Abstract
Saccharomyces cerevisiae var. boulardii is best known for its treatment efficacy against different gastrointestinal diseases. This probiotic yeast can significantly protect the normal microbiota of the human gut and inhibit the pathogenicity of different diarrheal infections. Several clinical investigations have declared S. cerevisiae var. boulardii a biotherapeutic agent due to its antibacterial, antiviral, anti-carcinogenic, antioxidant, anti-inflammatory and immune-modulatory properties. Oral or intramuscular administration of S. cerevisiae var. boulardii can remarkably induce health-promoting effects in the host body. Different intrinsic and extrinsic factors are responsible for its efficacy against acute and chronic gut-associated diseases. This review will discuss the clinical and beneficial effects of S. cerevisiae var. boulardii in the treatment and prevention of different metabolic diseases and highlight some of its health-promising properties. This review article will provide fundamental insights for new avenues in the fields of biotherapeutics, antimicrobial resistance and one health.
1. Introduction
According to the latest definition of the World Health Organization, probiotics are active microbes that stimulate the growth of other probiotic bacteria in the gut and possess beneficial health effects to the host [1]. These microorganisms are able to produce anti-carcinogenic, antioxidant and anti-mutagenic agents and induce protection against different bacterial diseases including diarrhea and respiratory tract infections. Saccharomyces cerevisiae var. boulardii is the most significant probiotic yeast species. S. cerevisiae var. boulardii is a eukaryotic organism that has been used in scientific investigations since the time of its discovery [2]. This model organism has unique importance because of its alterable and flexible genome. The genome of S. cerevisiae var. boulardii was completely sequenced in 1950 and a genome size of approximately 11.3 Mb was reported. It has approximately 6000 genes and 275 additional tRNA genes. Almost 23% of the S. cerevisiae var. boulardii’s genome is homologous to the hominid genome. This specific yeast is best known for its role in treating gastrointestinal diseases [3,4].
S. cerevisiae var. boulardii has gained the importance of the scientific community due to the production of different bioactive compounds [5]. This specie is an excellent protein source with high amino acid content, which is essential for the production of various foods and cosmetic supplements [6]. S. cerevisiae var. boulardii is also responsible for the formation of glutathione, an important antioxidant used in the food and drug industry [7]. The inactivated cells of S. cerevisiae var. boulardii are used as a rich protein source in probiotic feed supplements. Despite its high protein content and antioxidant nature, the thick and indigestible cell wall and high nucleic acid content limit the use of inactivated cells of S. cerevisiae var. boulardii in human food and nutrition. It can enhance its antioxidant properties by increasing the production of phytochemical constituents, such as isoflavones. S. cerevisiae var. boulardii is used preferably due to its unique digestible properties of starch and proteins. Reduction in trypsin-inhibitor activity and phytic acid content is responsible for its digestible behavior [8].
The oval to round cell shape of S. cerevisiae var. boulardii is composed of approx. 3 µm thickness and 2.5–10.5 µm length. This yeast is able to reproduce sexually and asexually by budding and unification [8]. The cell wall of S. cerevisiae var. boulardii is composed of a rigid inner polysaccharide layer with a 1,3-β-glucan branched structure while the outer layer is made up of mannoproteins. The total mass of S. cerevisiae var. boulardii in terms of dry weight is almost 30% and the estimated total polysaccharide and protein contents are 85% and 15%, respectively. Biochemical characterization of S. cerevisiae var. boulardii confirmed the presence of glucose, mannose and N-acetylglucosamine up to 90%, 20% and 2%, respectively. Glucose to glucose interaction is associated with β-1,3 and β-1,6 linkages. β-1,3 glucan is responsible for the elasticity and strength of the yeast cell wall. The lateral cell wall of S. cerevisiae var. boulardii is composed of straight chitin chains of 1–2% of total dry weight [9].
The nutritional value of S. cerevisiae var. boulardii is enhanced due to the presence of different minerals, vitamins and antioxidant compounds. Dietary yeast is composed of iron, manganese and copper, some trace minerals are also reported, i.e., ferric, manganic sulfate and cupric acetate [10]. Studies suggested that several toxic metals are easily accumulated by S. cerevisiae var. boulardii, which includes lead, cadmium, arsenic and mercury [11]. Nutritional yeast has the ability to enhance the energy level in an individual because of the presence of non-proteinaceous amino acids, proteinaceous amino acids and vitamin B, such as biotin, doxine, thiamin, vitamin B12 and riboflavin. It can also reduce antinutrient phytate levels and enhance the synthesis of folate. S. cerevisiae var. boulardii can also protect from bacterial infections along with increasing the glucose sensitivity to enhance the growth of skin, nails and hair [5].
Recently, it was observed that medical professionals are using nonpathogenic S. cerevisiae var. boulardii in the treatment of gut-related diseases. Clinical studies claimed that oral administration of S. cerevisiae var. boulardii can treat multiple gastrointestinal diseases including Traveler’s diarrhea [12], AIDS-associated diarrhea [13], antibiotic-associated diarrhea [14], Clostridium difficile-associated syndrome [15], Irritable Bowel Syndrome [16] and Crohn’s disease [17] (Figure 1). This yeast can be used for the treatment alone or can be administered in combination with other probiotics resulting in enhanced treatment efficiency. One hundred grams per day consumption of S. cerevisiae var. boulardii can induce beneficial effects on human health. S. cerevisiae var. boulardii cells have the ability to stick on the gastric and intestinal linings of the mucosa and actively prevail in the gastrointestinal tract of animals and humans [18]. The antineoplasmic effects of S. cerevisiae var. boulardii were reported with major findings. Oral administration of S. cerevisiae var. boulardii can inactivate epidermal growth factor receptor (EFR), which can further suppress EGFR-Erk and EGFR-Akt pathways resulting in induced apoptosis in tumor cells and reducing the level of cell colony formation and cancer cell proliferation. In vitro study claimed that S. cerevisiae var. boulardii consumption can inhibit the expression of HER2, HER-3 and IGF-1R genes which leads to the prevention of intestinal neoplasia [19]. Diarrhea caused by the continuous use of antibiotics can also be treated by S. cerevisiae var. boulardii in adults and children (Figure 1) [20]. A study claimed that this yeast can also effectively work against chronic permeability in patients with Crohn’s disease when administered orally for 3 months [21]. In HIV-linked diarrhea, exposure to a 3 g per day dose of S. cerevisiae var. boulardii can produce beneficial health effects [22]. The dose of S. cerevisiae var. boulardii in case of chronic diseases should be increased to meet the treatment criteria. Recently, an upsurge in multidrug-resistant organisms is reported due to the excessive consumption of antimicrobials [23]. Global healthcare authorities are trying to create awareness all over the globe via antibiotic stewardship programs, but the severity of antimicrobial resistance is continuously increasing [24]. To cope with this alarming situation, probiotics, especially S. cerevisiae and S. cerevisiae var. boulardii yeast, can be considered as an alternative method for the treatment of bacterial and fungal infections. A number of research and review articles describing the probiotic potentials of yeast and bacteria have been published in the last decade. A comprehensive review is needed to highlight the probiotic potential of S. cerevisiae var. boulardii in various aspects. Therefore, the aim of this review is to explore the diverse probiotic potential of S. cerevisiae var. boulardii through the combination of different meta-analyses. Utilization of S. cerevisiae var. boulardii as an alternate to antibiotics for the treatment and eradication of different metabolic diseases is investigated. Moreover, details of commercially available probiotic strains of S. cerevisiae var. boulardii and their clinical and beneficial detailed effects are provided. Finally, the significance of S. cerevisiae var. boulardii against cancer signaling cascades and safety attributes regarding its consumption among humans and livestock animals are thoroughly discussed.
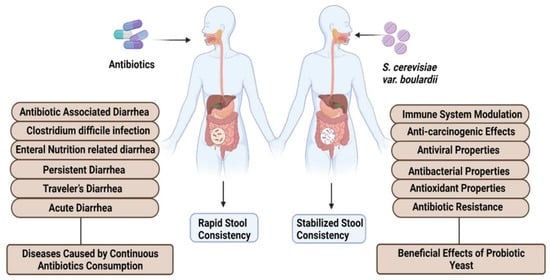
Figure 1.
Health-promoting effects of S. cerevisiae var. boulardii.
2. Enzymatic Potential of S. cerevisiae var. boulardii
S. cerevisiae var. boulardii can produce different enzymes which play a significant role in various industrial processes (Figure 2). Some active enzymes, i.e., maltase and invertase, have the potential to enhance the flavor of fermented products specifically in the food industry. Maltase is responsible for the conversion of malt sugar into normal sugar while invertase converts granulated sugar into regular sugar. Another enzyme, zymase, transforms normal sugar into CO2 and alcohol [25]. S. cerevisiae var. boulardii is able to produce intestinal enzymes including amylase, protease, cellulase and lipase and is unable to synthesize galactosidase, DNAase and gelatinase (Figure 2) [26]. S. cerevisiae var. boulardii has antibacterial properties due to the presence of extracellular protease enzymes and cell surface hydrophobicity [27]. This yeast enhances the concentration of the enzymes by the production of polyamines that trigger the cells of the intestine. Cell surface hydrophobicity is responsible for the adherence of S. cerevisiae var. boulardii yeast to the cell wall lining of the human intestine. S. cerevisiae var. boulardii is critically responsible for the production of ethanol in anaerobic conditions. This species can also show tolerance against a high level of ethanol and gastric discharge, including bile salts and intestinal acids, hence, eliminating toxic bacterial strains from the host body in the form of fecal matter [28]. Interestingly, this yeast can work against both Gram-positive and -negative bacteria and boost the host immunity. Despite its antibacterial properties, S. cerevisiae var. boulardii also showed resistance against all broad and narrow-spectrum antibiotic drugs and does not disturb the normal microbiota of the gastrointestinal tract of the host [28].
