Assimilation of Cholesterol by Monascus purpureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media Conditions
2.2. Submerged Culture Preparation
2.3. Cholesterol Assimilation
2.3.1. Cholesterol Reagents
2.3.2. Culture Preparation for Growing, Resting, Dead, and Control Conditions
2.3.3. Cholesterol Assimilation and Dry Weight Growth Curve
2.3.4. M. Purpureus Dormancy Experiment
2.3.5. Resting M. Purpureus Supplemented with Nitrogen Sources Experiment
2.3.6. Cholesterol Extraction
2.3.7. Gas Chromatography Methods
2.3.8. Calculations for Cholesterol Assimilation
2.4. Citrinin Production
2.4.1. Citrinin Reagents
2.4.2. Culture Preparation and Extraction for Citrinin Production
2.4.3. High-Performance Liquid Chromatography Methods
2.5. Statistical Analysis
3. Results
3.1. Cholesterol Assimilation
3.2. Growth of M. purpureus
3.3. Reactivating Dormant M. Purpureus
3.4. Citrinin Production in M. purpureus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, J.; Luo, J.; Ma, Z.; Sun, Q.; Wu, C.; Li, X. Quality and authenticity control of functional red yeast rice—a review. Molecules 2019, 24, 1944. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar] [CrossRef] [PubMed]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Fungal Metabolites. Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319250014. [Google Scholar]
- Patel, S. Functional food red yeast rice (RYR) for metabolic syndrome amelioration: A review on pros and cons. World J. Microbiol. Biotechnol. 2016, 32, 87. [Google Scholar] [CrossRef] [PubMed]
- National Center for Complementary and Integrative Health. Red Yeast Rice. 2013. Available online: https://nccih.nih.gov/health/redyeastrice (accessed on 31 October 2020).
- Souza Filho, P.F.; Nair, R.B.; Andersson, D.; Lennartsson, P.R.; Taherzadeh, M.J. Vegan-mycoprotein concentrate from pea-processing industry byproduct using edible filamentous fungi. Fungal Biol. Biotechnol. 2018, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Cao, X.; Wen, Q.; Chen, Z.; Cheng, Z.; Huang, X.; Zhang, Y.; Long, C.; Zhang, Y.; Huang, Z. An overview of the bioactivity of monacolin K/lovastatin. Food Chem. Toxicol. 2019, 131, 110585. [Google Scholar] [CrossRef]
- Endo, A. The origin of the statins. Atheroscler. Suppl. 2004, 5, 125–130. [Google Scholar] [CrossRef]
- Subhan, M.; Faryal, R.; Macreadie, I. Exploitation of Aspergillus terreus for the production of natural statins. J. Fungi 2016, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; van Pelt, A. The Effects of Red Yeast Rice Supplementation on Cholesterol Levels in Adults. Am. J. Nurs. 2017, 117, 46–53. [Google Scholar] [CrossRef]
- Nguyen, T.; Karl, M.; Santini, A. Red yeast rice. Foods 2017, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Mousa, S.A. The effect of red yeast rice (Monascus purpureus) in dyslipidemia and other disorders. Complement. Ther. Med. 2012, 20, 466–474. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.W.; Gerdes, V.E.A. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Burke, F.M. Red Yeast Rice for the Treatment of Dyslipidemia. Curr. Atheroscler. Rep. 2015, 17, 495. [Google Scholar] [CrossRef] [PubMed]
- Kriaa, A.; Bourgin, M.; Potiron, A.; Mkaouar, H.; Jablaoui, A.; Gérard, P.; Maguin, E.; Rhimi, M. Microbial impact on cholesterol and bile acid metabolism: Current status and future prospects. J. Lipid Res. 2019, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Observatory (GHO) Data: Raised Cholesterol. Available online: http://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/ (accessed on 31 October 2020).
- World Health Organization. Cardiovascular Disease. 2017. Available online: https://www.who.int/cardiovascular_diseases/about_cvd/en/ (accessed on 31 October 2020).
