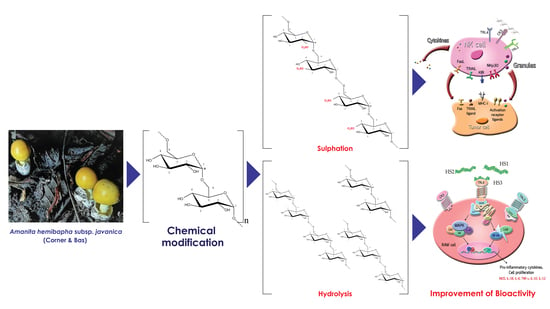
Sulphation and Hydrolysis Improvements of Bioactivities, and Immuno-Modulatory Properties of Edible Amanita hemibapha Subspecies javanica (Corner and Bas) Mucilage Polysaccharide as a Potential in Personalized Functional Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Cell Lines
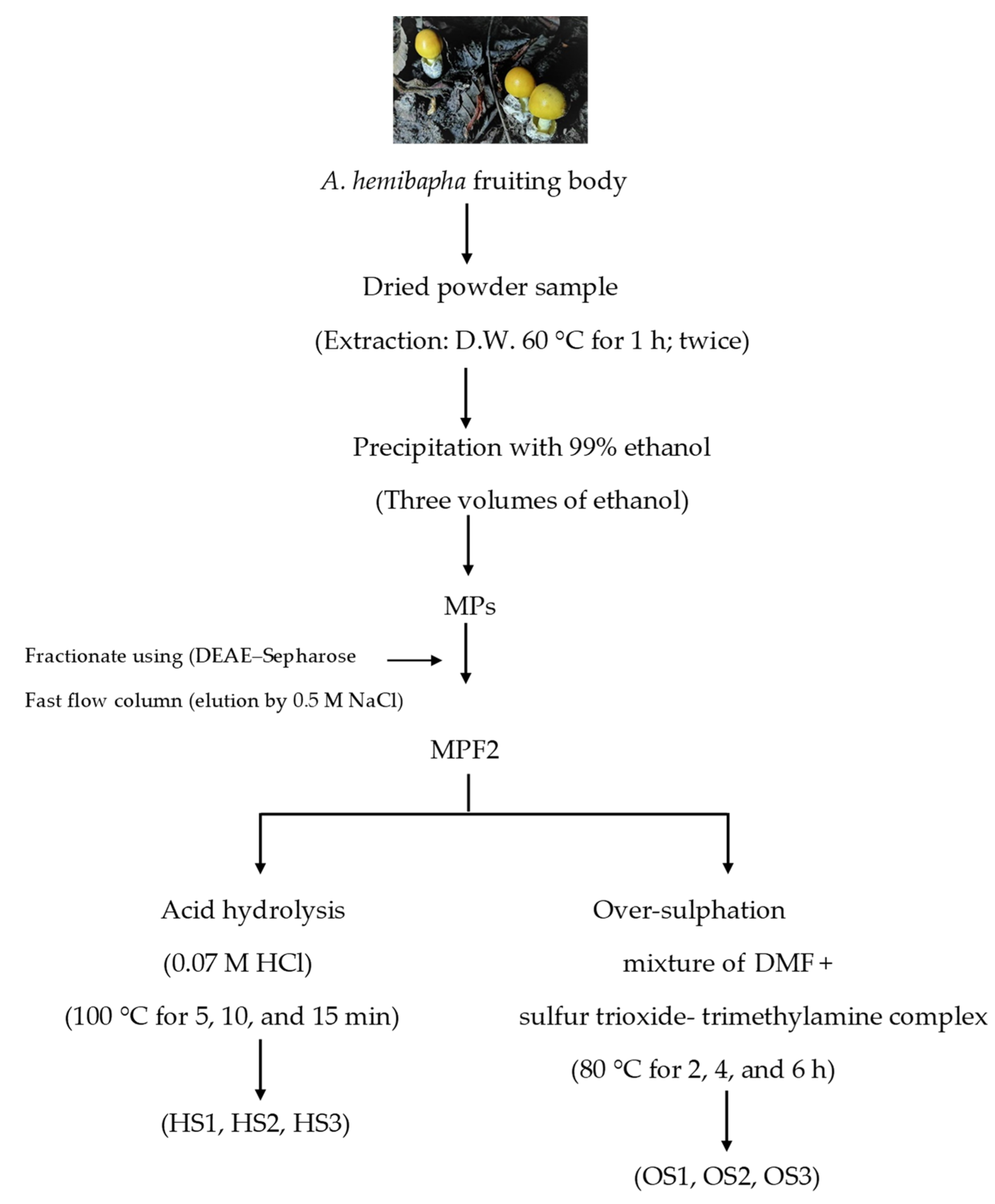
2.3. Extraction and Fractionation of Polysaccharide
2.4. Preparation of MPF2 Derivatives
2.5. Analytical Methods
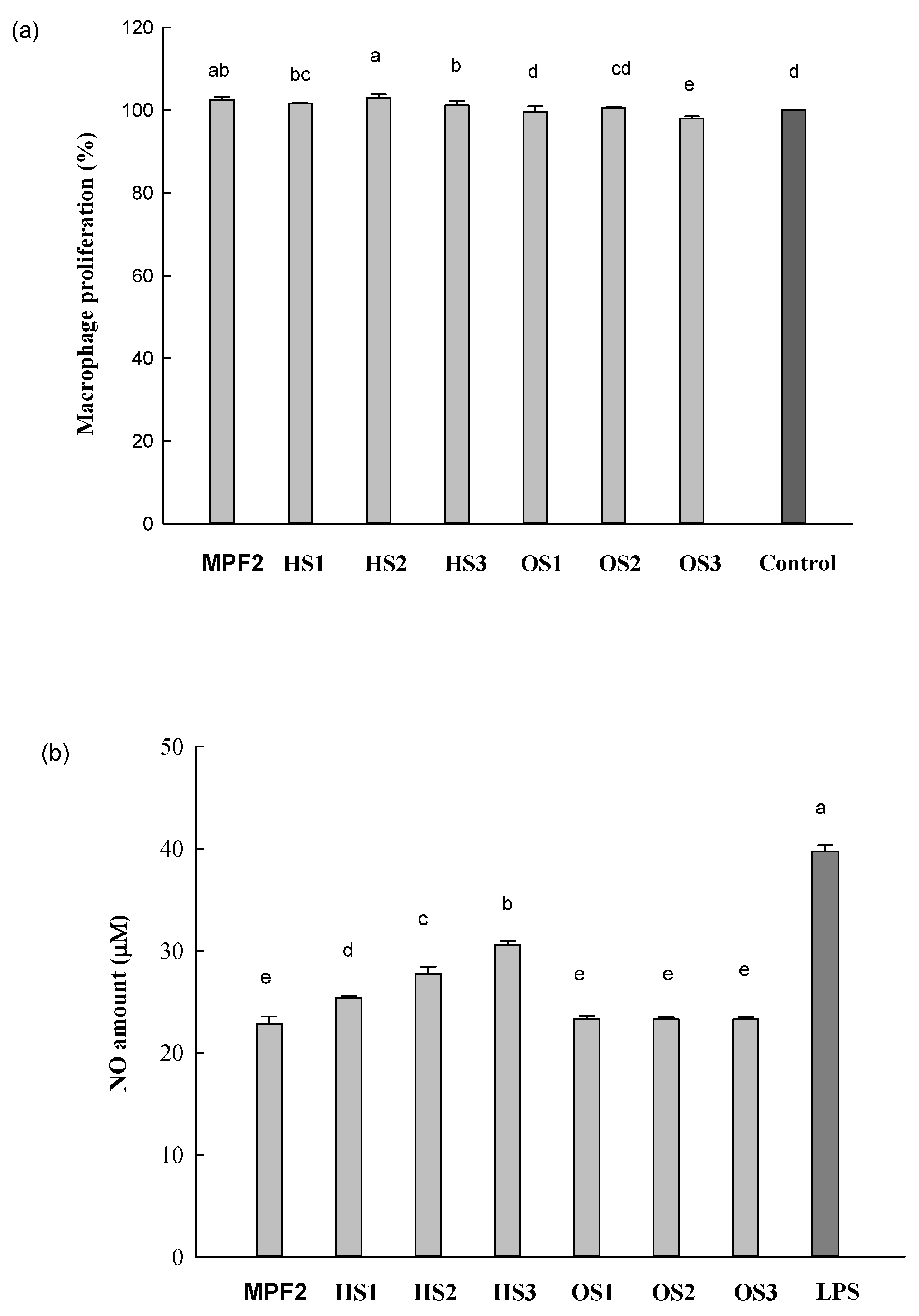
2.6. Macrophage Proliferation and Nitric Oxide Production Assay
2.7. NK Cell Activity Assay
2.8. Gene Expression by RT-PCR
2.9. Western Blot Analysis
2.10. Cell Binding Receptor
2.11. Statistical Analysis
3. Results and Discussion
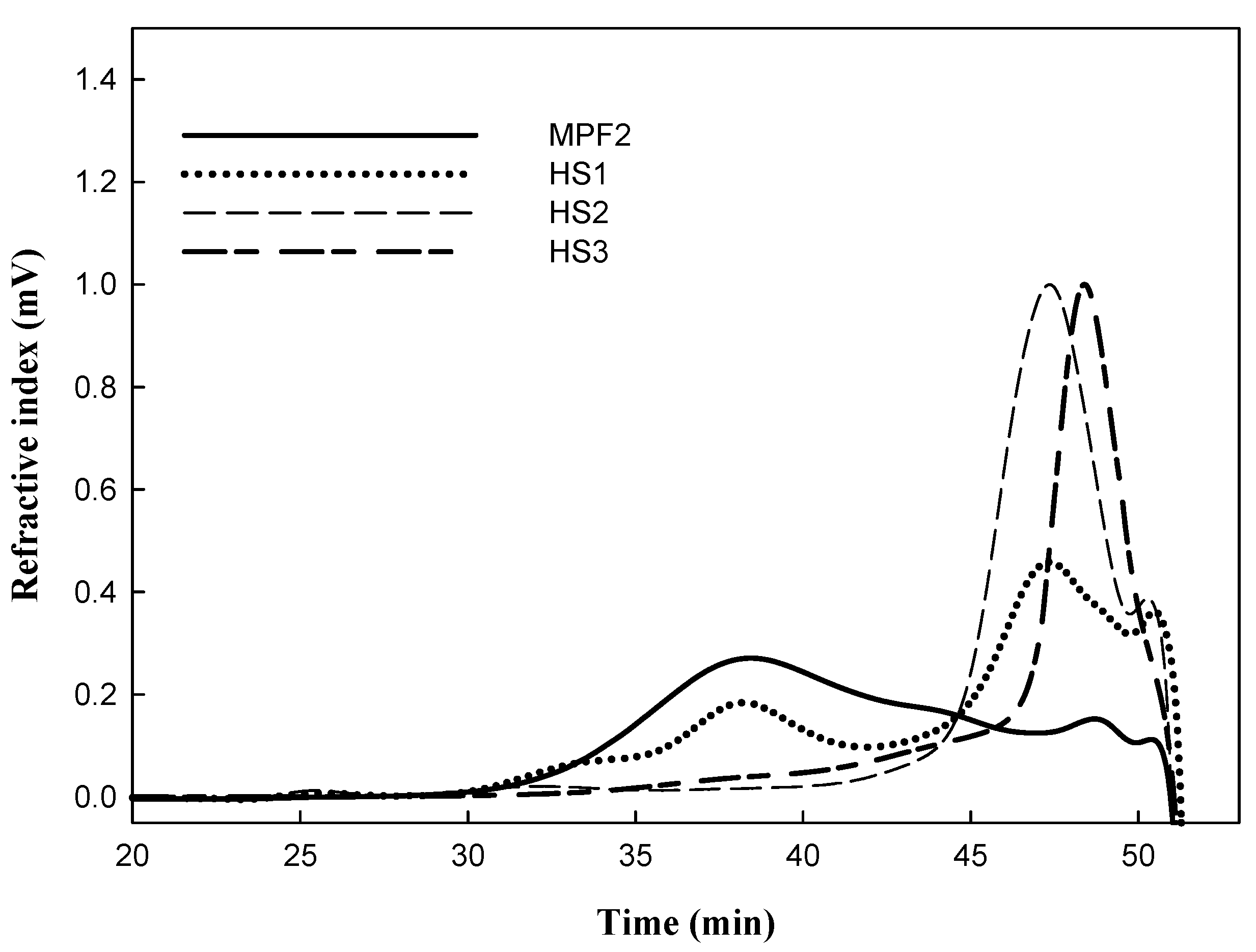
3.1. Preparation of MPF2 Derivatives
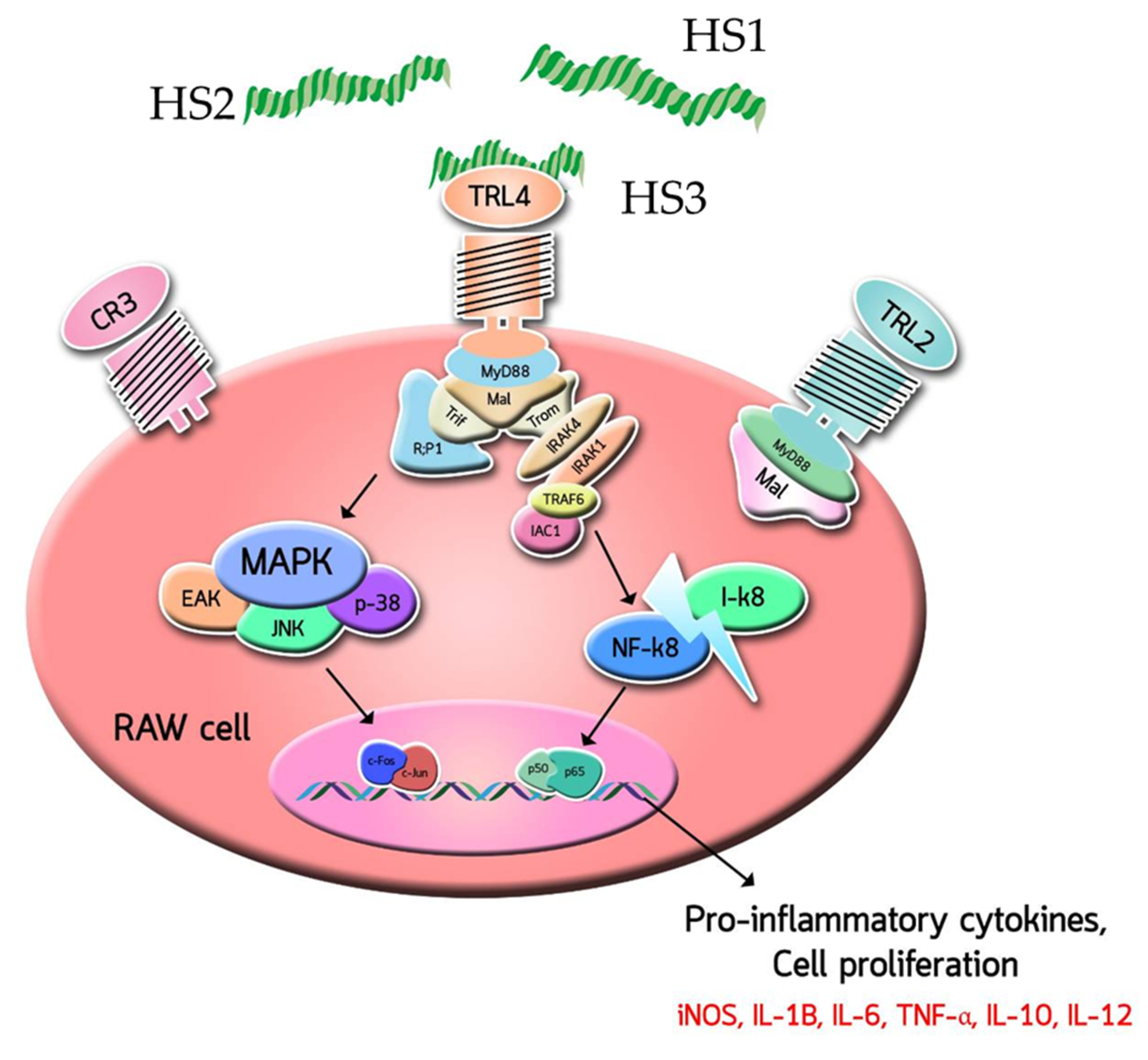
3.2. Effects of MPF2 Derivatives on RAW264.7 Cell Activation
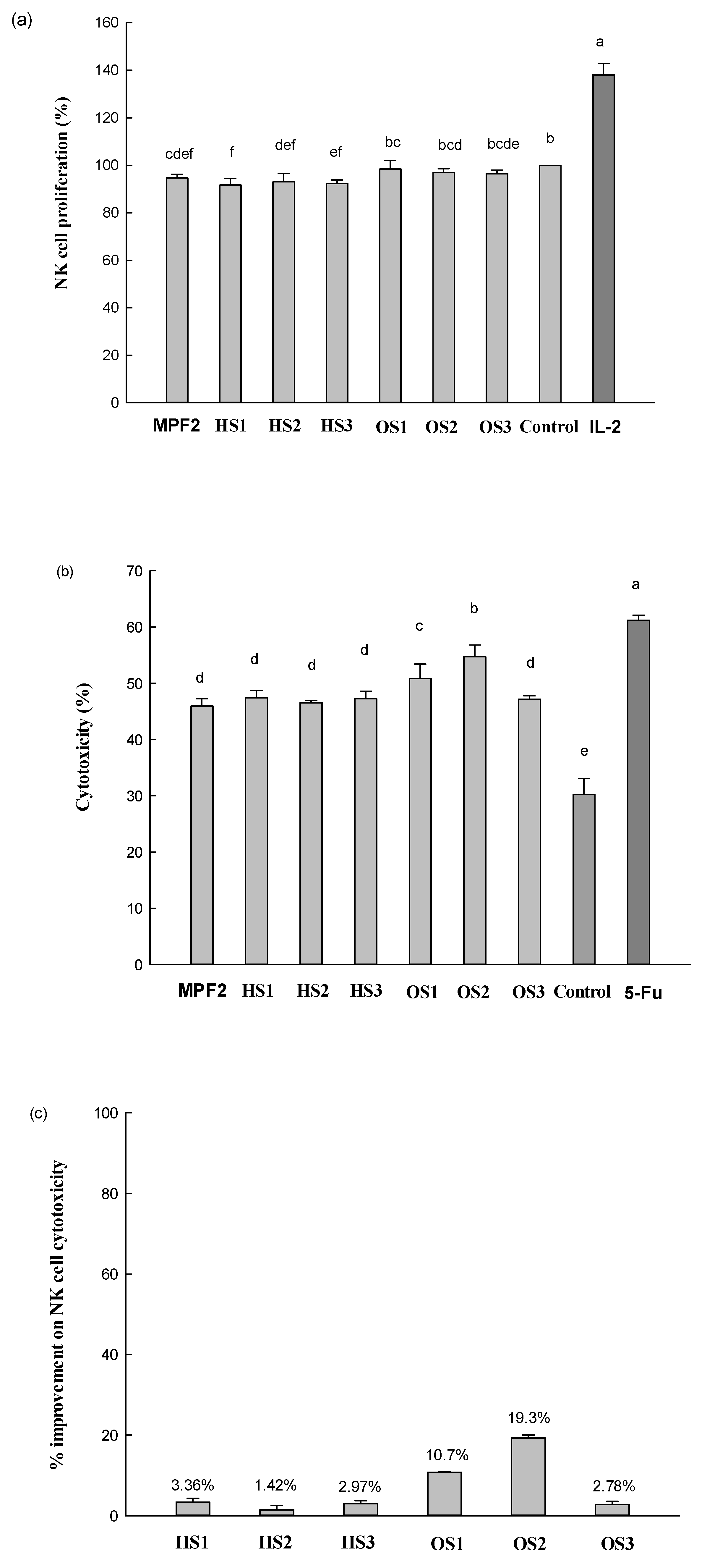
3.3. Effects of MPF2 Derivatives on NK Cell Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chien, R.C.; Yen, M.T.; Tseng, Y.H.; Mau, J.L. Chemical characteristics and anti-proliferation activities of Ganoderma tsugae polysaccharides. Carbohydr. Polym. 2015, 128, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Guillamon, E.; Garcia-Lafuente, A.; Lozano, M.D.; Arrigo, M.; Rostagno, M.A.; Villares, A.; Martinez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef]
- Siu, K.C.; Xu, L.; Chen, X.; Wu, J.Y. Molecular properties and antioxidant activities of polysaccharides isolated from alkaline extract of wild Armillaria ostoyae mushroom. Carbohydr. Polym. 2016, 137, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Wani, B.A.; Bodha, R.H.; Wani, A.H. Nutritional and medicinal importance of mushrooms. J. Med. Plants Res. 2010, 4, 2598–2604. [Google Scholar]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Wei, Y.; Ouyang, Z.; Su, Z. Polysaccharides purified from Cordyceps cicadae protects PC12 cells against glutamate-induced oxidative damage. Carbohydr. Polym. 2016, 153, 187–195. [Google Scholar] [CrossRef]
- Wang, Y.; He, P.; He, L.; Huang, Q.; Cheng, J.; Li, W.; Liu, Y.; Wei, C. Structural elucidation, antioxidant and immunomodulatory activities of a novel heteropolysaccharide from cultured Paecilomyces cicadae (Miquel.) Samson. Carbohydr. Polym. 2019, 216, 270–281. [Google Scholar] [CrossRef]