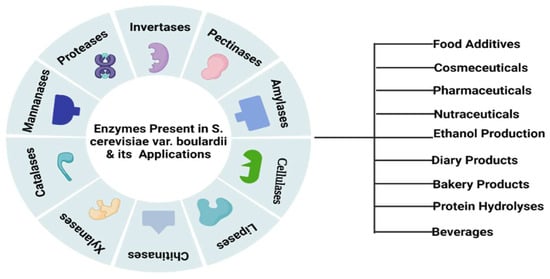
Figure 2.
Industrial significance of S. cerevisiae var. boulardii-based enzymes.
3. Factors Responsible for the Efficiency of S. cerevisiae var. boulardii as a Probiotic
Probiotics are being used to enhance treatment efficacy and to produce significant health benefits. S. cerevisiae var. boulardii is a unicellular, cost-effective active yeast species that has probiotic potential and is often used as a nutritional additive [29]. Different modes of actions were observed in favor of the host and against the antigenic microorganisms which include luminal action: (1) Antimicrobial activity: (a) Reduction in the intestinal bacterial growth [30], (b) lowering of gastrointestinal translocation of microbes [31], (c) nullifying the effect of bacterial pathogenicity [32], (d) reducing the binding affinity of the host cell with the bacterial population [33]. (2) Antitoxin effects: (a) obstructing the pathogenic receptor active sites [30], (b) enhancing the production of antibodies against Clostridium difficile toxin A [34], (c) mediating the synthesis of the phosphatases enzyme against Escherichia coli (E. coli) [35], (d) cleavage of pathogenic enzymatic proteins [35]. (3) Trophic action associated with intestinal linings: (a) reducing the expression of tumor necrosis factor-alpha (TNFα) gene and inhibiting programmed cell death [36], (b) enhancing the synthesis of glycoprotein in the intestinal brush border [37], (c) inducing the production of intestinal polyamines [37], (d) repairing fluid transport pathways [37], (e) stimulating the production of membrane enzymes (28). (4) Mediation of immune system: (a) stimulating the production of regulatory T cells [32], (b) enhancing the level of IgG antibody against Clostridium difficile toxin A [34], (c) improving the adherence of WBCs (White Blood Cells) to the endothelial cells [38,39].
Probiotics have gained global beneficial additive status to use as a potential feed supplement [40]. Human probiotic administration is based on the development and viability of probiotics in the intestinal lumen of the host organisms. Probiotic yeast has more survival chances in the stomach due to the presence of digestive enzymes, bile and gastrointestinal juices in comparison to probiotic bacteria [41]. The Food and Drug Administration (FDA) has approved certain probiotic strains which are potentially used for the benefit of humans, but S. cerevisiae and S. cerevisiae var. boulardii are the only probiotic yeast species that are commercially used for human benefits (Table 1) [42].

Table 1.
Commercially available probiotic yeast products.
S. cerevisiae var. boulardii has surpassed the affectivity of the commonest probiotic bacteria, i.e., lactobacillus due to its resistance against different antibiotics [43]. S. cerevisiae var. boulardii can be administered to patients as an alternative source of antibiotics due to its outrageous antibacterial properties. Probiotic consumption can also reduce the pathogenicity of harmful microbes present in the human gut [44]. The different strains of Saccharomyces sp., including S. boulardii, S. cerevisiae, and S. unisporus, also showed antibacterial and antiviral properties. These strains were used to enhance the probiotic potential of different human food supplements. These probiotic strains are also effective against acute and chronic diarrhea. The combination of S. cerevisiae var. boulardii with other probiotics of the same or different genus can also enhance the efficacy of human feed supplements [45].
Several intrinsic and extrinsic factors are directly implicated in the efficacy of S. cerevisiae var. boulardii as a probiotic (Figure 3).
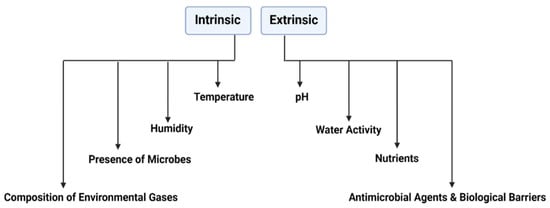
Figure 3.
Factors affecting the efficacy of S. cerevisiae var boulardii.
3.1. Temperature Fluctuations
S. cerevisiae var. boulardii strains can work effectively at a temperature range of 22–30 °C (Table 2), while other S. cerevisiae var. boulardii strains are functional at 37 °C temperature and some can survive below 20 °C temperature. As a probiotic, S. cerevisiae var. boulardii is present in the form of capsules. The heat-dried S. cerevisiae var. boulardii capsules could not survive at 25 °C after opening due to their reduced potency. They retain their efficacy when stored at a 4 °C refrigerator. Lyophilized S. cerevisiae var. boulardii capsules can survive at room temperature and are viable for 1 year approximately. Studies suggested that S. cerevisiae var. boulardii can grow best at 37 °C. However, the death phase of this yeast usually appeared at 55–56 °C [46,47].

Table 2.
Parameters for the survival of S. cerevisiae var. boulardii.
3.2. Water Activity aw and Relative Humidity
Water activity aw and relative humidity can produce synergistic effects. Survival of S. cerevisiae var. boulardii can be influenced by water activity. A study reported that the cells of S. cerevisiae var. boulardii showed a 0.98% value of water activity when refrigerated at −20 °C, which ultimately increased the survival rate of the yeast S. cerevisiae var. boulardii (Table 2). However, reduced water activity conditions can deteriorate the viability of S. cerevisiae var. boulardii’s cells. Water activity can also be influenced via relative humidity, specifically in the case of opened or uncovered foods. The viability of S. cerevisiae var. boulardii is reduced when the rate of water activity and environmental humidity decreases [48].
3.3. pH and Acidity
S. cerevisiae var. boulardii showed resistance against less pH and more acidic conditions. However, some yeast species are too fragile to bear such a hard environment. The ideal pH range for S. cerevisiae var. boulardii development and maturation is 2–8 (Table 2). Yeast species belonging to a genus other than Saccharomyces showed tolerance against extreme acidic and alkaline environments. Overall functionality of the yeast is better in the lyophilized form [29,47,51].
3.4. Antimicrobial Agents
S. cerevisiae var. boulardii showed antimicrobial properties due to these subsequent reasons: (i) synthesis of the extracellular enzyme, i.e., protease, it aids in the formation of colonic mucosa, (ii) excretion of toxins and SO2 gas, it can halt the efficacy of toxins released by Clostridium difficile, (iii) secretion of enzyme-based proteins, (iv) cell surface hydrophobicity and autoagglutination, it is responsible for the attachment of S. cerevisiae var. boulardii to the patient’s intestinal lining. The viability of different Gram-positive and -negative bacteria can easily be reduced by the negative influence of S. cerevisiae var. boulardii on the host organism [26].
3.5. Nutrient Media for the Growth of S. cerevisiae var. boulardii
Yeast can grow on different nutrient media including Yeast extract peptone dextrose media, Sabouraud dextrose agar, but Oxytetracyclin yeast agar media (OGA) is considered as the best medium for its growth (Table 2). Standard OGA media can be prepared by adding 8 g of yeast extract, 9 g of glucose, 12 g of nutrient agar and 0.1 uL of oxytetracycline in 500 mL of distilled H2O. Carbon (glucose, maltose, sucrose and fructose), nitrogen (Urea, peptone and powdered yeast extract) and a trace amount of minerals (zinc, copper, magnesium, sulfur) are required to enhance the growth of S. cerevisiae var. boulardii [49,50]. All these factors play a significant role in maintaining the viability of S. cerevisiae var. boulardii. The potential of this beneficial yeast may be disturbed when the optimum conditions of both intrinsic and extrinsic factors changes.
4. Clinical Significance of S. cerevisiae var. boulardii as a Probiotic in Acute and Chronic Diseases
4.1. Acute Diseases
4.1.1. Antibiotic-Associated Diarrhea
Antibiotic-associated diarrhea (AAD) occurs due to the continuous consumption of antibiotics for a longer period. The use of probiotics, mainly S. cerevisiae var. boulardii, is the commonest method for the treatment against AAD. A total of 8 (80%) out of 10 (100%) controlled experiments confirmed the efficacy of S. cerevisiae var. boulardii for the prohibition of AAD specifically in adult patients (Table 3). The beneficial impact of S. cerevisiae var. boulardii and the relative decline in AAD comparable to the control are categorized in the range of 7.4% and 25%, respectively [29]. The affectivity of S. cerevisiae var. boulardii against AAD in the pediatric population has also shown positive outcomes. Results of two meta-analyses confirmed the potential of S. cerevisiae var. boulardii against AAD with a pooled risk ratio of 0.47 and 0.43 and a 95% confidence interval [52].

