, QLD |
| BRIP 72287a | C. macadamiae | A16 | 3 | Maleny, QLD |
| BRIP 72301a | C. proteacearum | HAES 344 | 1 | Rosebank, NSW |
| Species | Isolate | GenBank Accession Numbers 1 | ||
|---|---|---|---|---|
| G3PDH | HSP60 | RPB2 | ||
| Botrytis aclada | MUCL8415 | AJ704992 | AJ716050 | AJ745664 |
| B. allii | MUCL403 | AJ704996 | AJ716055 | AJ745666 |
| B. byssoidea | MUCL94 | AJ704998 | AJ716059 | AJ745670 |
| B. californica | X655 | KJ937069 | KJ937059 | KJ937049 |
| B. calthae | MUCL1089 | AJ705000 | AJ716061 | AJ745672 |
| B. caroliniana | CB15 | JF811584 | JF811587 | JF811590 |
| B. cinerea | MUCL87 | AJ705004 | AJ716065 | AJ745676 |
| B. convoluta | MUCL11595 | AJ705008 | AJ716069 | AJ745680 |
| B. croci | MUCL436 | AJ705009 | AJ716070 | AJ745681 |
| B. deweyae | CBS 134649 | HG799521 | HG799519 | HG799518 |
| B. elliptica | BE9714 | AJ705012 | AJ716073 | AJ745684 |
| B. eucalypti | CERC 7170 | KX301020 | KX301024 | KX301028 |
| B. euroamericana | B83 | KC191677 | KC191678 | KC191679 |
| B. fabae | MUCL98 | AJ705014 | AJ716075 | AJ745686 |
| B. fabiopsis | BroadbeanBC–2 | EU519211 | EU514482 | EU514473 |
| B. ficariarum | MUCL376 | AJ705016 | AJ716077 | AJ745688 |
| B. fragariae | U14_P1 | KX429699 | KX429692 | KX429706 |
| B. galanthina | MUCL435 | AJ705018 | AJ716079 | AJ745689 |
| B. gladiolorum | MUCL3865 | AJ705020 | AJ716081 | AJ745692 |
| B. globose | MUCL444 | AJ705022 | AJ716083 | AJ745693 |
| B. hyacinthi | MUCL442 | AJ705024 | AJ716085 | AJ745696 |
| B. macadamiae | BRIP 72259a | MZ344223 | MZ344234 | MZ356230 |
| BRIP 72261a | MZ344224 | MZ344235 | MZ356231 | |
| BRIP 72276a | MZ344225 | MZ344236 | MZ356232 | |
| BRIP 72295a | MZ344226 | MZ344237 | MZ356233 | |
| B. medusae | B–555 | MH732861 | MH732866 | MH732870 |
| B. narcissicola | MUCL2120 | AJ705026 | AJ716087 | AJ745697 |
| B. paeoniae | MUCL16084 | AJ705028 | AJ716089 | AJ745700 |
| B. pelargonii | CBS497.50 | AJ704990 | AJ716046 | AM087030 |
| B. polyblastis | CBS287.38 | AJ705030 | AJ716091 | AJ745702 |
| B. porri | MUCL3234 | AJ705032 | AJ716093 | AJ745704 |
| B. prunorum | Bpru–21 | KP339980 | KP339994 | KP339987 |
| B. pseudocinerea | 10091 | JN692414 | JN692400 | JN692428 |
| B. pyriformis | SedsarBC–1 | KJ543484 | KJ543488 | KJ543492 |
| B. ranunculi | CBS178.63 | AJ705034 | AJ716095 | AJ745706 |
| B. sinoallii | OnionBC–23 | EU519217 | EU514488 | EU514479 |
| B. sinoviticola | GBC–5 | JN692413 | JN692399 | JN692427 |
| B. sphaerosperma | MUCL21481 | AJ705035 | AJ716096 | AJ745708 |
| B. squamosa | MUCL1107 | AJ705037 | AJ716098 | AJ745710 |
| B. tulipae | BT9830 | AJ705041 | AJ716102 | AJ745713 |
| Sclerotinia sclerotiorum | 484 | AJ705044 | AJ716048 | AJ745716 |
| Species | Isolate | GenBank Accession Numbers 1 | ||
|---|---|---|---|---|
| ITS | TEF1α | ACT | ||
| Cladosporium acalyphae | CBS 125982 T | HM147994 | HM148235 | HM148481 |
| C. alboflavescens | CBS 140690 T | LN834420 | LN834516 | LN834604 |
