MalaSelect: A Selective Culture Medium for Malassezia Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Broth Microdilution
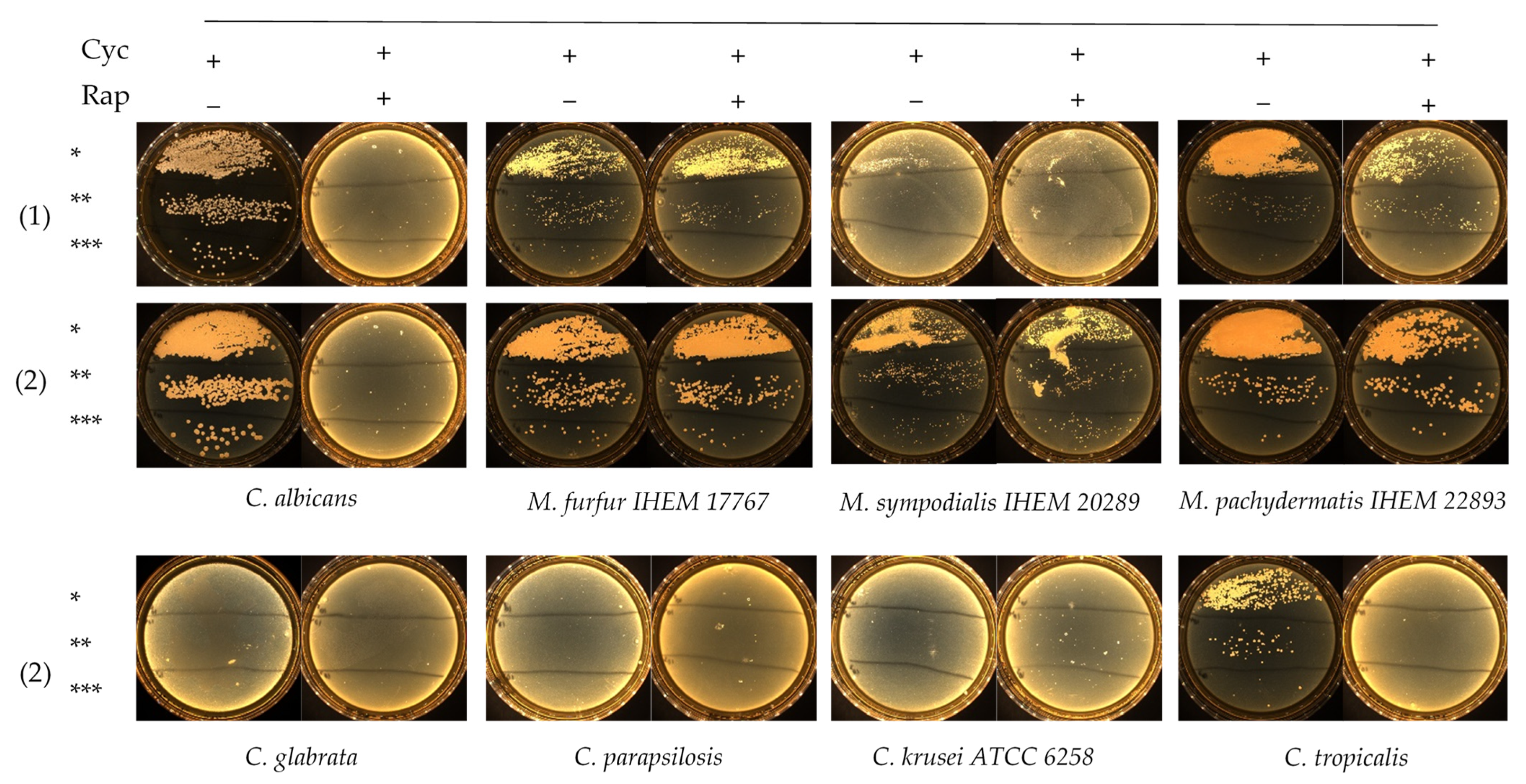
2.3. Growth Testing on Agar
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorch, J.M.; Palmer, J.M.; Vanderwolf, K.J.; Schmidt, K.Z.; Verant, M.L.; Weller, T.J.; Blehert, D.S. Malassezia vespertilionis sp. nov.: A new cold-tolerant species of yeast isolated from bats. Pers. Mol. Phylogeny Evol. Fungi 2018, 41, 56–70. [Google Scholar] [CrossRef]
- Gaitanis, G.; Magiatis, P.; Hantschke, M.; Bassukas, I.D.; Velegraki, A. The Malassezia Genus in Skin and Systemic Diseases. Clin. Microbiol. Rev. 2012, 25, 106–141. [Google Scholar] [CrossRef]
- Chryssanthou, E.; Broberger, U.; Petrini, B. Malassezia pachydermatis fungaemia in a neonatal intensive care unit. Acta Paediatr. 2007, 90, 323–327. [Google Scholar] [CrossRef]
- Al-Sweih, N.; Ahmad, S.; Joseph, L.; Khan, S.; Khan, Z. Malassezia pachydermatis fungemia in a preterm neonate resistant to fluconazole and flucytosine. Med. Mycol. Case Rep. 2014, 5, 9–11. [Google Scholar] [CrossRef]
- Iatta, R.; Cafarchia, C.; Cuna, T.; Montagna, O.; Laforgia, N.; Gentile, O.; Rizzo, A.; Boekhout, T.; Otranto, D.; Montagna, M.T. Bloodstream infections by Malassezia and Candida species in critical care patients. Med. Mycol. 2014, 52, 264–269. [Google Scholar] [CrossRef]
- Rhimi, W.; Theelen, B.; Boekhout, T.; Otranto, D.; Cafarchia, C. Malassezia spp. Yeasts of Emerging Concern in Fungemia. Front. Cell. Infect. Microbiol. 2020, 10, 370. [Google Scholar] [CrossRef]
- Celakovska, J.; Vankova, R.; Bukac, J.; Cermakova, E.; Andrys, C.; Krejsek, J. Atopic Dermatitis and Sensitisation to Molecular Components of Alternaria, Cladosporium, Penicillium, Aspergillus, and Malassezia—Results of Allergy Explorer ALEX 2. J. Fungi 2021, 7, 183. [Google Scholar] [CrossRef]
- Gomez-Moyano, E.; Crespo-Erchiga, V.; Martínez-Pilar, L.; Diaz, D.G.; Martínez-García, S.; Navarro, M.L.; Casaño, A.V. Do Malassezia species play a role in exacerbation of scalp psoriasis? J. Mycol. Méd. 2014, 24, 87–92. [Google Scholar] [CrossRef]
- Chua, K.B.; Chua, I.L.; Chua, I.E.; Chong, K.H.; Chua, K.H. A modified mycological medium for isolation and culture of Malassezia furfur. Malays. J. Pathol. 2005, 27, 99–105. [Google Scholar]
- Kaneko, T.; Makimura, K.; Onozaki, M.; Ueda, K.; Yamada, Y.; Nishiyama, Y.; Yamaguchi, H. Vital growth factors of Malassezia species on modified CHROMagar Candida. Med. Mycol. 2005, 43, 699–704. [Google Scholar] [CrossRef]
- Kaneko, T.; Makimura, K.; Sugita, T.; Yamaguchi, H. Tween 40-based precipitate production observed on modified chromogenic agar and development of biological identification kit for Malassezia species. Med. Mycol. 2006, 44, 227–231. [Google Scholar] [CrossRef]