Table 3.
Per capsule/tablet dose of S. cerevisiae var. boulardii for the treatment of different acute and chronic diseases.
4.1.2. Clostridium Difficile Infection (CDI)
Clostridium difficile (C. difficile) is a Gram-positive anaerobic rod-shaped bacteria that may cause antibiotic-associated Clostridium difficile diarrhea. It is responsible for the colon infection, it shows diarrhea (mild) to colon damage (severe) symptoms. Meta-analysis of six randomized control trials of different Saccharomyces strains including S. cerevisiae var. boulardii showed efficacy against CDI with a total risk ratio of 0.59 [53].
The beneficial impact of S. cerevisiae var. boulardii and the relative decline in CDI comparable to the control were categorized within the range of 19% and 33.3%, respectively (Table 3). S. cerevisiae var. boulardii can prevent diarrhea caused by toxin A. It can also suppress colon inflammation and can block the intestinal toxin receptor sites via protease liberation. It can modulate the immune response by stimulating the production of IgA immunoglobulins. Moreover, probiotic use can block the activation of several kinases, Erk1/2 and interleukin 8 expression (Figure 4) [53].
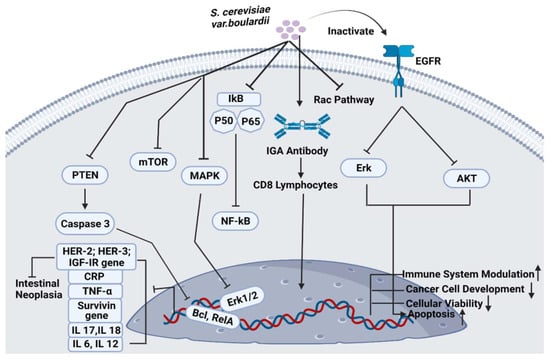
Figure 4.
Pathways associated with S. cerevisiae var. boulardii.
4.1.3. Acute Diarrhea
S. cerevisiae var. boulardii administered to patients involved in two randomized control group trials showed clinical efficacy against acute diarrhea as compared to the control (Table 3). S. cerevisiae var. boulardii consumption among 100 patients of an age less than 15 for 7 days resulted in reduced stool frequency and stabilized the normal stool condition [54]. A meta-analysis conducted among more than 600 patients that administered the S. cerevisiae var. boulardii probiotic strains for 60 days significantly reduce the rapid stool frequency [55]. Another meta-analysis of seven randomized controlled trials claimed to stabilize the childhood diarrhea consistency within 24 h as compared to the placebo treatment [56].
4.1.4. Persistent Diarrhea
Two randomized controlled trials suggested that S. cerevisiae var. boulardii significantly enhances treatment efficacy specifically in children with persistent diarrhea (Table 3). The beneficial impact of S. cerevisiae var. boulardii and the relative decline in persistent diarrhea comparable to the control was 50%, respectively. However, a meta-analysis of S. cerevisiae var. boulardii against persistent diarrhea among pediatric and young populations has not been performed up till now [57].
4.1.5. Enteral Nutrition-Related Diarrhea
Diarrhea is the major complication associated with total enteral nutrition (TEN) and can also cause fluctuations in short-chain fatty acids (SCFA). Diabetes, gastrointestinal infection and malabsorption-related disorders are responsible for diarrhea-associated TEN (Figure 5). Schneider et al. reported that patients who received S. cerevisiae var. boulardii can significantly enhance the levels of short-chain fatty acids in 10 TEN patients as compared to the normal controls. This treatment could increase the SCFA level in high stool frequency. The beneficial impact of S. cerevisiae var. boulardii and the relative decline in TEN-associated diarrhea comparable to control are categorized within the range of 5% and 8.2% in three randomized control trials, respectively [37].
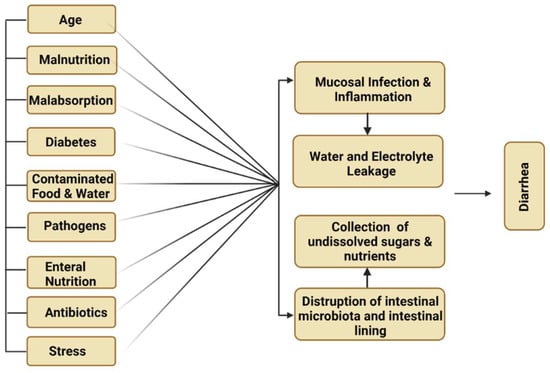
Figure 5.
Major causes of diarrhea.
4.1.6. Traveler’s Diarrhea
Traveler’s diarrhea is a common digestive illness that is responsible for frequent stool discharge. It occurs due to the intake of contaminated food or water (Figure 5). Twelve randomized control trials of S. cerevisiae var. boulardii and other probiotic strains significantly reduced the severity of the infection caused by Traveler’s diarrhea in children (Table 3). The beneficial impact of S. cerevisiae var. boulardii and the relative decline in Traveler’s diarrhea comparable to the control are categorized within the range of 5% and 11% in two randomized control trials, respectively [58].
4.2. Chronic Diseases
4.2.1. Cancer
S. cerevisiae var. boulardii is being potentially used to inhibit cancer cell development and progression (Figure 4). It was observed that this probiotic yeast can reduce the tumorigenic effects of colorectal cells in humans. In vivo, high-throughput metagenomic analysis of 281 stool samples confirmed that S. cerevisiae var. boulardii has significantly inhibited colorectal cancer metastasis by stimulating cancer cell apoptosis and promoting gastrointestinal health via immune modulation. S. cerevisiae var. boulardii significantly downregulates the expression of various tumor-causing genes including TNFα, Interleukin-1β and Interleukin-17, the expression of NF-kB and mTOR signaling cascades was also inhibited (Figure 4). However, the activity of different cytokines was not affected by S. cerevisiae var. boulardii treatment. HCT116 and DLD1 colorectal cell lines were used to analyze the apoptotic behavior of colorectal cells after the administration of S. cerevisiae var. boulardii. Results confirmed the presence of an enhanced percentage of apoptosis in probiotic yeast-treated cells [59].
4.2.2. Ulcerative Colitis
Broad-spectrum antibiotics are conventionally used to treat ulcerative colitis but due to antibiotic resistance, their efficacy has been reduced by a substantial level. Probiotics, especially S. cerevisiae var. boulardii and its derivatives, act as an alternative method for the maintenance of normal gut microbiota and help to treat chronic colitis diseased patients [60]. Studies suggested that the pathogenic strain of E. coli, known as adherent-invasive E. coli (AIEC), showed a strong binding affinity with the small intestinal lining of Crohn’s disease patients. This Gram-negative bacteria can easily invade the intestine of patients. Patients with Crohn’s disease showed strong adherence between AIEC bacteria due to its FimH adhesion potential and overexpressed mannose residues, which are present on the surface of intestinal glycoprotein CEACAM6 (carcinoembryonic antigen-related cell adhesion molecule). In vivo results reported that S. cerevisiae var. boulardii significantly blocked the adherence potential of LF82 to the intestinal brush border. Probiotic yeast also lowered the pro-inflammatory cytokine level and was confirmed to treat the pathogenesis of ulcerative colitis [61].
4.2.3. Crohn’s Disease (CD)
Typically, Crohn’s disease is a part of chronic inflammatory bowel disease, which can cause digestive inflammation, abdominal pain, weight loss, watery stool and malnutrition. Individuals with chronic Crohn’s disease may also come across inflammation of skin, liver, joints, anemia, kidney stones and maldevelopment. Bacteria associated with Crohn’s disease can damage the gastrointestinal tract (GI), especially the small intestine, colon and can cause erratic and multiwall GI inflammation. Dalmasso and colleagues reported that consumption of S. cerevisiae var. boulardii can significantly reduce the level of CD, can control chronic inflammation and reinforce epithelial reformation [62]. In a pilot study, 31 patients with Crohn’s disease were randomly treated with S. cerevisiae var. boulardii or an antimicrobial drug for 12 weeks. Patients treated with probiotics considerably reduced colonic permeability as compared to the antimicrobial drug-treated patients. Another pilot study of 20 patients with Crohn’s disease, who were administered S. cerevisiae var. boulardii for 49 days, showed remarkable improvement in the patient’s health (Table 3). S. cerevisiae var. boulardii consumption after steroidal therapy does not produce health-promoting effects on Crohn’s disease patients [22].
4.2.4. Vaginal Candidiasis
Vaginal Candidiasis can be considered the most common fungus-associated vaginal infection globally. Candida albicans is the major causative agent of this disease. Vaginal Candidiasis is typically caused by large antibiotic consumption, which produces fluctuations in the normal composition of the vaginal microbiota. Studies have reported the efficacy of oral and intramuscular administration of S. cerevisiae var. boulardii-based probiotics [63] (Table 3). Vaginal inoculation of S. cerevisiae var. boulardii live yeast or inactivated whole yeast can significantly lower the growth of Candida albicans in mice vaginas. Both these yeast types cause S. cerevisiae var. boulardii and fungus interaction which results in prohibiting the cohesion of Candida albicans to the vaginal epithelial cells. Probiotic administration can significantly reduce the pathogenicity of Candida albicans by lowering its ability to transform itself from yeast to mycelium and the capability of exhibiting aspartyl proteases. However, the efficacy of live yeast is greater as compared to the inactivated whole yeasts [63].