| C. angulosum | CBS 140692 T | LN834425 | LN834521 | LN834609 |
| C. angustisporum | CBS 125983 T | HM147995 | HM148236 | HM148482 |
| C. angustiterminale | CBS 140480 T | KT600379 | KT600476 | KT600575 |
| C. anthropophilum | CBS 140685 T | LN834437 | LN834533 | LN834621 |
| C. arenosum | CHFC–EA 566 | MN879328 | MN890011 | MN890008 |
| C. asperulatum | CBS 126340 T | HM147998 | HM148239 | HM148485 |
| C. australiense | CBS 125984 T | HM147999 | HM148240 | HM148486 |
| C. austroafricanum | CBS 140481 T | KT600381 | KT600478 | KT600577 |
| C. chalastosporoides | CBS 125985 T | HM148001 | HM148242 | HM148488 |
| C. chasmanthicola | CBS 142612 T | KY646221 | KY646227 | KY646224 |
| C. chubutense | CBS 124457 T | FJ936158 | FJ936161 | FJ936165 |
| C. cladosporioides | CBS 112388 T | HM148003 | HM148244 | HM148490 |
| C. colocasiae | CBS 386.64 T | HM148067 | HM148310 | HM148555 |
| C. colombiae | CBS 274.80B T | FJ936159 | FJ936163 | FJ936166 |
| C. crousii | CBS 140686 T | LN834431 | LN834527 | LN834615 |
| C. cucumerinum | CBS 171.52 T | HM148072 | HM148316 | HM148561 |
| C. devikae | BRIP 72278a T | MZ303808 | MZ344193 | MZ344212 |
| C. endoviticola | JZB390018 T | MN654960 | MN984228 | MN984220 |
| C. europaeum | CBS 134914 T | HM148056 | HM148298 | HM148543 |
| C. exasperatum | CBS 125986 T | HM148090 | HM148334 | HM148579 |
| C. exile | CBS 125987 T | HM148091 | HM148335 | HM148580 |
| C. flabelliforme | CBS 126345 T | HM148092 | HM148336 | HM148581 |
| C. flavovirens | CBS 140462 T | LN834440 | LN834536 | LN834624 |
| C. funiculosum | CBS 122129 T | HM148094 | HM148338 | HM148583 |
| C. gamsianum | CBS 125989 T | HM148095 | HM148339 | HM148584 |
| C. globisporum | CBS 812.96 T | HM148096 | HM148340 | HM148585 |
| C. grevilleae | CBS 114271 T | JF770450 | JF770472 | JF770473 |
| C. herbarum | CBS 121621 T | EF679363 | EF679440 | EF679516 |
| C. hillianum | CBS 125988 T | HM148097 | HM148341 | HM148586 |
| C. inversicolor | CBS 401.80 T | HM148101 | HM148345 | HM148590 |
| C. ipereniae | CBS 140483 T | KT600394 | KT600491 | KT600589 |
| C. iranicum | CBS 126346 T | HM148110 | HM148354 | HM148599 |
| C. kenpeggii | CBS 142613 T | KY646222 | KY646228 | KY646225 |
| C. licheniphilum | CBS 125990 T | HM148111 | HM148355 | HM148600 |
| C. longicatenatum | CBS 140485 T | KT600403 | KT600500 | KT600598 |
| C. macadamiae | BRIP 72269a T | MZ303810 | MZ344195 | MZ344214 |
| BRIP 72287a | MZ303811 | MZ344196 | MZ344215 | |
| C. montecillanum | CBS 140486 T | KT600406 | KT600504 | KT600602 |
| C. myrtacaearum | CBS 126350 T | HM148117 | HM148361 | HM148606 |
| C. needhamense | CBS 143359 T | MF473142 | MF473570 | MF473991 |
| C. neerlandicum | CBS 143360 T | KP701887 | KP701764 | KP702010 |
| C. neopsychrotolerans | CGMCC3.18031 T | KX938383 | KX938400 | KX938366 |
| C. oxysporum | CBS 125991 | HM148118 | HM148362 | HM148607 |
