Four New Species of Hemileccinum (Xerocomoideae, Boletaceae) from Southwestern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Morphological Study
2.2. Molecular Procedures and Phylogenetic Analyses
2.3. Phylogenetic Analyses
| Species | Voucher | Locality | GenBank Accession Number | References | ||||
|---|---|---|---|---|---|---|---|---|
| ITS | nrLSU | rpb2 | rpb1 | tef1-α | ||||
| Hemileccinum rugosum | KUN-HKAS84355 | China | - | KT990578 | KT990413 | KT990931 | KT990774 | [8] |
| Hemileccinum rugosum | KUN-HKAS84970 | China | - | KT990577 | KT990412 | - | KT990773 | [8] |
| Hemileccinum rugosum | KUN-HKAS50284 | China | - | KT990576 | KT990411 | - | KT990772 | [8] |
| Hemileccinum subglabripes | MICH:KUO-07230802 | USA | - | MK601738 | MK766300 | - | MK721092 | [10] |
| Hemileccinum subglabripes | MICH:KUO-07070702 | USA | - | MK601737 | MK766299 | - | MK721091 | [10] |
| Hemileccinum subglabripes | MICH:KUO-08301402 | USA | - | MK601739 | MK766301 | - | MK721093 | [10] |
| Hemileccinum subglabripes | 72206 | USA | - | KF030303 | - | KF030374 | KF030404 | [36] |
| Hemileccinum subglabripes | 294169 | USA | MN128237 | - | - | - | - | from GenBank |
| Hemileccinum subglabripes | 3660 | - | KM248936 | - | - | - | - | from GenBank |
| Hemileccinum depilatum | 2137333 | USA | AY127032 | - | - | - | - | from GenBank |
| Hemileccinum depilatum | AF2845 | Belgium | - | - | MG212633 | - | MG212591 | [37] |
| Hemileccinum depilatum | Bd1 | - | - | AF139712 | - | - | - | [5] |
| Hemileccinum impolitum | Bim 1 | Germany | - | AF139715 | - | KF030375 | JQ327034 | [36] |
| Hemileccinum impolitum | 47698 | Portugal | AJ419187 | - | - | - | - | [38] |
| Hemileccinum impolitum | BI57407 | Thailand | KM235997 | - | - | - | - | from GenBank |
| Hemileccinum impolitum | BI57408 | Thailand | KM235998 | - | - | - | - | from GenBank |
| Hemileccinum impolitum | 17173 | USA | JF907783 | - | - | - | - | [39] |
| Hemileccinum impolitum | KUN-HKAS84869 | Germany | - | KT990575 | KT990410 | KT990930 | KT990771 | [8] |
| Hemileccinum indecorum | KUN-HKAS63126 | China | - | KF112440 | - | - | - | [7] |
| Hemileccinum indecorum | OR0863 | Thailand | - | - | MH614772 | - | MH614726 | [16] |