4.3. Health Benefits of S. cerevisiae var. boulardii as a Probiotic
4.3.1. Antibacterial and Antiviral Properties
The efficacy of S. cerevisiae var. boulardii on gastrointestinal microbiota has been critically investigated. S. cerevisiae var. boulardii can opt for different modes of action for antibacterial and antiviral activities in the human gut, which includes: (i) direct inhibition of pathogenic intestinal microbes and normalizing the pH of the gastrointestinal tract by reducing the pathogenicity of toxic microorganisms, (ii) producing an indirect impact on the gut microenvironment, (iii) producing an immunomodulatory effect on the host body [64]. The antibacterial effects of S. cerevisiae var. boulardii against different Gram-positive and -negative bacterial and viral pathogens including Bacillus anthracis, Shigella, E. coli, Vibrio cholera, Helicobacter pylori, C. difficile, Salmonella and Rotavirus have been previously reported [65]. S. cerevisiae var. boulardii can adhere to the toxin released by Vibrio cholera and inhibit its activity. The enhanced fluidity of sodium and chloride produced by Vibrio cholera can significantly be reduced by S. cerevisiae var. boulardii via inhibition of cyclic adenosine monophosphate-induced chloride secretion. Therefore, probiotic yeast can directly treat C. difficile disease by targeting its toxins and receptors. This infection can also be prevented by the action of the protease enzyme of S. cerevisiae var. boulardii against receptors and bacterial toxins. Moreover, this yeast can also block the pro-inflammatory pathways which are triggered by the toxins of C. difficile. It can also inhibit the expression of IL-8 and Erk1/2 genes and the activity of the NF-KB pathway (Figure 4) [66]. Anthrax is a bacterial infection that is produced by virulence factors with a protective antigen, lethal factors and edematogenic factors, these peptides are responsible for causing morphological changes in the epithelial cells of the host [67]. The probiotic potential of S. cerevisiae var. boulardii against Salmonella enterica Typhimurium has been analyzed previously. Studies suggested that probiotic yeast can reduce the morbidity and mortality rate of the disease caused by pathogenic S. Typhimurium bacteria. It can limit the entry of bacteria into the host intestinal epithelial cells by inactivating the Rac pathway (Figure 4). S. cerevisiae var. boulardii sticks to the surface of pathogenic bacteria and reduces its multiplication and growth by accelerating bacterial excretion via the stools [68]. S. cerevisiae var. boulardii can also produce antibacterial properties against peptic ulcer disease caused by Gram-negative Helicobacter pylori bacteria, which cause gastrointestinal tract infection and chronic gastric inflammation in the infected stomach. S. cerevisiae var. boulardii decreases the cytokine and chemokine levels into the stomach and significantly produces IgA antibodies against the toxic Helicobacter pylori [66]. This probiotic yeast is also effective against infection caused by viruses including rotavirus. It can suppress the level of oxidative stress in the host cells that are infected with rotavirus and also reduce the Cl- excretion caused by rotavirus [69].
4.3.2. Immune System Modulation
The probiotic effect of S. cerevisiae var. boulardii on the human immune system has been thoroughly investigated. The mechanisms that are mediated by the action of S. cerevisiae var. boulardii yeast are: (i) stimulation in the host immune activity, (ii) production of immunoglobulins, (iii) synthesis of cytokines and chemokines, (iv) assistance in the development of immune cells and (v) stimulate immune priming [70]. In a clinical study, S. cerevisiae var. boulardii administered to a child suffering from gastroenteritis showed a considerable rise in the IgA levels and a reduction in CRP (C-reactive protein) levels. After a 7-day treatment, a significant increase in the rate of CD8 lymphocytes in the S. cerevisiae var. boulardii-treated group as compared to the control group (Figure 4) [71]. A combination of yeast and bacterial probiotic is capable of treating child-associated diarrhea. Results showed a significant increase in immune system modulation and CD3+, CD4+ and Th1/Th2 levels. Moreover, the clinical outcomes of the diarrheal disease were remarkably increased in the probiotic-treated group [72]. When the pathogenic bacteria enter into the gastrointestinal tract of the host, S. cerevisiae var. boulardii releases IgA antibodies which bind to the bacteria and excrete it from the host’s body via feces. S. cerevisiae var. boulardii consumption frequently increased the release of IgA antibodies when the host is exposed specifically to C. difficile toxin A [73]. A study on germ-free mice reported that yeast raised the level of IgM, cytokines and the total number of liver macrophages and cleared the infection caused by pathogenic bacteria from the host intestine in the treated group [74]. Briefly, S. cerevisiae var. boulardii can mediate different hormonal and molecular responses which are responsible to inhibit the activity of intestinal pathogens. Generally, the defensive mechanism of probiotic yeasts against several toxins is executed by stimulating the production of cytokines and interleukin (IL)-1β, IL-12, IL-6, TNFα, and IL-10 [75]. The in vitro and in vivo studies of S. cerevisiae var. boulardii showed significant modulation in the host early immune response, through this, the host body can show resistance against most microbial communities. It can also keep the equilibrium between pro and anti-inflammatory immune responses by the upregulation of several cytokines and inhibit the immune cell proliferation and maturation [8].
4.3.3. Antioxidant Properties
S. cerevisiae var. boulardii showed comprehensive antioxidant properties in the previous studies. S. cerevisiae var. boulardii extracted from the fermentation of guajillo pepper showed 66.1% alleviation in cholesterol level when placed in the incubator for 2 days. In a study, DPPH (1,1-diphenyl-2-picryl-hydrazyl free radical) assay calculated 63% of the total antioxidant potential of S. cerevisiae var. boulardii [34]. A DPPH scavenging assay of S. cerevisiae var. boulardii yeast also showed 2.3 mgTE/L antioxidant activity, which is beneficial for the manufacturing of beer with enhanced probiotic potential. Moreover, this assay also exhibited a 40% antioxidant level of S. cerevisiae var. boulardii yeast extracted from different Brazilian local fermented foods [76,77]. Studies suggested that S. cerevisiae var. boulardii whole cells possess superior antioxidant properties as compared to its extracts, it can be due to the presence of a high level of 1/3-b-D-glucan in the S. cerevisiae var. boulardii cell wall structure. Insoluble glucan and metabolites including phenyl ethyl alcohol, vitamin B6, cinnamic acid, vanillic acid and erythromycin are responsible for high antioxidant properties [78]. In a comparative study, raw and miscellaneous S. cerevisiae var. boulardii extracts were investigated for antioxidant level by DPPH test, superoxide radical scavenging assay by the total number of bioactive compounds. Results of this study confirmed the maximum antioxidant potential of S. cerevisiae var. boulardii raw extracts as compared to other extracts. S. cerevisiae var. boulardii antioxidant properties also showed beneficial effects on clinical therapeutics [79]. Another study demonstrated that S. cerevisiae var. boulardii can induce antioxidant activities of gastrointestinal-induced oxidative stress. In a human organ culture study, S. cerevisiae var. boulardii showed a reduction in the level of oxidative stress specifically in the rotavirus infected cells via human gastrointestinal examination [80].
4.3.4. Control of Antibiotic Resistance
S. cerevisiae var. boulardii showed resistance against both broad and narrow-spectrum antibiotic drugs. However, it cannot resist antifungal drugs and therapies. It is the most suitable and active therapeutic agent for the prevention and medication of all diarrheal-associated diseases which are specifically caused by the fluctuation in the normal gastrointestinal microbiota in patients with continuous antibiotics consumption for a longer run. Furthermore, bacterial probiotics are unable to exhibit such properties [34]. In order to increase the quality of functional food, different probiotic strains have been added to human foods and dietary supplements. The safety of human administered probiotics is significant due to their resistance against various antimicrobials [43]. Antibiotics are considered the fundamental tool to fight against pathogenic bacteria. These pathogenic microorganisms can acquire antibiotic resistance genes against advanced antibiotic drugs [81]. This resistance mechanism can negatively affect the treatment strategy against common bacterial infections [82]. Studies suggested that probiotic bacteria and yeast can act as the reservoir of antibiotic resistance genes. Antibiotic resistance in probiotic strains due to intrinsic or extrinsic mutations does not harm the host gastrointestinal tract. Moreover, they are useful to regain the lost gut microbiota of the host after continuous antibiotic intake. However, these probiotics can horizontally transpose resistance genes in harmful microbes. Tetracyclin and vancomycin resistance genes have been observed in various food and gut microbes [83].
5. Mechanisms of Action of S. cerevisiae var. boulardii Yeast
The responsibility of the host gut microbiota is not limited to just providing protection against pathogenic microbes [84]. It can also contribute to various mechanisms including cellular adhesion, reestablishment of lost gut microbiota, mediation of cancer signaling cascades, competition with pathogenic microbes, mucin production and regulation of nutritional and trophic effects. Adequate administration of S. cerevisiae var. boulardii can target and eliminate disease-causing microbes from the gastrointestinal tract of the host [85].