- Quintero-Cabello, K.P.; Lugo-Flores, M.A.; Rivera-Palafox, P.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Esqueda, M.; Gaitán-Hernández, R.; Ayala-Zavala, J.F. Antioxidant properties and industrial uses of edible polyporales. J. Fungi 2021, 7, 196. [Google Scholar] [CrossRef]
- Panda, B.C.; Maity, P.; Nandi, A.K.; Pattanayak, M.; Manna, D.K.; Mondal, S.; Tripathy, S.; Roy, S.; Acharya, K.; Islam, S.S. Heteroglycan of an edible Mushroom Pleurotus cystidiosus: Structural characterization and study of biological activities. Int. J. Biol. Macromol. 2017, 95, 833–842. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Delgado-Andrade, C. The beneficial role of edible mushrooms in human health. Curr. Opin. Food Sci. 2017, 14, 122–128. [Google Scholar] [CrossRef]
- GIaVaSIS, I. Polysaccharides from medicinal mushrooms for potential use as nutraceuticals. In Polysaccharides: Natural Fibers in Food and Nutrition; CRC Press: Boca Raton, FL, USA, 2014; Volume 1, pp. 171–206. [Google Scholar]
- Xu, Z.; Yan, X.; Song, Z.; Li, W.; Zhao, W.; Ma, H.; Du, J.; Li, S.; Zhang, D. Two heteropolysaccharides from Isaria cicadae Miquel differ in composition and potentially immunomodulatory activity. Int. J. Biol. Macromol. 2018, 117, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Yodkeeree, S. Skin Wound-Healing Potential of Polysaccharides from Medicinal Mushroom Auricularia auricula-judae (Bull.). J. Fungi 2021, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Jesus, L.I.; Smiderle, F.R.; Cordeiro, L.M.C.; Freitas, R.A.; Griensven, L.J.L.D.V.; Iacomini, M. Simple and effective purification approach to dissociate mixed water insoluble α- and β-D-glucans and its application on the medicinal mushroom Fomitopsis betulina. Carbohydr. Polym. 2018, 200, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Manna, D.K.; Nandi, A.K.; Pattanayak, M.; Maity, P.; Tripathy, S.; Mandal, A.K.; Roy, S.; Tripathy, S.S.; Gupta, N.; Islam, S.S. A water soluble β-glucan of an edible mushroom Termitomyces heimii: Structural and biological investigation. Carbohydr. Polym. 2015, 134, 75–384. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.K.; Nandi, D.K.; Manna, M.; Pattanayak, I.K.; Sen, S.K.; Bhanja, S.; Samanta, B.C.; Panda, S.; Paloi, K.; Acharia, S.S. Islam Structural characterization and antioxidant activity of a glucan from Meripilus giganteus. Carbohydr. Polym. 