| Hemileccinum rubropunctum | JLF56666 | USA | MH190826 | - | - | - | - | from GenBank |
| Hemileccinum rubropunctum | MES256 | USA | FJ480428 | - | - | - | - | [40] |
| Hemileccinum rubropunctum | NY-792788REH-8501 | USA | - | MK601768 | MK766327 | - | MK721122 | [10] |
| Hemileccinum rubropunctum | NY-01193924REH-9597 | USA | - | MK601769 | MK766328 | - | MK721123 | [10] |
| Hemileccinum sp. | KUN-HKAS53421 | China | - | KF112432 | KF112751 | KF112565 | KF112235 | [7] |
| Hemileccinum hortonii | MICH KUO-07050706 | USA | - | MK601821 | MK766377 | - | MK721175 | [10] |
| Hemileccinum albidum | KUN-HKAS87225 | China | MZ923777 | MZ923774 | MZ936317 | MZ936334 | MZ936351 | This study |
| Hemileccinum albidum | KUN-HKAS83355 | China | MZ923778 | MZ923775 | MZ936321 | MZ936340 | MZ936357 | This study |
| Hemileccinum albidum (T) | KUN-HKAS81120 | China | MZ923782 | MZ923766 | MZ936320 | MZ936339 | MZ936352 | This study |
| Hemileccinum albidum | KUN-HKAS50503 | China | MZ923781 | MZ923767 | MZ936319 | MZ936335 | MZ936355 | This study |
| Hemileccinum albidum | KUN-HKAS50350 | China | MZ923779 | MZ923768 | MZ936323 | MZ936342 | MZ936359 | This study |
| Hemileccinum albidum | KUN-HKAS84554 | China | MZ923780 | - | MZ936318 | MZ936336 | MZ936358 | This study |
| Hemileccinum albidum | KUN-HKAS85753 | China | MZ923786 | - | MZ936325 | MZ936337 | MZ936353 | This study |
| Hemileccinum albidum | KUN-HKAS87105 | China | - | MZ923769 | MZ936327 | MZ936338 | MZ936356 | This study |
| Hemileccinum albidum | KUN-HKAS83333 | China | MZ923784 | - | MZ936326 | MZ936344 | MZ936361 | This study |
| Hemileccinum albidum | KUN-HKAS83400 | China | MZ923783 | MZ923770 | MZ936324 | MZ936341 | MZ936354 | This study |
| Hemileccinum albidum | KUN-HKAS115749 | China | MZ923785 | - | MZ936322 | MZ936343 | MZ936360 | This study |
| Hemileccinum brevisporum (T) | KUN-HKAS89150 | China | MZ923788 | MZ923764 | MZ936328 | MZ936345 | MZ936362 | This study |
| Hemileccinum brevisporum | KUN-HKAS59445 | China | - | KT990579 | KT990414 | KT990932 | KT990775 | [8] |
| Hemileccinum brevisporum | KUN-HKAS67896 | China | MZ923787 | MZ923765 | MZ936329 | MZ936346 | MZ936363 | This study |
| Hemileccinum ferrugineipes (T) | KUN-HKAS115554 | China | MZ923792 | MZ923773 | MZ936330 | MZ936350 | MZ973011 | This study |