5.1. General Mode of Actions
Host gut dysbiosis due to the pathogenic microbial attack may reduce the overall probiotic bacterial load in the host gastrointestinal tract which may cause inflammation and secondary infections [86]. The adhesion potential of S. cerevisiae var. boulardii against pathogenic microbes may actively contribute to neutralizing the mechanism of antigen translocation from the gastrointestinal tract to other parts of the host body [87]. Continuous administration of S. cerevisiae var. boulardii for several weeks can stabilize the host gut microenvironment by reducing the severity of the disease and eventually eradicating the disease from the host body. Some probiotics are frequently eliminated from the host body, but before the elimination, they would have significantly modulated the host immune system. While other probiotics may recognize and bind to the active sites of the host intestinal mucosal layers [88]. Mucin production by intestinal epithelial cells of the host may also be influenced by the presence of probiotics in the host gut. Both pathogenic and beneficial microbes compete for binding to the gastrointestinal tract of the host. Cell wall proteins and mannose residues of S. cerevisiae var. boulardii are responsible for the direct binding of probiotic yeast to the intestinal receptors and reducing the probability of pathogenic microbes binding to the active sites [89]. However, if pathogens already adhere to the active sites, then probiotic administration may significantly induce the expression of exogenous sugars which can obstruct the binding of pathogenic microbes to the intestinal mucosal layers.
5.2. Mechanisms of Cancer Signaling Cascades
Cancer is considered as a major public health concern globally [90]. It is the leading cause of death not only in developing countries but also in developed countries. This deadly disease is a combination of more than 100 different diseases [91]. There are different types of cancer; all types have the same origin which is the abnormal growth of the cells. The growth rate of healthy and cancerous cells are different, healthy cells grow and proliferate in a controlled manner resulting to keep the body alive, while tumor cells grow in an abnormal fashion leading to cause anti-apoptotic effects [92]. To reduce the expression of oncogenes and protooncogenes, several clinical therapeutics including anticancer drugs, chemotherapy, radiotherapy and other strategies are conventionally used, however, the use of probiotics acts as an alternative treatment method for cancer prevention.
S. cerevisiae var. boulardii can significantly induce cancer signaling cascades by upregulating the expression of apoptotic proteins and downregulating the expression of protooncogenes and oncogenes. A recent study investigated the anti-tumorigenic activity of S. cerevisiae var. boulardii against gastric cancer cell lines and analyzed total cellular viability, apoptotic effects and activity of survivin gene after 3 days. Results of this study reported that targeted probiotic yeast significantly reduced the level of cellular viability, which stimulate apoptosis and lowered the activity of the survivin gene in gastric cancer cells (Figure 4). This study strongly recommends the use of S. cerevisiae var. boulardii as a potential anti-gastric-cancer treatment therapy [93]. The probiotic potential of this yeast was also reported against human colorectal cancer cell lines (HT-29) and animal models. To evaluate the efficacy of S. cerevisiae var. boulardii on cell growth, development and apoptosis, this yeast was thoroughly spread over the HT-29 cells by using 4′,6-diamidino-2-phenylindole (DAPI) dye and 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The expression profiles of PTEN/caspase-3, Bclxl and RelA genes were evaluated by real-time PCR [94]. After 24 h, the activity of PTEN and caspase-3 gene was increased. However, the expression of Bclxl and RelA genes was significantly reduced (Figure 4). After 2 days, the MTT assay showed inhibition in the growth of probiotic treated HT-29 cells. In another study, 1,3-beta-glucan part of the S. cerevisiae var. boulardii yeast showed anti-neoplastic effects on rat colon cancer cells when treated with dimethylhydrazine and S. cerevisiae var. boulardii orally [94]. Chen et al. reported that S. cerevisiae var. boulardii consumption can significantly block the activity of epidermal growth factor receptors when exposed to the targeted yeast and inhibit the Erk and Akt pathway (Figure 4). According to the results, S. cerevisiae var. boulardii reduced the growth and proliferation of cancer cells and induces cancer cell apoptosis [19].
6. Discussion
Probiotics are non-digestible constituents of food, and when added in food or diet, confer useful and healthy effects to the host and stimulate the growth of a confined quantity of colon bacteria [95]. Natural strains of S. cerevisiae var. boulardii observed harsh environmental conditions as compared to the strains artificially cultured in the lab. This probiotic yeast has advanced conventional survival strategies which ensure its viability for the long run [96]. Mostly, the natural strains of this beneficial yeast are present in the nutrient-enriched soil environment. Some other environmental habitats of S. cerevisiae var. boulardii are the leaves and trunk surfaces of different medicinal and non-medicinal plants. It is also naturally present in intact grapes and other citrus fruits [27]. The natural transmission of this yeast to the human body is possible by the consumption of grapes, grape wine and different fruits. Studies suggested that this yeast is also insect-borne and is observed in wasps, Drosophila and other insects. These insects absorbed S. cerevisiae var. boulardii by feeding on the grapes and other fruits [97]. S. cerevisiae var. boulardii has shown direct and indirect effects on functional (fermented) food stuff. Direct effect indicates host-organism relationship, while indirect effects demonstrate the biogenic upshot (due to taking of microbial metabolites as a result of fermentation). This advances towards the efficient consequences of probiotics that seem to be applied in non-dairy food items as products related to chocolate, chewing gum, biscuit, honey, cereals, cakes, dressing, sweetness and tea [98]. In general, S. cerevisiae var. boulardii in the food industry somehow has difficulty in its multiplication and survival rate because of the distress conditions of the gastrointestinal tract [99].
To ensure the shelf-life of probiotics, novel probiotics are being designed through microencapsulation technology that opposes environmental conditions. Various factors can contribute to the beneficial aspects of probiotics but its proper mechanism of action is still vague. Studies suggested that lactation performance of the dairy animals was improved by S. cerevisiae var. boulardii yeast supplementation. It is found that the increased milk yield might be due to the stimulatory effect of probiotic yeast on the animal microbiota, which in turn increases cellulose digestion [100].
The presence of functional food in animal diets is responsible for increasing the productivity of livestock. Livestock can significantly improve human nutrition by providing essential nutrients in the form of milk, meat, and eggs [101]. An inadequate diet can drastically damage the health of livestock and reduced the overall yield. Due to poor feeding, animals are generally suffering from digestive and respiratory diseases leading to insufficient digestion and consequently retarded growth and productive performance. Dietary supplementation of S. cerevisiae var. boulardii is a viable and safe option for farmers to enhance the production of lactating dairy cattle and heifers [102]. This yeast has gained the Generally Recognized As Safe (GRAS) status from the Food and FDA, thus, can significantly be used to improve the animal feed supplements [103]. Moreover, the probiotic dose administered to animals is dependent on the (i) composition of feed, (ii) age of the animal, (iii) physical health of the animal and (iv) nature of the digestive system of the animal [29].
S. cerevisiae var. boulardii showed its applications in the wine industry for the benefit of humans. The natural grape was considered as a potential habitat of S. cerevisiae var. boulardii due to its high sugar content and acidic pH [104]. This yeast can significantly cope with all the fermentation stresses of the environment and has gained “the wine yeast” status and it is considered an important component of the wine industry globally. Moreover, the biosynthesis of primary and secondary alcohols is responsible due to their fermenting ability [105]. Theobroma cacao grains are significantly used for the manufacturing of chocolate. Fermentation of cacao grains can reduce the bitter and acrid effects of these grains. Direct exposure of cacao grains to probiotic yeast can induce fermentation reaction resulting in the production of ethanol and useful secondary metabolites [106]. The pectinolytic enzymes of S. cerevisiae var. boulardii can potentially metabolize citric acid produced by cacao grains. The increased growth of S. cerevisiae var. boulardii under high pH and stress conditions contributed to ethanol production [104]. S. cerevisiae and S. cerevisiae var. boulardii have remarkable applications in the bread and bakery industry. Sourdough, water and flour mixture are required for the manufacturing of bread. Various types of flours are commercially available which include spelt, barley, maize, einkorn, rye, khorasan, sorghum and many others [107]. Probiotic yeast and lactic acid bacteria are the main components of sourdough. A total of 2% of the fermenting yeast is added for the biosynthesis of bread. Atmospheric oxygen enters into the dough during dough mixing, which is adequately consumed by the yeast cells. Moreover, in an oxygen-limited environment, the rate of yeast cell reproduction was hindered and dough started to rise due to the fermentation process [108].
7. Conclusions
Continuous upsurge in multidrug-resistant organisms is responsible for causing millions of deaths annually. To control the spread of antimicrobial resistance, probiotic yeast S. cerevisiae var. boulardii can be considered as an alternative method for the treatment of bacterial and fungal infections. Several clinical and therapeutic studies confirmed the efficacy of S. cerevisiae var. boulardii against different pathogenic gastrointestinal diseases. The probiotic nature of this yeast has surpassed the effectiveness of different probiotic bacteria due to its gut microbiota protection potential. This probiotic yeast can actively participate in the manufacturing of bread, bakery products, wine, chocolate and large-scale bioethanol production. The consumption of S. cerevisiae var. boulardii in adequate amounts can also enhance the overall yield of milk and meat in poultry and livestock. The prescribed S. cerevisiae var. boulardii dose can also reduce the probability of co-morbidities that are caused by the continuous consumption of antibiotics for a long period. The combination of S. cerevisiae var. boulardii with other probiotics can enhance the treatment efficacy and reduce the pathogenicity of the disease. Despite its beneficial aspects, the use of this probiotic yeast should be according to the prescription of a physician. Moreover, this study will open up new insights for the development of novel probiotic strains, which will reduce the transmission of antimicrobial resistance genes among humans and farm animals.