| C. paracladosporioides | CBS 171.54 T | HM148120 | HM148364 | HM148609 |
| C. parapenidielloides | CBS 140487 T | KT600410 | KT600508 | KT600606 |
| C. perangustum | CBS 125996 T | HM148121 | HM148365 | HM148610 |
| C. phaenocomae | CBS 128769 T | JF499837 | JF499875 | JF499881 |
| C. phyllactiniicola | CBS 126355 T | HM148153 | HM148397 | HM148642 |
| C. phyllophilum | CBS 125992 T | HM148154 | HM148398 | HM148643 |
| C. pini-ponderosae | CBS 124456 T | FJ936160 | FJ936164 | FJ936167 |
| C. proteacearum | BRIP 72301a T | MZ303809 | MZ344194 | MZ344213 |
| C. pseudochalastosporoides | CBS 140490 T | KT600415 | KT600513 | KT600611 |
| C. pseudocladosporioides | CBS 125993 T | HM148158 | HM148402 | HM148647 |
| C. rectoides | CBS 125994 T | HM148193 | HM148438 | HM148683 |
| C. rugulovarians | CBS 140495 T | KT600459 | KT600558 | KT600656 |
| C. scabrellum | CBS 126358 T | HM148195 | HM148440 | HM148685 |
| C. silenes | CBS 109082 T | EF679354 | EF679429 | EF679506 |
| C. sinuatum | CGMCC3.18096 T | KX938385 | KX938402 | KX938368 |
| C. subuliforme | CBS 126500 T | HM148196 | HM148441 | HM148686 |
| C. tenuissimum | CBS 125995 T | HM148197 | HM148442 | HM148687 |
| C. tianshanense | CGMCC3.18033 T | KX938381 | KX938398 | KX938364 |
| C. uredinicola | CPC 5390 | AY251071 | HM148467 | HM148712 |
| C. uwebraunianum | CBS 143365 T | MF473306 | MF473729 | MF474156 |
| C. varians | CBS 126362 T | HM148224 | HM148470 | HM148715 |
| C. verrucocladosporioides | CBS 126363 T | HM148226 | HM148472 | HM148717 |
| C. vicinum | CBS 143366 T | MF473311 | MF473734 | MF474161 |
| C. vignae | CBS 121.25 | HM148227 | HM148473 | HM148718 |
| C. welwitschiicola | CPC 18648 T | KY646223 | KY646229 | KY646226 |
| C. westerdijkiae | CBS 113746 T | HM148061 | HM148303 | HM148548 |
| C. xanthocromaticum | CBS 140691 T | LN834415 | LN834511 | LN834599 |
| C. xylophilum | CBS 125997 T | HM148230 | HM148476 | HM148721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasannath, K.; Shivas, R.G.; Galea, V.J.; Akinsanmi, O.A. Novel Botrytis and Cladosporium Species Associated with Flower Diseases of Macadamia in Australia. J. Fungi 2021, 7, 898. https://doi.org/10.3390/jof7110898
Prasannath K, Shivas RG, Galea VJ, Akinsanmi OA. Novel Botrytis and Cladosporium Species Associated with Flower Diseases of Macadamia in Australia. Journal of Fungi. 2021; 7(11):898. https://doi.org/10.3390/jof7110898
Chicago/Turabian StylePrasannath, Kandeeparoopan, Roger G. Shivas, Victor J. Galea, and Olufemi A. Akinsanmi. 2021. "Novel Botrytis and Cladosporium Species Associated with Flower Diseases of Macadamia in Australia" Journal of Fungi 7, no. 11: 898. https://doi.org/10.3390/jof7110898
APA StylePrasannath, K., Shivas, R. G., Galea, V. J., & Akinsanmi, O. A. (2021). Novel Botrytis and Cladosporium Species Associated with Flower Diseases of Macadamia in Australia. Journal of Fungi, 7(11), 898. https://doi.org/10.3390/jof7110898