| Hemileccinum ferrugineipes | KUN-HKAS75054 | China | - | KF112377 | KF112749 | KF112563 | KF112234 | [7] |
| Hemileccinum ferrugineipes | KUN-HKAS93310 | China | MZ923791 | - | MZ936331 | MZ936347 | MZ973012 | This study |
| Hemileccinum parvum | KUN-HKAS99764 | China | MZ923789 | MZ923771 | MZ936332 | MZ936349 | MZ973009 | This study |
| Hemileccinum parvum (T) | KUN-HKAS115553 | China | MZ923790 | MZ923772 | MZ936333 | MZ936348 | MZ973010 | This study |
| Heimioporus sp. | KUN-HKAS53451 | China | - | KF112345 | KF112805 | KF112616 | KF112226 | [7] |
| Heimioporus aff. japonicus | KUN-HKAS52236 | China | - | KF112346 | KF112807 | KF112617 | KF112227 | [7] |
| Heimioporus japonicas | KUN-HKAS52237 | China | - | KF112347 | KF112806 | KF112618 | KF112228 | [7] |
| Aureoboletus tenuis | KUN-HKAS75104 | China | - | KT990518 | KT990359 | KT990897 | KT990722 | [8] |
| Aureoboletus thibetanus | KUN-HKAS76655 | China | - | KF112420 | KF112752 | KF112626 | KF112236 | [7] |
| Pulchroboletus roseoalbidus | AMB 12757 | Italy | - | NG_060126 | - | - | KJ729512 | [27] |
| Alessioporus ichnusanus | AMB 12756 | Italy | - | NG_057044 | - | - | KJ729513 | [27] |
| Phylloporus rubrosquamosus | KUN-HKAS52552 | China | - | KF112391 | KF112780 | - | KF112289 | [7] |
| Phylloporus rubrosquamosus | KUN-HKAS54559 | China | NR120124 | NG_042668 | - | - | JQ967175 | [24,25] |
| Phylloporus rubeolus | KUN-HKAS52573 | China | JQ967259 | JQ967216 | - | - | JQ967172 | [24,25] |
| Xerocomus fraternus | KUN-HKAS55328 | China | - | KT990681 | KT990497 | - | KT990869 | [8] |
| Xerocomus velutinus | KUN-HKAS68135 | China | - | KT990673 | - | KT991011 | KT990861 | [8] |
| Hourangia cheoi | Yang 5153 | China | KP136997 | KP136947 | KP136975 | KP136966 | KP136924 | [26] |
| Hourangia pumila | REH8063 | Indonesia | JQ003626 | NG_060636 | - | - | - | [28] |
| Boletellus indistinctus | KUN-HKAS77623 | China | - | KT990531 | KT990371 | - | KT990733 | [8] |
| Boletellus indistinctus | KUN-HKAS80681 | China | - | KT990532 | KT990368 | KT990903 | KT990734 | [8] |
| Leccinum variicolor | KUN-HKAS57758 | China | - | KF112445 | KF112725 | KF112591 | KF112251 | [7] |