Author Contributions
R.A., H.W. and S.G. conceptualization, data analysis and wrote the first draft of the manuscript; G.M.A. revised the tabulated data and helped in graphical work; R.A. helped in data collection and drawing figures; J.A. critically revised the article; A.M.E. and S.H.A. provided funding and su-pervision. They also critically revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This article received external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
A.M.E. extends his appreciation to the Deanship of Scientific Research at Jouf University for funding his work through Research Grant number DSR-2021-01-0363.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Abhari, K.; Eş, I.; Soares, M.B.; Oliveira, R.B.A.; Hosseini, H.; Rezaei, M.; Balthazar, C.F.; Silva, R.; Cruz, A.G.; et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Batista, T.M.; Marques, E.T.A., Jr.; Franco, G.R.; Douradinha, B. Draft genome sequence of the probiotic yeast Saccharomyces cerevisiae var. boulardii strain ATCC MYA-796. Genome Announc. 2014, 2, e01345-14. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, P.B.; Tarasov, O.V.; Matveenko, A.G.; Radchenko, E.A.; Sopova, J.V.; Polev, D.E.; Inge-Vechtomov, S.G.; Dobrynin, P. V Genome sequencing and comparative analysis of Saccharomyces cerevisiae strains of the Peterhof genetic collection. PLoS ONE 2016, 11, e0154722. [Google Scholar] [CrossRef] [PubMed]
- Lazo-Vélez, M.A.; Serna-Saldívar, S.O.; Rosales-Medina, M.F.; Tinoco-Alvear, M.; Briones-García, M. Application of Saccharomyces cerevisiae var. boulardii in food processing: A review. J. Appl. Microbiol. 2018, 125, 943–951. [Google Scholar] [CrossRef]
- The Benefits of Yeast. Available online: https://www.exploreyeast.com/article/the-benefits-of-yeast (accessed on 10 March 2022).
- Badr, H.; El-Baz, A.; Mohamed, I.; Shetaia, Y.; El-Sayed, A.S.A.; Sorour, N. Bioprocess optimization of glutathione production by Saccharomyces boulardii: Biochemical characterization of glutathione peroxidase. Arch. Microbiol. 2021, 203, 6183–6196. [Google Scholar] [CrossRef]
- Ansari, F.; Samakkhah, S.A.; Bahadori, A.; Jafari, S.M.; Ziaee, M.; Khodayari, M.T.; Pourjafar, H. Health-promoting properties of Saccharomyces cerevisiae var. boulardii as a probiotic; characteristics, isolation, and applications in dairy products. Crit. Rev. Food Sci. Nutr. 2021, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H. The Ideal Yeast Cell Wall Product for Young Animals. Available online: https://www.poultryworld.net/Specials/Articles/2021/6/The-ideal-yeast-cell-wall-product-for-young-animals-754687E/ (accessed on 8 March 2022).
- Łukaszewicz, M. Saccharomyces cerevisiae var. boulardii—Probiotic Yeast. In Probiotics; IntechOpen: London, UK, 2012. [Google Scholar]
- Średnicka, P.; Juszczuk-Kubiak, E.; Wójcicki Michałand Akimowicz, M.; Roszko, M. Probiotics as a biological detoxification tool of food chemical contamination: A review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef]
- Bisson, J.-F.; Hidalgo, S.; Rozan, P.; Messaoudi, M. Preventive effects of different probiotic formulations on travelers’ diarrhea model in wistar rats. Dig. Dis. Sci. 2010, 55, 911–919. [Google Scholar] [CrossRef]
- Saint-Marc, T.; Blehaut, H.; Musial, C.; Touraine, J.L. AIDS-related diarrhea: A double-blind trial of Saccharomyces boulardii. Sem. Hop. Paris 1995, 71, 735–741. [Google Scholar]
- Ehrhardt, S.; Guo, N.; Hinz, R.; Schoppen, S.; May, J.; Reiser, M.; Schroeder, M.P.; Schmiedel, S.; Keuchel, M.; Reisinger, E.C.; et al. Saccharomyces boulardii to prevent antibiotic-associated diarrhea: A randomized, double-masked, placebo-controlled trial. Open Forum Infect. Dis. 2016, 3, ofw011. [Google Scholar] [CrossRef]
- Carstensen, J.W.; Chehri, M.; Schønning, K.; Rasmussen, S.C.; Anhøj, J.; Godtfredsen, N.S.; Andersen, C.Ø.; Petersen, A.M. Use of prophylactic Saccharomyces boulardii to prevent Clostridium difficile infection in hospitalized patients: A controlled prospective intervention study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Leventogiannis, K.; Gkolfakis, P.; Spithakis, G.; Tsatali, A.; Pistiki, A.; Sioulas, A.; Giamarellos-Bourboulis, E.J.; Triantafyllou, K. Effect of a preparation of four probiotics on symptoms of patients with irritable bowel syndrome: Association with intestinal bacterial overgrowth. Probiotics Antimicrob. Proteins 2019, 11, 627–634. [Google Scholar] [CrossRef]
- Guslandi, M.; Mezzi, G.; Sorghi, M.; Testoni, P.A. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig. Dis. Sci. 2000, 45, 1462–1464. [Google Scholar] [CrossRef]
- Tomičić, Z.M.; Čolović, R.R.; Čabarkapa, I.S.; Vukmirović, Đ.M.; Đuragić, O.M.; Tomičić, R.M. Beneficial properties of probiotic yeast Saccharomyces boulardii. Food Feed Res. 2016, 43, 103–110. [Google Scholar] [CrossRef]
- Chen, X.; Fruehauf, J.; Goldsmith, J.D.; Xu, H.; Katchar, K.K.; Koon, H.-W.; Zhao, D.; Kokkotou, E.G.; Pothoulakis, C.; Kelly, C.P. Saccharomyces boulardii inhibits EGF receptor signaling and intestinal tumor growth in Apcmin mice. Gastroenterology 2009, 137, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G. Antibiotic-associated diarrhea. N. Engl. J. Med. 2002, 346, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Garcia Vilela, E.; De Lourdes De Abreu Ferrari, M.; Oswaldo Da Gama Torres, H.; Guerra Pinto, A.; Carolina Carneiro Aguirre, A.; Paiva Martins, F.; Marcos Andrade Goulart, E.; Sales Da Cunha, A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Pothoulakis, C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap. Adv. Gastroenterol. 2012, 5, 111–125. [Google Scholar] [CrossRef]
- Waseem, H.; Ali, J.; Sarwar, F.; Khan, A.; Rehman, H.S.U.; Choudri, M.; Arif, N.; Subhan, M.; Saleem, A.R.; Jamal, A.; et al. Assessment of knowledge and attitude trends towards antimicrobial resistance (AMR) among the community members, pharmacists/pharmacy owners and physicians in district Sialkot, Pakistan. Antimicrob. Resist. Infect. Control 2019, 8, 67. [Google Scholar] [CrossRef]
- Waseem, H.; Jameel, S.; Ali, J.; Jamal, A.; Ali, M.I. Recent advances in treatment technologies for antibiotics and antimicrobial resistance genes. In Antibiotics and Antimicrobial Resistance Genes; Springer: Cham, Switzerland, 2020; pp. 395–413. [Google Scholar]
- BC Cook Articulation Committee. Understanding Ingredients for the Canadian Baker; BCcampus: Victoria, BC, Canada, 2014. [Google Scholar]
- Capitán-Cañadas, F. New Insights into the Mechanisms of Prebiotics and Microbiota on Intestinal Defense; Universidad de Granada: Granada, Spain, 2014; ISBN 9788490289358. [Google Scholar]
- Cosme, F.; Inês, A.; Vilela, A. Consumer’s acceptability and health consciousness of probiotic and prebiotic of non-dairy products. Food Res. Int. 2022, 151, 110842. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, Z.; Zupan, J.; Matos, T.; Raspor, P. Probiotic yeast Saccharomyces boulardii (nom. nud.) modulates adhesive properties of Candida glabrata. Sabouraudia 2016, 54, 835–845. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L. V Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. WJG 2010, 16, 2202. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Vallance, B.A.; Boyer, L.; Bergstrom, K.S.B.; Walker, J.; Madsen, K.; O’Kusky, J.R.; Buchan, A.M.; Jacobson, K. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am. J. Physiol. Liver Physiol. 2008, 294, G295–G306. [Google Scholar]
- Geyik, M.F.; Aldemir, M.; Hosoglu, S.; Ayaz, C.; Satilmis, S.; Buyukbayram, H.; Kokoglu, O.F. The effects of Saccharomyces boulardii on bacterial translocation in rats with obstructive jaundice. Ann. R. Coll. Surg. Engl. 2006, 88, 176–180. [Google Scholar] [CrossRef]
- Jahn, H.-U.; Ullrich, R.; Schneider, T.; Liehr, R.-M.; Schieferdecker, H.L.; Holst, H.; Zeitz, M. Immunological and Trophical Effects of Saccharomyces boulardii on the Small Intestine in Healthy Human Volunteers. Digestion 1996, 57, 95–104. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. yeast as probiotics--Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Qamar, A.; Aboudola, S.; Warny, M.; Michetti, P.; Pothoulakis, C.; LaMont, J.T.; Kelly, C.P. Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect. Immun. 2001, 69, 2762–2765. [Google Scholar] [CrossRef]