2017, 157, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Manna, D.K.; Maity, P.; Nandi, A.K.; Pattanayak, M.; Panda, B.C.; Mandal, A.K.; Tripathy, S.; Acharya, K.; Sahoo, A.K.; Gupta, N.; et al. Structural elucidation and immunostimulating property of a novel polysaccharide extracted from an edible mushroom Lentinus fusipes. Carbohydr. Polym. 2017, 157, 1657–1665. [Google Scholar] [CrossRef]
- Rai, M.; Sen, S.; Acharya, K. Antimicrobial activity of four wild edible mushrooms from Darjeeling hills, West Bengal, India. Int. J. Pharmtech. Res. 2013, 5, 949–956. [Google Scholar]
- Ren, L.; Perera, C.; Hemar, Y. Antitumor activity of mushroom polysaccharides: A review. Food Funct. 2012, 3, 1118–1130. [Google Scholar] [CrossRef]
- Meng, Y.; Yan, J.; Yang, G.; Han, Z.; Tai, G.; Cheng, H.; Zhou, Y. Structural characterization and macrophage activation of a hetero-galactan isolated from Flammulina velutipes. Carbohydr. Polym. 2018, 183, 207–218. [Google Scholar] [CrossRef]
- Nandi, A.K.; Sen, I.K.; Samanta, S.; Maity, K.; Devi, K.S.P.; Mukherjee, S.; Maiti, T.K.; Acharya, K.; Islam, S.S. Glucan from hot aqueous extract of an ectomycorrhizal edible mushroom, Russula albonigra (Krombh.) Fr.: Structural characterization and study of immunoenhancing properties. Carbohydr. Res. 2012, 363, 43–50. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Li, Y. Antitumor and immunomodulatory activity of polysaccharide isolated from Trametes orientalis. Carbohydr. Polym. 2015, 131, 248–254. [Google Scholar] [CrossRef]
- Wang, D.; Sun, S.; Wu, W.; Yang, S.; Tan, J. Characterization of a water-soluble polysaccharide from Boletus edulis and its antitumor and immunomodulatory activities on renal cancer in mice. Carbohydr. Polym. 2014, 105, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, E.K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharm. 2011, 11, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P.S. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, C.; Shang, J.Y.; Fu, H.T.; Chen, J.X.; Liu, H.Y.; Chen, J.H. Mechanism of the immunostimulatory activity by a polysaccharide from Dictyophora indusiata. Int. J. Biol. Macromol. 2016, 91, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.K.; Chan, J.Y.; Wu, S.B.; Cheng, L.; Ho, G.K.; Lau, C.P.; Kennelly, E.J.; Leung, P.C.; Fung, K.P.; Lau, C.B. Anti-inflammatory activities of an active fraction isolated from the root of Astragalus membranaceus in RAW 264.7 macrophages. Phytother. Res. 2014, 28, 395–404. [Google Scholar] [CrossRef]