| Leccinum aff. scabrum | KUN-HKAS57266 | China | - | KF112442 | KF112722 | KF112590 | KF112248 | [7] |
| Leccinum monticola | KUN-HKAS76669 | China | - | KF112443 | KF112723 | KF112592 | KF112249 | [7] |
| Leccinellum cremeum | KUN-HKAS90639 | China | - | - | KT990420 | KT990936 | KT990781 | [8] |
| Leccinellum sp. | KUN-HKAS53410 | China | - | KT990585 | KT990421 | KT990937 | - | [8] |
3. Results
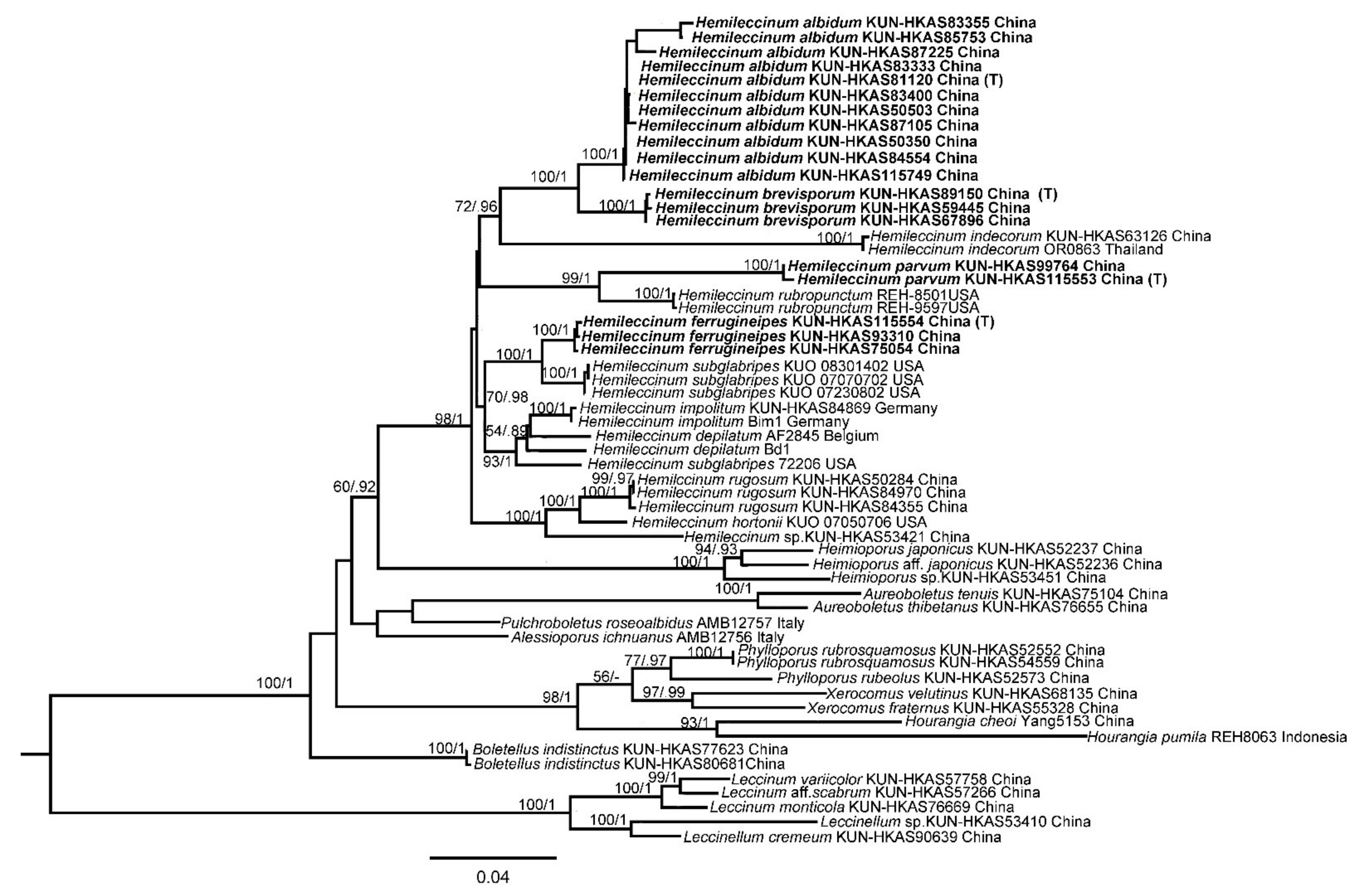
3.1. Molecular Phylogenetic Analysis
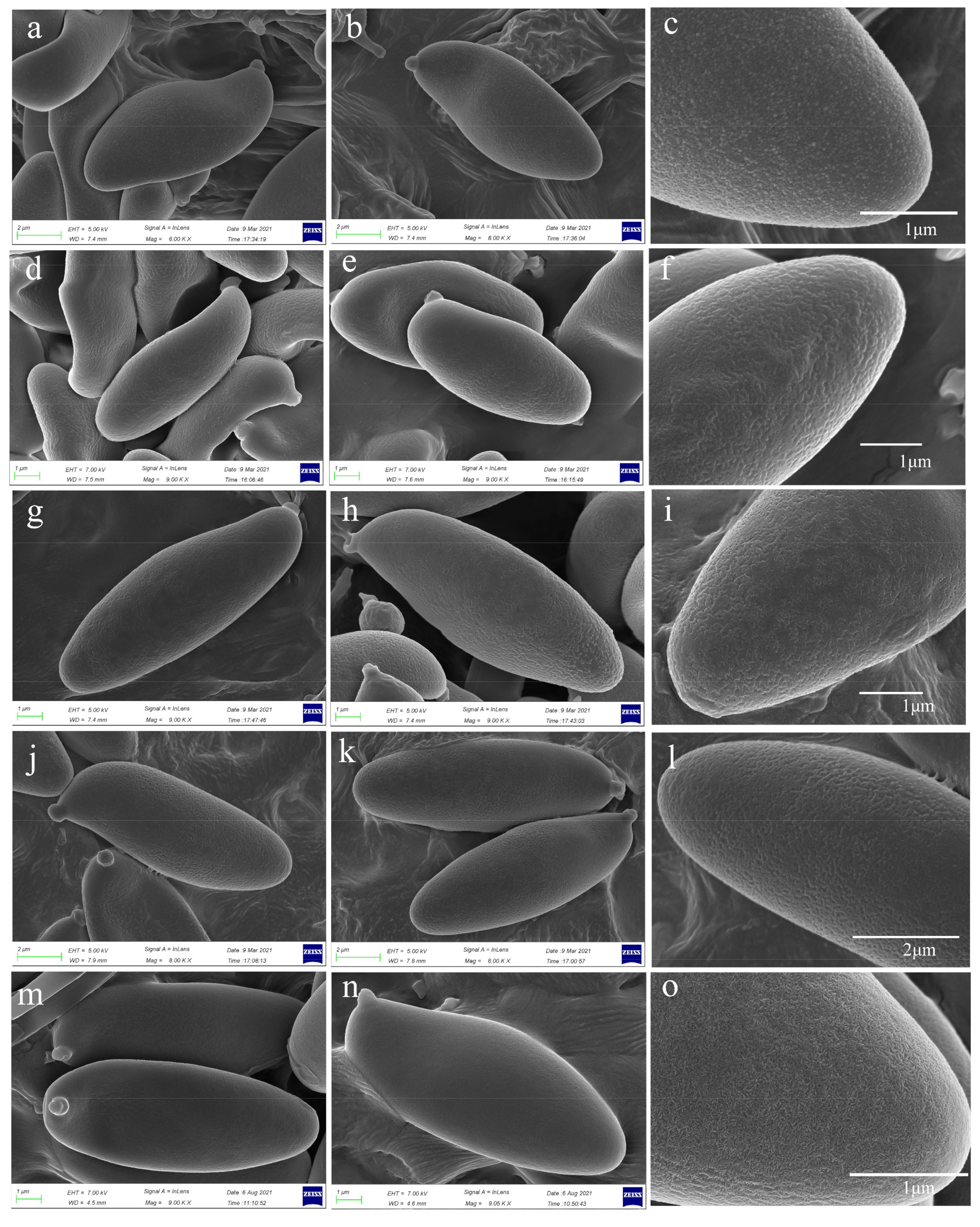
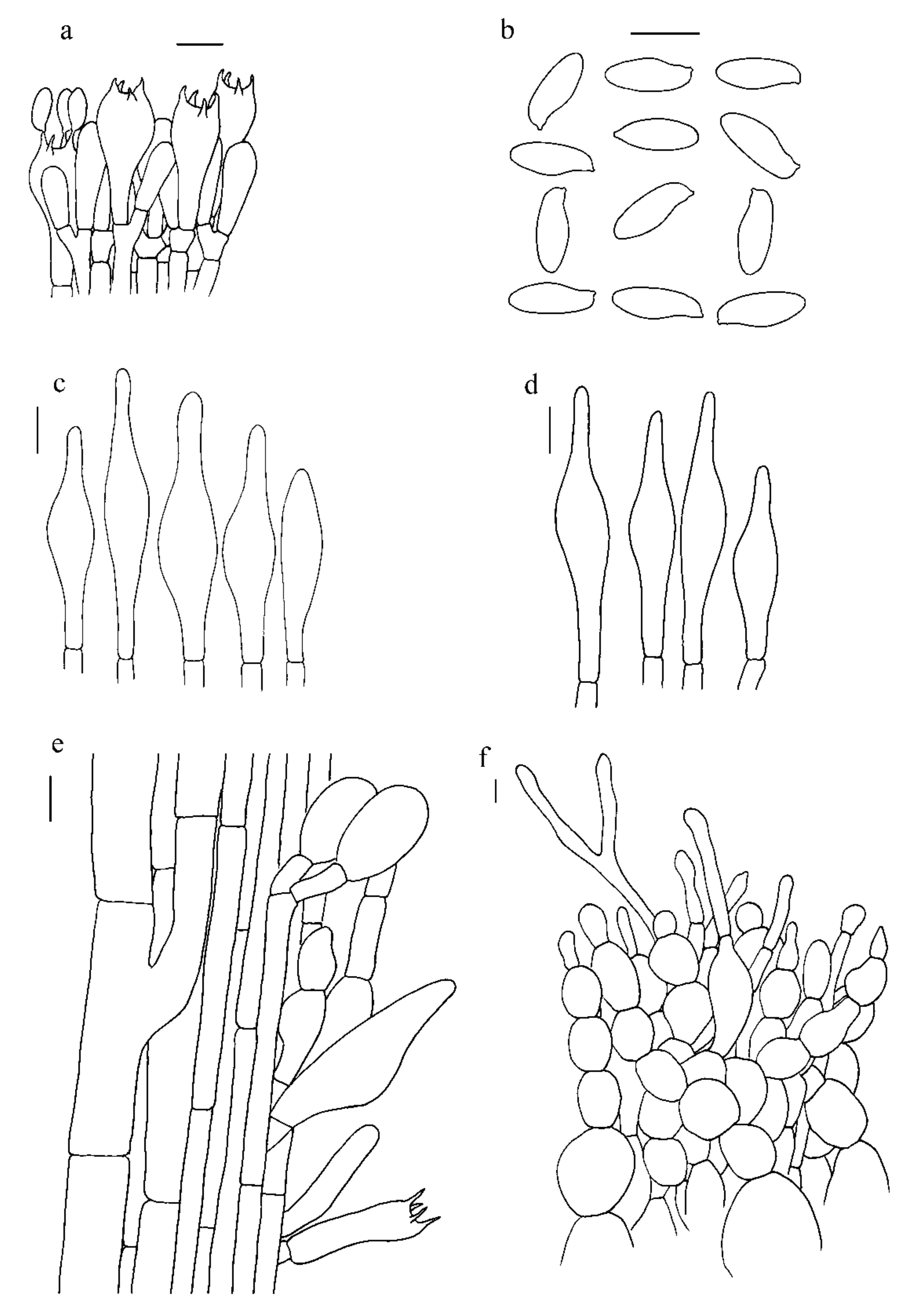
3.2. Taxonomy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Key to known species of Hemileccinum in the world | |
| 1. Pileus surface pale brown, brown to reddish brown, more or less even | 2 |
| 1′. Pileus surface orange to reddish orange, distinctively wrinkled | 9 |
| 2. Pileus surface slightly subtomentose, without small conical to subconical to irregularly shaped squamules, known in subtropical and temperate regions in the Northern Hemisphere… | 3 |
| 2′. Pileus surface with whitish to dirty white, small conical to subconical to irregularly shaped squamules, known from tropical southeast Asia… | H. indecorum |
| 3. Basidioma varied in size, yellowish context without color change when bruised… | 4 |
| 3′. Basidioma usually small in size (≤ 4 cm in daim), the pale yellow context staining pale blue very slowly when bruised, known from subtropical areas… | H. parvum |
| 4. Stipe surface covered with obvious ornaments… | 5 |
| 4′. Stipe surface covered with small scales, not obvious | 6 |
| 5. Stipe surface ornamented with distinctly reddish brown longitudinal streaks, known from East Asia | H. ferrugineipes |
| 5′. Stipe surface often covered with red dense fine-grained scales, known from North America… | H. rubropunctum |
| 6. Basidiospores longer in length (> 11 µm), and in other parts of the world… | 7 |
| 6′. Basidiospores shorter in length (≤ 11 µm), and distributed in subtropical forests in southwestern China.… | H. brevisporum |
| 7. Stipe stout (> 2.5 cm in diam.); pileipellis an trichoderm, collapsed when mature, restricted to Europe | H. impolitum |
| 7′. Stipe slender (≤ 2.5 cm in diam.); pileipellis an hyphoepithelium | 8 |