- Lin, C.C.; Li, T.C.; Lai, M.M. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. Eur. J. Endocrinol. 2005, 153, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Klimek, M.; Wang, S.; Ogunkanmi, A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. Pharm. Ther. 2009, 34, 313–317. [Google Scholar]
- Miremadi, F.; Sherkat, F.; Stojanovska, L. Hypocholesterolaemic effect and anti-hypertensive properties of probiotics and prebiotics: A review. J. Funct. Foods 2016, 25, 497–510. [Google Scholar] [CrossRef]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef]
- Hassan, A.; Din, A.U.; Zhu, Y.; Zhang, K.; Li, T.; Wang, Y.; Luo, Y.; Wang, G. Updates in understanding the hypocholesterolemia effect of probiotics on atherosclerosis. Appl. Microbiol. Biotechnol. 2019, 103, 5993–6006. [Google Scholar] [CrossRef]
- Bordoni, A.; Amaretti, A.; Leonardi, A.; Boschetti, E.; Danesi, F.; Matteuzzi, D.; Roncaglia, L.; Raimondi, S.; Rossi, M. Cholesterol-lowering probiotics: In vitro selection and in vivo testing of bifidobacteria. Appl. Microbiol. Biotechnol. 2013, 97, 8273–8281. [Google Scholar] [CrossRef]
- Fava, F.; Lovegrove, J.A.; Gitau, R.; Jackson, K.G.; Tuohy, K.M. The Gut Microbiota and Lipid Metabolism: Implications for Human Health and Coronary Heart Disease. Curr. Med. Chem. 2006, 13, 3005–3021. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization Agriculture of the United Nations. Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 31 October 2020).
- De Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Adrio, J.L.; Demain, A.L. Fungal biotechnology. Int. Microbiol. 2003, 6, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.J.; Loret, M.O.; Goma, G.; Rangueil, C.S. De Production of citrinin by various species of Monascus. Biotechnol. Lett. 1995, 17, 291–294. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.S.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef]
- Srianta, I.; Ristiarini, S.; Nugerahani, I.; Sen, S.K.; Zhang, B.B.; Xu, G.R.; Blanc, P.J. Recent research and development of Monascus fermentation products. Int. Food Res. J. 2014, 21, 1–12. [Google Scholar]
- Ishimwe, N.; Daliri, E.B.; Lee, B.H.; Fang, F.; Du, G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol. Nutr. Food Res. 2015, 59, 94–105. [Google Scholar] [CrossRef]
- Lye, H.S.; Rusul, G.; Liong, M.T. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J. Dairy Sci. 2010, 93, 1383–1392. [Google Scholar] [CrossRef]
- Reis, S.A.; Conceição, L.L.; Rosa, D.D.; Siqueira, N.P.; Peluzio, M.C.G. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutr. Res. Rev. 2017, 30, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Psomas, E.I.; Fletouris, D.J.; Litopoulou-Tzanetaki, E.; Tzanetakis, N. Assimilation of cholesterol by yeast strains isolated from infant feces and feta cheese. J. Dairy Sci. 2003, 86, 3416–3422. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Mizumachi, K.; Nomura, M.; Kobayashi, M.; Fujita, Y.; Okamoto, T.; Suzuki, I.; Tsuji, N.M.; Kurisaki, J.I.; Ohmomo, S. Lactococcus sp. as potential probiotic lactic acid bacteria. Jpn. Agric. Res. Q. 2007, 41, 181–189. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kumar, S.S.; Ghosh, A.R. Probiotic Enterococcus faecalis AG5 effectively assimilates cholesterol and produces fatty acids including propionate. FEMS Microbiol. Lett. 2019, 366, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Bhat, A.A.; Thakare, J.M.; Pawar, P.B.; Zope, D.G.; Jain, R.M.; Chaudhari, B.L. Cholesterol assimilation and biotransformation by Lactobacillus helveticus. Biotechnol. Lett. 2012, 34, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Miremadi, F.; Ayyash, M.; Sherkat, F.; Stojanovska, L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J. Funct. Foods 2014, 9, 295–305. [Google Scholar] [CrossRef]
- Kelesidis, T.; Pothoulakis, C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap. Adv. Gastroenterol. 2012, 5, 111–125. [Google Scholar] [CrossRef]