- Onoprienko, L. Molecular mechanisms of regulation of the macrophage activity. Russ. J. Bioorg. Chem. 2011, 37, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, M.; Muroi, M.; Tanamoto, K.; Suzuki, T.; Azuma, H.; Ikeda, H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: Roles of the receptor complex. Pharmacol. Ther. 2003, 100, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Han, Y.; Huang, J.; Yu, X.; Jiao, C.; Yang, X.; Yang, B.B. Purification and identification of a polysaccharide from medicinal mushroom Amauroderma rude with immunomodulatory activity and inhibitory effect on tumor growth. Oncotarget 2015, 6, 17777–17791. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Qi, C.; Peng, G.; Liu, Y.; Zhang, X.Y.; Meng, Z.L. Immune-enhancing effects of a polysaccharide PRG1-1 from Russula griseocarnosa on RAW264.7 macrophage cells via the MAPK and NF-κB signalling pathways. Food Agric. Immunol. 2018, 29, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Yang, Y.; Bian, Z.; Xu, B. Molecular weight and helix conformation determine intestinal anti-inflammatory effects of exopolysaccharide from Schizophyllum commune. Carbohydr. Polym. 2017, 172, 68–77. [Google Scholar] [CrossRef]
- Vivier, E.; Nunes, J.A.; Vely, F. Natural killer cell signaling pathways. Science 2004, 306, 1517–1519. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Jinushi, M.; Takehara, T.; Tatsumi, T.; Hiramatsu, N.; Sakamori, R.; Yamaguchi, S.; Hayashi, N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J. Hepatol. 2005, 43, 1013–1020. [Google Scholar] [CrossRef]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.; Yagita, H.; Takeda, K.; van Dommelen, S.L.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK cell cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and moisturizing properties of carboxymethyl chitosan with different molecular weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Kolsi, R.B.A.; Fakhfakh, J.; Krichen, F.; Jribi, I.; Chiarore, A.; Patti, F.P.; Blecker, C.; Allouche, N.; Belghith, H.; Belghith, K. Structural characterization and functional properties of antihypertensive Cymodocea nodosa sulfated polysaccharide. Carbohydr. Polym. 