| 8. Stipe surface whitish, covered with small, granular scales only at the base, distributed in subtropical China | H. albidum |
| 8′. Stipe surface yellowish, nearly smooth, covered with indistinctive tiny scales, known from eastern North America | H. subglabripes |
| 9. Basidiospores smaller (10–12 × 4–5 µm), distributed in subtropical and tropical China | H. rugosum |
| 9′. Basidiospores larger (≥ 12 µm in length), distributed in temperate regions… | 10 |
| 10. Basidiospores narrower (12.0–15.0 × 3.5–4.5 µm), known from North America… | H. hortonii |
| 10′. Basidiospores wider (12.0–15.0 × 5–6 µm), known from Europe… | H. depilatum |
References
- Šutara, J. Xerocomus s. l. in the light of the present state of knowledge. Czech Mycol. 2008, 60, 29–62. [Google Scholar] [CrossRef]
- Fries, E.M. Epicrisis Systematis Mycologici, seu Synopsis Hymenomycetum; Typographia Academica: Munich, Germany, 1839; p. 421. [Google Scholar]
- Bertault, R. Amanites du Maroc (Troisième contribution). Bull. Société Mycol. Fr. 1980, 96, 271–287. [Google Scholar]
- Šutara, J. The delimitation of the genus Leccinum. Czech Mycol. 1989, 43, 1–12. [Google Scholar]
- Binder, M.; Besl, H. 28S rDNA sequence data and chemotaxonomical analyses on the generic concept of Leccinum (Boletales). Micologia 2000, 71–82. [Google Scholar]
- Binder, M.; Hibbett, D.S. Molecular systematics and biological diversification of Boletales. Mycology 2006, 98, 971–981. [Google Scholar] [CrossRef]
- Wu, G.; Feng, B.; Xu, J.; Zhu, X.-T.; Li, Y.-C.; Zeng, N.-K.; Hosen, I.; Yang, Z.L. Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers. 2014, 69, 93–115. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.-C.; Zhu, X.-T.; Zhao, K.; Han, L.-H.; Cui, Y.-Y.; Li, F.; Xu, J.-P.; Yang, Z.L. One hundred noteworthy boletes from China. Fungal Divers. 2016, 81, 25–188. [Google Scholar] [CrossRef]
- Halling, R.E.; Fechner, N.; Nuhn, M.; Osmundson, T.; Soytong, K.; Arora, D.; Binder, M.; Hibbett, D. Evolutionary relationships of Heimioporus and Boletellus (Boletales), with an emphasis on Australian taxa including new species and new combinations in Aureoboletus, Hemileccinum and Xerocomus. Aust. Syst. Bot. 2015, 28, 1–22. [Google Scholar] [CrossRef]