- Aridogan, I.A.; Ilkit, M.; Izol, V.; Ates, A.; Demirhindi, H. Glans penis and prepuce colonisation of yeast fungi in a paediatric population: Pre- and postcircumcision results. Mycoses 2009, 52, 49–52. [Google Scholar] [CrossRef]
- Hamad, I.; Ranque, S.; Azhar, E.; Yasir, M.; Jiman-Fatani, A.A.; Tissot-Dupont, H.; Raoult, D.; Bittar, F. Culturomics and Amplicon-based Metagenomic Approaches for the Study of Fungal Population in Human Gut Microbiota. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Iskit, S.; Ilkit, M.; Turaç-Biçer, A.; Demirhindi, H.; Türker, M. Effect of circumcision on genital colonization of Malassezia spp. in a pediatric population. Med. Mycol. 2006, 44, 113–117. [Google Scholar] [CrossRef]
- Mayser, P.; Schütz, M.; Schuppe, H.-C.; Jung, A.; Schill, W.-B. Frequency and spectrum of Malassezia yeasts in the area of the prepuce and glans penis. BJU Int. 2001, 88, 554–558. [Google Scholar] [CrossRef]
- Singh, S.; Banerjee, T.; Tilak, R.; Chaudhary, R. Prevalence of different Malassezia species in pityriasis versicolor in central India. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 159–164. [Google Scholar] [CrossRef]
- Talaee, R.; Katiraee, F.; Ghaderi, M.; Erami, M.; Alavi, A.K.; Nazeri, M. Molecular Identification and Prevalence of Malassezia Species in Pityriasis Versicolor Patients from Kashan, Iran. Jundishapur J. Microbiol. 2014, 7, e11561. [Google Scholar] [CrossRef]
- Zeinali, E.; Sadeghi, G.; Yazdinia, F.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Clinical and epidemiological features of the genus Malassezia in Iran. Iran. J. Microbiol. 2014, 6, 354–360. [Google Scholar]
- Zhao, Y.; Li, L.; Wang, J.-J.; Kang, K.F.; Zhang, Q.Q. Cutaneous malasseziasis: Four case reports of atypical dermatitis and onychomycosis caused by Malassezia. Int. J. Dermatol. 2010, 49, 141–145. [Google Scholar] [CrossRef]
- Cleland, E.J.; Bassiouni, A.; Boase, S.; Dowd, S.; Vreugde, S.; Wormald, P.-J. The fungal microbiome in chronic rhinosinusitis: Richness, diversity, postoperative changes and patient outcomes. Int. Forum Allergy Rhinol. 2014, 4, 259–265. [Google Scholar] [CrossRef]
- Hamad, I.; Abdallah, R.A.; Ravaux, I.; Mokhtari, S.; Tissot-Dupont, H.; Michelle, C.; Stein, A.; Lagier, J.-C.; Raoult, D.; Bittar, F. Metabarcoding analysis of eukaryotic microbiota in the gut of HIV-infected patients. PLoS ONE 2018, 13, e0191913. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef]
- Willger, S.D.; Grim, S.L.; Dolben, E.L.; Shipunova, A.; Hampton, T.H.; Morrison, H.G.; Filkins, L.M.; O’Toole, G.A.; Moulton, L.A.; Ashare, A.; et al. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome 2014, 2, 40. [Google Scholar] [CrossRef]
- Van Woerden, H.C.; Gregory, C.; Brown, R.; Marchesi, J.R.; Hoogendoorn, B.; Matthews, I.P. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: A community based case control study. BMC Infect. Dis. 2013, 13, 69. [Google Scholar] [CrossRef]