2016, 151, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Surin, S.; Surayot, U.; Seesuriyachan, P.; You, S.G.; Phimolsiripol, Y. Antioxidant and immunomodulatory activities of sulphated polysaccharides from purple glutinous rice bran (Oryza sativa L.). Int. J. Food Sci. Technol. 2018, 53, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wu, W.; Xu, X.; Zhang, L.; Liu, Y.; Wang, K. Chain conformation and anti-tumor activity of derivatives of polysaccharide from Rhizoma Panacis Japonici. Carbohydr. Polym. 2014, 105, 308–316. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Chen, Z. Sulfated modification of the polysaccharides obtained from defatted rice bran and their antitumor activities. Int. J. Biol. Macromol. 2009, 44, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huang, M.; Shen, M.; Wen, P.; Wu, T.; Hong, Y.; Yu, Q.; Chen, Y.; Xie, J. Sulfated modification enhanced the antioxidant activity of Mesona chinensis Benth polysaccharide and its protective effect on cellular oxidative stress. Int. J. Biol. Macromol. 2019, 136, 1000–1006. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Zhang, Y.; Qin, L.; Zhang, J. Sulfated modification and immunomodulatory activity of water-soluble polysaccharides derived from fresh Chinese persimmon fruit. Int. J. Biol. Macromol. 2010, 46, 67–71. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Jin, W.; Guo, Y. Immunomodulatory effects of a low-molecular weight polysaccharide from Enteromorpha prolifera on RAW 264.7 macrophages and cyclophosphamide-induced immunosuppression mouse models. Mar. Drugs 2020, 18, 340. [Google Scholar] [CrossRef]
- Kouakou, K.; Schepetkin, I.A.; Jun, S.; Kirpotina, L.N.; Yapi, A.; Khramova, D.S.; Pascual, D.W.; Ovodov, Y.S.; Jutila, M.A.; Quinn, M.T. Immunomodulatory activity of polysaccharides isolated from Clerodendrum splendens: Beneficial effects in experimental autoimmune encephalomyelitis. BMC Complementary Altern. Med. 2013, 13, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of polysaccharides and resulting bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Surayot, U.; Wangtueai, S.; You, S.; Palanisamy, S.; Krusong, W.; Brennan, C.S.; Barba, F.J.; Phimolsiripol, Y.; Seesuriyachan, P. Extraction, structural characterisation, and immunomodulatory properties of edible Amanita hemibapha subspecies javanica (Corner and Bas) mucilage polysaccharide as a potential of functional food. J. Fungi 2021, 7, 683. [Google Scholar] [CrossRef]