- Kuo, M.; Ortiz-Santana, B. Revision of leccinoid fungi, with emphasis on North American taxa, based on molecular and morphological data. Mycology 2020, 112, 197–211. [Google Scholar] [CrossRef]
- Singer, R. The Agaricales in Modern Taxonomy, 4th ed.; Koeltz Scientific Books: Koenigstein, Germany, 1986; pp. 1–981. [Google Scholar]
- Agerer, R. Fungal relationships and structural identity of their ectomycorrhizae. Mycol. Prog. 2006, 5, 67–107. [Google Scholar] [CrossRef]
- Tedersoo, L.; May, T.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2009, 20, 217–263. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, M.; Matheny, P.B. Asynchronous origins of ectomycorrhizal clades of Agaricales. Proc. R. Soc. B Boil. Sci. 2011, 279, 2003–2011. [Google Scholar] [CrossRef]
- Zeng, N.-K.; Cai, Q.; Yang, Z.L. Corneroboletus, a new genus to accommodate the southeastern Asian Boletus indecorus. Mycology 2012, 104, 1420–1432. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Lumyong, S.; Raspé, O. Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys 2019, 54, 1–29. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1967; pp. 1–252. [Google Scholar]
- Li, Y.C.; Yang, Z.L.; Tolgor, B. Phylogenetic and biogeographic relationships of Chroogomphus species as inferred from molecular and morphological data. Fungal Divers. 2009, 38, 85–104. [Google Scholar]
- Zhou, M.; Dai, Y.-C.; Vlasák, J.; Yuan, Y. Molecular Phylogeny and Global Diversity of the Genus Haploporus (Polyporales, Basidiomycota). J. Fungi 2021, 7, 96. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press Inc.: New York, NY, USA, 1990; p. 315. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Kauff, F.; Schoch, C.L.; Matheny, P.B.; Hofstetter, V.; Cox, C.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.-K.; Tang, L.-P.; Li, Y.-C.; Tolgor, B.; Zhu, X.-T.; Zhao, Q.; Yang, Z.L. The genus Phylloporus (Boletaceae, Boletales) from China: Morphological and multilocus DNA sequence analyses. Fungal Divers. 2012, 58, 73–101. [Google Scholar] [CrossRef]