- Buts, J.-P.; Dekeyser, N.; Stilmant, C.; Delem, E.; Smets, F.; Sokal, E. Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatr. Res. 2006, 60, 24–29. [Google Scholar] [CrossRef]
- Barc, M.-C.; Charrin-Sarnel, C.; Rochet, V.; Bourlioux, F.; Sandré, C.; Boureau, H.; Doré, J.; Collignon, A. Molecular analysis of the digestive microbiota in a gnotobiotic mouse model during antibiotic treatment: Influence of Saccharomyces boulardii. Anaerobe 2008, 14, 229–233. [Google Scholar] [CrossRef]
- Schneider, S.M.; Girard-Pipau, F.; Filippi, J.; Hébuterne, X.; Moyse, D.; Hinojosa, G.C.; Pompei, A.; Rampal, P. Effects of Saccharomyces boulardii on fecal short-chain fatty acids and microflora in patients on long-term total enteral nutrition. World J. Gastroenterol. WJG 2005, 11, 6165. [Google Scholar] [CrossRef] [PubMed]
- Zanello, G.; Meurens, F.; Berri, M.; Salmon, H. Saccharomyces boulardii effects on gastrointestinal diseases. Curr. Issues Mol. Biol. 2009, 11, 47–58. [Google Scholar] [PubMed]
- Dalmasso, G.; Cottrez, F.; Imbert, V.; Lagadec, P.; Peyron, J.-F.; Rampal, P.; Czerucka, D.; Groux, H. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology 2006, 131, 1812–1825. [Google Scholar] [CrossRef]
- Salim, H.M.; Huque, K.S.; Kamaruddin, K.M.; Haque Beg, A. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018, 101, 52–75. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Characterization of yeasts isolated from traditional kefir grains for potential probiotic properties. J. Funct. Foods 2019, 58, 56–66. [Google Scholar] [CrossRef]
- Nayak, S.K. Biology of eukaryotic probiotics. In Probiotics; Springer: Berlin/Heidelberg, Germany, 2011; pp. 29–55. [Google Scholar]
- Sharifi-Rad, J.; Rodrigues, C.F.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Milton Prabu, S.; et al. Probiotics: Versatile bioactive components in promoting human health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.-T. Probiotics: Biology, Genetics and Health Aspects; Springer Science & Business Media: Cham, Switzerland, 2011; ISBN 978-3-642-20837-9. [Google Scholar]
- Banik, A.; Halder, S.K.; Ghosh, C.; Mondal, K.C. Fungal probiotics: Opportunity, challenge, and prospects. In Recent Advancement in White Biotechnology Through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 101–117. [Google Scholar]
- Du, L.P.; Hao, R.X.; Xiao, D.G.; Guo, L.L.; Gai, W.D. Research on the Characteristics and Culture Conditions of Saccharomyces boulardii. Adv. Mater. Res. 2012, 343, 594–598. [Google Scholar]
- McFarland, L. V Common organisms and probiotics: Saccharomyces boulardii. In The Microbiota in Gastrointestinal Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 145–164. [Google Scholar]
- Pardo, S.; Galvagno, M.A.; Cerrutti, P. Studies of viability and vitality after freezing of the probiotic yeast Saccharomyces boulardii: Physiological preconditioning effect. Rev. Iberoam. Micol. 2009, 26, 155–160. [Google Scholar] [CrossRef]
- Lohith, K.; Anu Appaiah, K.A. In vitro probiotic characterization of yeasts of food and environmental origin. Int. J. Probiotics Prebiotics 2014, 9, 87–92. [Google Scholar]
- Salimi, M.; Mahzonieh, M. Preparation of proper culture medium for Saccharomyces cerevisiae var. boulardii with Molasses and Animal Serum. J. Med. Microbiol. Infect. Dis. 2015, 3, 18–22. [Google Scholar]
- Lei, Z.; Chen, H.; Huang, D.; Zhai, Y.; Shu, G. Optimization of medium compositions for Saccharomyces Boulardii by Box-Behnken design. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2016, 17, 405. [Google Scholar]
- McFarland, L. V Unraveling the causes of negative studies: A case of S. boulardii for the prevention of antibiotic-associated diarrhea. Rev. Med. Chile 2009, 137, 719–720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McFarland, L. V Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Off. J. Am. Coll. Gastroenterol. ACG 2006, 101, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Mansour-Ghanaei, F.; Dehbashi, N.; Yazdanparast, K.; Shafaghi, A. Efficacy of Saccharomyces boulardii with antibiotics in acute amoebiasis. World J. Gastroenterol. WJG 2003, 9, 1832. [Google Scholar] [CrossRef]
- Billoo, A.G.; Memon, M.A.; Khaskheli, S.A.; Murtaza, G.; Iqbal, K.; Shekhani, M.S.; Siddiqi, A.Q. Role of a probiotic (Saccharomyces boulardii) in management and prevention of diarrhoea. World J. Gastroenterol. WJG 2006, 12, 4557. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Skórka, A. Saccharomyces boulardii for treating acute gastroenteritis in children: Updated meta-analysis of randomized controlled trials. Aliment. Pharmacol. Ther. 2009, 30, 960–961. [Google Scholar] [CrossRef] [PubMed]
- Gaon, D.; Garcia, H.; Winter, L.; Rodríguez, N.; Quintás, R.; González, S.N.; Oliver, G. Effect of Lactobacillus strains and Saccharomyces boulardii on persistent diarrhea in children. Medicina 2003, 63, 293–298. [Google Scholar]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med. Infect. Dis. 2007, 5, 97–105. [Google Scholar] [CrossRef]
- Li, J.Q.; Li, J.L.; Xie, Y.H.; Wang, Y.; Shen, X.N.; Qian, Y.; Han, J.X.; Chen, Y.X.; Fang, J.-Y. Saccharomyces cerevisiae may serve as a probiotic in colorectal cancer by promoting cancer cell apoptosis. J. Dig. Dis. 2020, 21, 571–582. [Google Scholar] [CrossRef]
- Guslandi, M.; Giollo, P.; Testoni, P.A. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2003, 15, 697–698. [Google Scholar] [CrossRef]
- Sivignon, A.; de Vallée, A.; Barnich, N.; Denizot, J.; Darcha, C.; Pignède, G.; Vandekerckove, P.; Darfeuille-Michaud, A. Saccharomyces cerevisiae CNCM I-3856 prevents colitis induced by AIEC bacteria in the transgenic mouse model mimicking Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Profir, A.-G.; Buruiana, C.-T.; Vizireanu, C. Effects of S. cerevisiae var. boulardii in gastrointestinal disorders. J. Agroaliment. Proc. Technol. 2015, 21, 148–155. [Google Scholar]
- Pericolini, E.; Gabrielli, E.; Ballet, N.; Sabbatini, S.; Roselletti, E.; Cayzeele Decherf, A.; Pélerin, F.; Luciano, E.; Perito, S.; Jüsten, P.; et al. Therapeutic activity of a Saccharomyces cerevisiae-based probiotic and inactivated whole yeast on vaginal candidiasis. Virulence 2017, 8, 74–90. [Google Scholar] [CrossRef]
- Basavaprabhu, H.N.; Sonu, K.S.; Prabha, R. Mechanistic insights into the action of probiotics against bacterial vaginosis and its mediated preterm birth: An overview. Microb. Pathog. 2020, 141, 104029. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Ruszkowski, J.; Fic, M.; Folwarski, M.; Makarewicz, W. Saccharomyces boulardii CNCM I-745: A non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr. Microbiol. 2020, 77, 1987–1996. [Google Scholar] [CrossRef]
- Thyab Gddoa Al-sahlany, S.; Altemimi, A.B.; Al-Manhel, A.J.A.; Niamah, A.K.; Lakhssassi, N.; Ibrahim, S.A. Purification of bioactive peptide with antimicrobial properties produced by Saccharomyces cerevisiae. Foods 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Czerucka, D.; Rampal, P. Diversity of Saccharomyces boulardii CNCM I-745 mechanisms of action against intestinal infections. World J. Gastroenterol. 2019, 25, 2188. [Google Scholar] [CrossRef]
- Sivignon, A.; Yu, S.-Y.; Ballet, N.; Vandekerckove, P.; Barnich, N.; Guerardel, Y. Heteropolysaccharides from S. cerevisiae show anti-adhesive properties against E. coli associated with Crohn’s disease. Carbohydr. Polym. 2021, 271, 118415. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; Pontier-Bres, R.; Larbret, F.; Rekima, A.; Verhasselt, V.; Blin-Wakkach, C.; Czerucka, D. Saccharomyces boulardii strain CNCM I-745 modifies the mononuclear phagocytes response in the small intestine of mice following Salmonella typhimurium infection. Front. Immunol. 2019, 10, 643. [Google Scholar] [CrossRef]