- Schöniger, W. The rapid microanalytical determination of halogens and sulfur in organic substances. Microchim. Acta 1956, 44, 869–876. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, R.A.; Ji, R.; Tabarsa, M.; Zhang, J.; Meng, L.; Zhang, C.; Zhang, J.; Wang, L.; Wu, R.; Wang, C.; et al. Purification, characterization and immunostimulatory effects of polysaccharides from Anemarrhena asphodeloides rhizomes. Int. J. Biol. Macromol. 2021, 172, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Yelithao, K.; Surayot, U.; Park, W.; Lee, S.; Lee, D.H.; You, S. Effect of sulfation and partial hydrolysis of polysaccharides from Polygonatum sibiricum on immune-enhancement. Int. J. Biol. Macromol. 2019, 122, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ouyang, K.; Li, J.; Liu, X.; An, Q.; Zhao, M.; Chen, S.; Li, X.; Ye, X.; Zhao, Z.; et al. Sulfated modification, characterization, immunomodulatory activities and mechanism of the polysaccharides from Cyclocarya paliurus on dendritic cells. Int. J. Biol. Macromol. 2020, 159, 108–116. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Sulfated modification, characterization and bioactivities of an acidic polysaccharide fraction from an edible mushroom Pleurotus eous (Berk.) Sacc. Heliyon 2021, 7, e05964. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Park, G.M.; Shin, I.S.; Lee, E.; Kim, J.K.; You, S. Structure-activity relationships of sulfated glycoproteins from Codium fragile on nitric oxide releasing capacity from RAW264.7 cells. Mar. Biotechnol. 2015, 17, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.; Yan, X.H.; Zhang, J.L.; Wang, L.Y.; Xue, H.; Jiang, G.C.; Ma, X.T.; Liu, X.J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Surayot, U.; Wang, J.; Seesuriyachan, P.; Kuntiya, A.; Tabarsa, M.; Lee, Y.; Kim, J.K.; Park, W.; You, S. Exopolysaccharides from lactic acid bacteria: Structural analysis, molecular weight effect on immunomodulation. Int. J. Biol. Macromol. 2014, 68, 233–240. [Google Scholar] [CrossRef]
- Kakar, M.U.; Kakar, I.U.; Mehboob, M.Z.; Zada, S.; Soomro, H.; Umair, M.; Iqbal, I.; Umer, M.; Shaheen, S.; Syed, S.F.; et al. A review on polysaccharides from Artemisia sphaerocephala Krasch seeds, their extraction, modification, structure, and applications. Carbohydr. Polym. 2021, 252, 117113. [Google Scholar] [CrossRef]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.G.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Huyan, T.; Li, Q.; Yang, H.; Jin, M.L.; Zhang, M.J.; Ye, L.J.; Li, J.; Huang, Q.S.; Yin, D.C. Protective effect of polysaccharides on simulated microgravity-induced functional inhibition of human NK cells. Carbohydr. Polym. 2014, 101, 819–827. [Google Scholar] [CrossRef]