- Schoch, C.L.; Robbertse, B.; Robert, V.; Vu, D.; Cardinali, G.; Irinyi, L.; Meyer, W.; Nilsson, R.H.; Hughes, K.; Miller, A.N.; et al. Finding needles in haystacks: Linking scientific names, reference specimens and molecular data for Fungi. Database 2014, 2014, bau061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-T.; Wu, G.; Zhao, K.; Halling, R.E.; Yang, Z.L. Hourangia, a new genus of Boletaceae to accommodate Xerocomus cheoi and its allied species. Mycol. Prog. 2015, 14, 1–10. [Google Scholar] [CrossRef]
- Gelardi, M.; Simonini, G.; Ercole, E.; Vizzini, A. Alessioporusand Pulchroboletus (Boletaceae, Boletineae), two novel genera for Xerocomus ichnusanus and X. roseoalbidusfrom the European Mediterranean basin: Molecular and morphological evidence. Mycology 2014, 106, 1168–1187. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.A.; Binder, M.; Halling, R.; Hibbett, D.; Soytong, K. The phylogeny of selected Phylloporus species, inferred from NUC-LSU and ITS sequences, and descriptions of new species from the Old World. Fungal Divers. 2012, 55, 109–123. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A Rapid Bootstrap Algorithm for the RAxML Web Servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Smith, S.A.; Dunn, C. Phyutility: A phyloinformatics tool for trees, alignments and molecular data. Bioinformatics 2008, 24, 715–716. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Nuhn, M.E.; Binder, M.; Taylor, A.F.; Halling, R.E.; Hibbett, D.S. Phylogenetic overview of the Boletineae. Fungal Biol. 2013, 117, 479–511. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Raspé, O.; Lumyong, S. Phylogenetic affinities of the sequestrate genus Rhodactina (Boletaceae), with a new species, R. rostratispora from Thailand. MycoKeys 2018, 29, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Raidl, S. The taxonomic position of Rhizopogon melanogastroides (Boletales). Mycotaxon 2002, 84, 221–228. [Google Scholar]
- Osmundson, T.W.; Robert, V.A.; Schoch, C.L.; Baker, L.J.; Smith, A.; Robich, G.; Mizzan, L.; Garbelotto, M.M. Filling Gaps in Biodiversity Knowledge for Macrofungi: Contributions and Assessment of an Herbarium Collection DNA Barcode Sequencing Project. PLoS ONE 2013, 8, e62419. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Pfister, D.H. Tuberculate ectomycorrhizae of angiosperms: The interaction betweenBoletus rubropunctus (Boletaceae) and Quercusspecies (Fagaceae) in the United States and Mexico. Am. J. Bot. 2009, 96, 1665–1675. [Google Scholar] [CrossRef][Green Version]