- Stier, H.; Bischoff, S.C. Influence of Saccharomyces boulardii CNCM I-745on the gut-associated immune system. Clin. Exp. Gastroenterol. 2016, 9, 269. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, H.J.; Chi, S.G. Saccharomyces boulardii reduced intestinal inflammation in mice model of 2, 4, 6-trinitrobencene sulfonic acid induced colitis: Based on microarray. Korean J. Gastroenterol. 2010, 55, 33–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, G.; Feng, D. Therapeutic effect of Saccharomyces boulardii combined with Bifidobacterium and on cellular immune function in children with acute diarrhea. Exp. Ther. Med. 2019, 18, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.X.; Li, Z.; Su, C.; Hu, G.H. Clinical observation of Saccharomyces boulardii combined with cetirizine hydrochloride in children allergic rhinitis. J. Clin. Otorhinolaryngol. Head Neck Surg. 2017, 31, 1649–1652. [Google Scholar]
- Bahgat, M.; Maghraby, A.S.; OM, A.D.-F.; Elshafei, A.M. Immunization of mice with crude extract of Saccharomyces boulardii yeast induces cross-reactive immune responses with antigenic preparations from different developmental stages of the Schistosoma mansoni and reduces the parasite worm burden. J. Egypt. Soc. Parasitol. 2005, 35, 563–580. [Google Scholar] [PubMed]
- Fidan, I.; Kalkanci, A.; Yesilyurt, E.; Yalcin, B.; Erdal, B.; Kustimur, S.; Imir, T. Effects of Saccharomyces boulardii on cytokine secretion from intraepithelial lymphocytes infected by Escherichia coli and Candida albicans. Mycoses 2009, 52, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.G.T.; Ramos, C.L.; Cenzi, G.; Melo, D.S.; Dias, D.R.; Schwan, R.F. Probiotic potential, antioxidant activity, and phytase production of indigenous yeasts isolated from indigenous fermented foods. Probiotics Antimicrob. Proteins 2020, 12, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-S.; Ma, Y.; Maubois, J.-L.; Chen, L.-J.; Liu, Q.-H.; Guo, J.-P. Identifcation of yeasts from raw milk and selection for some specific antioxidant properties. Int. J. Dairy Technol. 2010, 63, 47–54. [Google Scholar] [CrossRef]
- Suryavanshi, A.; Agarwal, A.; Kaler, A.; Bihade, U.; Kaur, J.; Tikoo, K.B.; Banerjee, U.C. Comparative studies on the antioxidant potential of vanillin-producing Saccharomyces boulardii extracts. Oxid. Antioxid. Med. Sci. 2013, 2, 201–209. [Google Scholar] [CrossRef]
- Buccigrossi, V.; Laudiero, G.; Russo, C.; Miele, E.; Sofia, M.; Monini, M.; Ruggeri, F.M.; Guarino, A. Chloride secretion induced by rotavirus is oxidative stress-dependent and inhibited by Saccharomyces boulardii in human enterocytes. PLoS ONE 2014, 9, e99830. [Google Scholar] [CrossRef] [PubMed]
- Waseem, H.; Williams, M.R.; Stedtfeld, R.D.; Hashsham, S.A. Antimicrobial resistance in the environment. Water Environ. Res. 2017, 89, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Pruden, A.; Larsson, D.G.J.; Amézquita, A.; Collignon, P.; Brandt, K.K.; Graham, D.W.; Lazorchak, J.M.; Suzuki, S.; Silley, P.; Snape, J.R.; et al. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ. Health Perspect. 2013, 121, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic resistance among commercially available probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]
- Palma, M.L.; Zamith-Miranda, D.; Martins, F.S.; Bozza, F.A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E.T.A.; Douradinha, B. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: Is there room for improvement? Appl. Microbiol. Biotechnol. 2015, 99, 6563–6570. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Mondal, O.; Khanna, D.; Panwar, S.; Negi, S.; Basu, S. Systematic Review on Therapeutic Applications of Yeast ‘Saccharomyces’. Int. J. Sci. Res. Sci. Technol. 2021, 8, 174–197. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Roussel, C.; Sivignon, A.; De Vallee, A.; Garrait, G.; Denis, S.; Tsilia, V.; Ballet, N.; Vandekerckove, P.; de Wiele, T.; Barnich, N.; et al. Anti-infectious properties of the probiotic Saccharomyces cerevisiae CNCM I-3856 on enterotoxigenic E. coli (ETEC) strain H10407. Appl. Microbiol. Biotechnol. 2018, 102, 6175–6189. [Google Scholar] [CrossRef]
- Youlden, D.R.; Cramb, S.M.; Dunn, N.A.M.; Muller, J.M.; Pyke, C.M.; Baade, P.D. The descriptive epidemiology of female breast cancer: An international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012, 36, 237–248. [Google Scholar] [CrossRef]
- da Costa Vieira, R.A.; Biller, G.; Uemura, G.; Ruiz, C.A.; Curado, M.P. Breast cancer screening in developing countries. Clinics 2017, 72, 244–253. [Google Scholar] [CrossRef]
- Elenbaas, B.; Spirio, L.; Koerner, F.; Fleming, M.D.; Zimonjic, D.B.; Donaher, J.L.; Popescu, N.C.; Hahn, W.C.; Weinberg, R.A. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001, 15, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Pishkhan Dibazar, S.; Allahyari, S.; Javadi, M.; Farasat, A.; Darzi, S. Probiotic Saccharomyces cerevisiae var. boulardii supernatant inhibits survivin gene expression and induces apoptosis in human gastric cancer cells. Food Sci. Nutr. 2021, 9, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Fortin, O.; Aguilar-Uscanga, B.; Vu, K.D.; Salmieri, S.; Lacroix, M. Cancer chemopreventive, antiproliferative, and superoxide anion scavenging properties of Kluyveromyces marxianus and Saccharomyces cerevisiae var. boulardii cell wall components. Nutr. Cancer 2018, 70, 83–96. [Google Scholar] [CrossRef]
- Martín, R.; Langella, P. Emerging health concepts in the probiotics field: Streamlining the definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.; Dahabieh, M.; Krogerus, K.; Jouhten, P.; Magalhães, F.; Pereira, R.; Siewers, V.; Vidgren, V. Adaptive laboratory evolution of ale and lager yeasts for improved brewing efficiency and beer quality. Annu. Rev. Food Sci. Technol. 2020, 11, 23–44. [Google Scholar] [CrossRef]
- Stefanini, I.; Dapporto, L.; Berná, L.; Polsinelli, M.; Turillazzi, S.; Cavalieri, D. Social wasps are a Saccharomyces mating nest. Proc. Natl. Acad. Sci. USA 2016, 113, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Broadway, P.R.; Carroll, J.A.; Sanchez, N.C.B. Live yeast and yeast cell wall supplements enhance immune function and performance in food-producing livestock: A review. Microorganisms 2015, 3, 417–427. [Google Scholar] [CrossRef]
- Sharma, M.; Devi, M. Probiotics: A comprehensive approach toward health foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 537–552. [Google Scholar] [CrossRef]
- Burdick Sanchez, N.C.; Broadway, P.R.; Carroll, J.A. Influence of yeast products on modulating metabolism and immunity in cattle and swine. Animals 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.T.; Bule, M.; Rahman Ullah, M.N.; Asif, S.; Niaz, K. The antioxidant components of milk and their role in processing, ripening, and storage: Functional food. Vet. World 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Niu, J.; Wu, Y.; Zhang, W. Effects of Saccharomyces cerevisiae var. boulardii on growth, incidence of diarrhea, serum immunoglobulins, and rectal microbiota of suckling dairy calves. Livest. Sci. 2022, 258, 104875. [Google Scholar] [CrossRef]
- Poloni, V.; Salvato, L.; Pereyra, C.; Oliveira, A.; Rosa, C.; Cavaglieri, L.; Keller, K.M. Bakery by-products based feeds borne-Saccharomyces cerevisiae strains with probiotic and antimycotoxin effects plus antibiotic resistance properties for use in animal production. Food Chem. Toxicol. 2017, 107, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Leo, V.V.; Viswanath, V.; Deka, P.; Ramji, D.R.; Pachuau, L.; Carrie, W.; Malvi, Y.; Singh, G.; Singh, B.P. Saccharomyces and Their Potential Applications in Food and Food Processing Industries. In Industrially Important Fungi for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2021; pp. 393–427. [Google Scholar]
- Walker, G.M.; Dijck, P. Van Physiological and molecular responses of yeasts to the environment. In Yeasts in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2006; pp. 111–152. [Google Scholar]
- Jedlińska, A.; Samborska, K.; Janiszewska, E.; Witrowa-Rajchert, D.; Seuvre, A.M.; Voilley, A. Physicochemical characteristic of industrial aromas in a powder form. Biol. Act. Compd. Food 2017, 21. [Google Scholar]
- Ogrodowczyk, A.M.; Drabinska, N. Crossroad of tradition and innovation-the application of lactic acid fermentation to increase the nutritional and health-promoting potential of plant-based food products-a review. Pol. J. Food Nutr. Sci. 2021, 71, 107–134. [Google Scholar]
- Nevoigt, E. Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2008, 72, 379–412. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).