- Surayot, U.; Lee, S.; You, S. Effects of sulfated fucan from the sea cucumber Stichopus japonicus on natural killer cell activation and cytotoxicity. Int. J. Biol. Macromol. 2018, 108, 177–184. [Google Scholar] [CrossRef]
- Zhao, X.; Jiao, G.; Yang, Y.; Li, M.; Li, Q.; Wang, X.; Cai, C.; Li, G.; Hao, J.; Yu, G. Structure and immunomodulatory activity of a sulfated agarose with pyruvate and xylose substitutes from Polysiphonia senticulosa Harvey. Carbohydr. Polym. 2017, 176, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, H.; Han, M.; Cao, Q.; Liang, H.; Yuan, R.; Sun, J.; Zhang, L.; Wu, Y. Chemical modification and antioxidant activity of the polysaccharide from Acanthopanax leucorrhizus. Carbohydr. Res. 2020, 487, 107890. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L. Physicochemical properties and antitumor activities for sulfated derivatives of lentinan. Carbohydr. Res. 2009, 344, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signalling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]






| Primer Sequences (5′→3′) | ||
|---|---|---|
| iNOS | Forward | CTGCAGCACTTGGATCAGGAACCTG |
| Reverse | GGGAGTAGC CTGTGTGCACCTGGAA | |
| IL-1β | Forward | ATGGCAACTATTCCAGAACTCAACT |
| Reverse | CAGGACAGGTATAGATTCTTTCCTTT | |
| TNF-α | Forward | AGGTTCTGTCCCTTTCACTCACTG |
| Reverse | AGAAGACCTGGGAGTCAAGGTA | |
| IL-6 | Forward | TTCCTCTCTGCAAGAGACT |
| Reverse | TGTATCTCTCTGAAGGACT | |
| IL-10 | Forward | TACCTGGTAGAAGTGATGCC |
| Reverse | CATCATGTATGCTTCTATGC | |
| β-actin | Forward | ATGTGCAAAAAGCTGGCTTTG |
| Reverse | ATTTGTGGTGGATGATGGAGG | |
| IFN-γ | Forward | GATGCTCTTCGACCTCGAAACAGCAT |
| Reverse | ATGAAATATACAAGTTATAATCTTGGCTTT | |
| Granzyme-B | Forward | AGATCGAAAGTGCGAATCTGA |
| Reverse | TTCGTCCATAGGAGACAATGC | |
| Perforin | Forward | AGTCCTCCACCTCGTTGTCCGTGA |
| Reverse | AAAGTCAGCTCCACTGAAGCTGTG | |
| NKp30 | Forward | TCTATTACCAGGGCAAATGTGAAGT |
| Reverse | GTCACTGGGGTCTAGAATCACTCAT | |
| FasL | Forward | CCAGAGAGAGCTCAGATACGTTGAC |
| Reverse | ATGTTTCAGCTCTTCCACCTACAGA | |