- Breitenbach, J.; Kränzlin, F. Fungi of Switzerland 3; Boletales and Agaricales Mykologia: Luzern, Switzerland, 1991; pp. 1–361. [Google Scholar]
- Courtecuisse, R.; Duhem, B. Mushroom and Toadstools of Britain and Europe; Harper Collins Publishers: New York, NY, USA, 1995; p. 432. [Google Scholar]
- Peck, C.H. Boleti of the United States; Bulletin of the New York State Museum: New York, NY, USA, 1889; Volume 2, p. 112. [Google Scholar]
- Bessette, A.E.; Bessette, A.R.; Fisher, D.W. Mushroom of Northeastern North America; Syracause University Press: New York, NY, USA, 1997; p. 328. [Google Scholar]
- Phillips, R. Mushrooms and Other Fungi of North America; Firefly Books: New York, NY, USA, 2010; p. 263. [Google Scholar]
- Wu, G.; Zhao, K.; Li, Y.-C.; Zeng, N.-K.; Feng, B.; Halling, R.E.; Yang, Z.L. Four new genera of the fungal family Boletaceae. Fungal Divers. 2015, 81, 1–24. [Google Scholar] [CrossRef]
- Smith, A.H.; Thiers, H.D. The Boletes of Michigan; University of Michigan Press: Ann Arbor, MI, USA, 1971; p. 428. [Google Scholar]
- Peck, C.H. Report of the state botanist. Ann. Rep. N. Y. St. Mus. Nat. Hist. 1896, 50, 77–159. [Google Scholar]
- Kuo, M. Retrieved from the Mushroom Expert. Com 2020. Available online: http://www.mushroomexpert.com/ (accessed on 30 September 2021).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.-X.; Wu, G.; Yang, Z.L. Four New Species of Hemileccinum (Xerocomoideae, Boletaceae) from Southwestern China. J. Fungi 2021, 7, 823. https://doi.org/10.3390/jof7100823
Li M-X, Wu G, Yang ZL. Four New Species of Hemileccinum (Xerocomoideae, Boletaceae) from Southwestern China. Journal of Fungi. 2021; 7(10):823. https://doi.org/10.3390/jof7100823
Chicago/Turabian StyleLi, Mei-Xiang, Gang Wu, and Zhu L. Yang. 2021. "Four New Species of Hemileccinum (Xerocomoideae, Boletaceae) from Southwestern China" Journal of Fungi 7, no. 10: 823. https://doi.org/10.3390/jof7100823
APA StyleLi, M.-X., Wu, G., & Yang, Z. L. (2021). Four New Species of Hemileccinum (Xerocomoideae, Boletaceae) from Southwestern China. Journal of Fungi, 7(10), 823. https://doi.org/10.3390/jof7100823