- Vézina, C.; Kudelski, A.; Sehgal, S.N. Rapamycin (AY-22,989), A new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 1975, 28, 721–726. [Google Scholar] [CrossRef]
- Thomson, A.W.; Starzl, T.E. New Immunosuppressive Drugs: Mechanistic Insights and Potential Therapeutic Advances. Immunol. Rev. 1993, 136, 71–97. [Google Scholar] [CrossRef]
- Cruz, M.C.; Goldstein, A.L.; Blankenship, J.; Del Poeta, M.; Perfect, J.R.; McCusker, J.H.; Bennani, Y.L.; Cardenas, M.E.; Heitman, J. Rapamycin and Less Immunosuppressive Analogs Are Toxic to Candida albicans and Cryptococcus neoformans via FKBP12-Dependent Inhibition of TOR. Antimicrob. Agents Chemother. 2001, 45, 3162–3170. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.K.; Griffith, S.; Kojima, I.; Demain, A.L. Antifungal activities of rapamycin and its derivatives, prolylrapamycin, 32-desmethylrapamycin, and 32-desmethoxyrapamycin. J. Antibiot. 1998, 51, 487–491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ianiri, G.; Clancey, S.A.; Lee, S.C.; Heitman, J. FKBP12-Dependent Inhibition of Calcineurin Mediates Immunosuppressive Antifungal Drug Action in Malassezia. mBio 2017, 8, e01752-17. [Google Scholar] [CrossRef] [PubMed]
- Bittar, F.; Gouriet, F.; Khelaifia, S.; Raoult, D.; Ranque, S. FastFung: A novel medium for the culture and isolation of fastidious fungal species from clinical samples. J. Microbiol. Methods 2020, 180, 106108. [Google Scholar] [CrossRef] [PubMed]
- Abdillah, A.; Khelaifia, S.; Raoult, D.; Bittar, F.; Ranque, S. Comparison of Three Skin Sampling Methods and Two Media for Culturing Malassezia Yeast. J. Fungi 2020, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Gümral, R.; Döğen, A.; Ilkit, M.M. Comparison of the contamination rates of culture media used for isolation and identification of dermatophytes. Turk. J. Med. Sci. 2015, 45, 587–592. [Google Scholar] [CrossRef] [PubMed]
| Strains | Rapamycin MIC (mg/L) | |
|---|---|---|
| 24 h | 48 h | |
| C. albicans | 0.25 | 0.5 |
| C. glabrata | 0.5 | 2.0 |
| C. tropicalis | 0.25 | 0.5 |
| C. parapsilosis | 0.5 | 1.0 |
| C. krusei ATCC 6258 | 32.0 | >32.0 |
| M. furfur IHEM 17767 | - | >32.0 |
| M. furfur IHEM 19320 | - | >32.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdillah, A.; Ranque, S. MalaSelect: A Selective Culture Medium for Malassezia Species. J. Fungi 2021, 7, 824. https://doi.org/10.3390/jof7100824
Abdillah A, Ranque S. MalaSelect: A Selective Culture Medium for Malassezia Species. Journal of Fungi. 2021; 7(10):824. https://doi.org/10.3390/jof7100824
Chicago/Turabian StyleAbdillah, Abdourahim, and Stéphane Ranque. 2021. "MalaSelect: A Selective Culture Medium for Malassezia Species" Journal of Fungi 7, no. 10: 824. https://doi.org/10.3390/jof7100824
APA StyleAbdillah, A., & Ranque, S. (2021). MalaSelect: A Selective Culture Medium for Malassezia Species. Journal of Fungi, 7(10), 824. https://doi.org/10.3390/jof7100824