| β-actin | Forward | CATCTCTTGCTCGAAGTCCA |
| Reverse | ATCATGTTTGAGACCTTCAACA |
| Sample | Temperature (°C) | Reaction Time (h) | DS | Sulphate Content (%) |
|---|---|---|---|---|
| MPF2 | - | - | - | 9.30 ± 0.20 d |
| OS1 | 80 | 2 | 0.72 | 9.85 ± 0.05 c |
| OS2 | 80 | 4 | 0.89 | 11.3 ± 0.21 b |
| OS3 | 80 | 6 | 1.31 | 14.2 ± 0.21 a |
| Sample | Temperature (°C) | Reaction Time (min) | Yield (%) | Mw (×103 g/mol) |
|---|---|---|---|---|
| MPF2 | - | - | - | 104.0 ± 13.1 a |
| HS1 | 100 | 5 | 80 | 88.1 ± 3.52 b |
| HS2 | 100 | 10 | 81 | 50.7 ± 5.20 c |
| HS3 | 100 | 15 | 77 | 32.8 ± 3.87 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surayot, U.; Wangtueai, S.; You, S.; Techapun, C.; Phimolsiripol, Y.; Leksawasdi, N.; Krusong, W.; Barba, F.J.; Seesuriyachan, P. Sulphation and Hydrolysis Improvements of Bioactivities, and Immuno-Modulatory Properties of Edible Amanita hemibapha Subspecies javanica (Corner and Bas) Mucilage Polysaccharide as a Potential in Personalized Functional Foods. J. Fungi 2021, 7, 847. https://doi.org/10.3390/jof7100847
Surayot U, Wangtueai S, You S, Techapun C, Phimolsiripol Y, Leksawasdi N, Krusong W, Barba FJ, Seesuriyachan P. Sulphation and Hydrolysis Improvements of Bioactivities, and Immuno-Modulatory Properties of Edible Amanita hemibapha Subspecies javanica (Corner and Bas) Mucilage Polysaccharide as a Potential in Personalized Functional Foods. Journal of Fungi. 2021; 7(10):847. https://doi.org/10.3390/jof7100847
Chicago/Turabian StyleSurayot, Utoomporn, Sutee Wangtueai, Sangguan You, Charin Techapun, Yuthana Phimolsiripol, Noppol Leksawasdi, Warawut Krusong, Francisco J. Barba, and Phisit Seesuriyachan. 2021. "Sulphation and Hydrolysis Improvements of Bioactivities, and Immuno-Modulatory Properties of Edible Amanita hemibapha Subspecies javanica (Corner and Bas) Mucilage Polysaccharide as a Potential in Personalized Functional Foods" Journal of Fungi 7, no. 10: 847. https://doi.org/10.3390/jof7100847
APA StyleSurayot, U., Wangtueai, S., You, S., Techapun, C., Phimolsiripol, Y., Leksawasdi, N., Krusong, W., Barba, F. J., & Seesuriyachan, P. (2021). Sulphation and Hydrolysis Improvements of Bioactivities, and Immuno-Modulatory Properties of Edible Amanita hemibapha Subspecies javanica (Corner and Bas) Mucilage Polysaccharide as a Potential in Personalized Functional Foods. Journal of Fungi, 7(10), 847. https://doi.org/10.3390/jof7100847