- Gil-Rodríguez, A.M.; Carrascosa, A.V.; Requena, T. Yeasts in foods and beverages: In vitro characterisation of probiotic traits. LWT Food Sci. Technol. 2015, 64, 1156–1162. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Chen, L.S.; Ma, Y.; Maubois, J.L.; He, S.H.; Chen, L.J.; Li, H.M. Screening for the potential probiotic yeast strains from raw milk to assimilate cholesterol. Dairy Sci. Technol. 2010, 90, 537–548. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Production of ethanol and biomass from thin stillage using food-grade Zygomycetes and Ascomycetes filamentous fungi. Energies 2014, 7, 3872–3885. [Google Scholar] [CrossRef]
- Yudianto, D.; Nainggolan, E.A.; Millati, R.; Hidayat, C.; Lennartsson, P.; Taherzadeh, M.J.; Niklasson, C. Bioconversion of pretreated wheat straw to ethanol by monascus purpureus CBS 109.07 and fusarium venenatum ATCC 20334 using simultaneous saccharification and fermentation. Biodiversitas 2019, 20, 2229–2235. [Google Scholar] [CrossRef]
- Fletouris, D.J.; Botsoglou, N.A.; Psomas, I.E.; Mantis, A.I. Rapid Determination of Cholesterol in Milk and Milk Products by Direct Saponification and Capillary Gas Chromatography. J. Dairy Sci. 1998, 81, 2833–2840. [Google Scholar] [CrossRef]
- Liu, R.; Xu, B. Optimization of Extraction Conditions of Citrinin from Red Yeast Rice by Orthogonal Design and Quantification of Citrinin by High-Performance Liquid Chromatography. Food Anal. Methods 2013, 6, 677–682. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Q.; Lee, J.; Jia, X.; Sung, C. HPLC analysis of citrinin in red yeast rice. Food Sci. Biotechnol. 2003, 12, 376–380. [Google Scholar]
- Pazouki, M.; Panda, T. Understanding the morphology of fungi. Bioprocess Eng. 2000, 22, 127–143. [Google Scholar] [CrossRef]
- Veiter, L.; Rajamanickam, V.; Herwig, C. The filamentous fungal pellet—Relationship between morphology and productivity. Appl. Microbiol. Biotechnol. 2018, 102, 2997–3006. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U. Evaluation of Epicoccum nigrum for growth, morphology and production of natural colorants in liquid media and on a solid rice medium. Biotechnol. Lett. 2008, 30, 2183–2190. [Google Scholar] [CrossRef]
- Castillo, N.A.; Valdez, A.L.; Fariña, J.I. Microbial production of scleroglucan and downstream processing. Front. Microbiol. 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, T.; Zhao, J.; Lv, Y.; Ren, R. Simultaneous removal of carbon and nitrogen by mycelial pellets of a heterotrophic nitrifying fungus-Penicillium sp. L1. J. Biosci. Bioeng. 2017, 123, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, J.; Mao, J.; Zhang, L.; He, C.; Chen, G.; Parkin, I.P.; Lai, Y. In vivo and in vitro efficient textile wastewater remediation by Aspergillus niger biosorbent. Nanoscale Adv. 2019, 1, 168–176. [Google Scholar] [CrossRef]
- Dikshit, R.; Tallapragada, P. Monascus purpureus: A potential source for natural pigment production. J. Microbiol. Biotechnol. Res. 2011, 1, 164–174. [Google Scholar]
- Witherden, E.A.; Shoaie, S.; Hall, R.A.; Moyes, D.L. The human mucosal mycobiome and fungal community interactions. J. Fungi 2017, 3, 56. [Google Scholar] [CrossRef]
- Ajdari, Z.; Ebrahimpour, A.; Abdul Manan, M.; Hamid, M.; Mohamad, R.; Ariff, A.B. Nutritional requirements for the improvement of growth and sporulation of several strains of monascus purpureus on solid state cultivation. J. Biomed. Biotechnol. 2011, 2011, 487329. [Google Scholar] [CrossRef]
- Nimnoi, P.; Lumyong, S. Improving Solid-State Fermentation of Monascus purpureus on Agricultural Products for Pigment Production. Food Bioprocess Technol. 2011, 4, 1384–1390. [Google Scholar] [CrossRef]
- Madani, G.; Mirlohi, M.; Yahay, M.; Hassanzadeh, A. How much in vitro cholesterol reducing activity of Lactobacilli predicts their in vivo cholesterol function? Int. J. Prev. Med. 2013, 4, 404–413. [Google Scholar]
- Kimoto, H.; Ohmomo, S.; Okamoto, T. Cholesterol removal from media by lactococci. J. Dairy Sci. 2002, 85, 3182–3188. [Google Scholar] [CrossRef]
- Tomaro-Duchesneau, C.; Jones, M.L.; Shah, D.; Jain, P.; Saha, S.; Prakash, S. Cholesterol Assimilation by Lactobacillus Probiotic Bacteria: An In Vitro Investigation. BioMed Res. Int. 2014, 2014, 380316. [Google Scholar] [CrossRef]
- Tok, E.; Aslim, B. Cholesterol removal by some lactic acid bacteria that can be used as probiotic. Microbiol. Immunol. 2010, 54, 257–264. [Google Scholar] [CrossRef]
- Pereira, D.I.A.; Gibson, G.R. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, S.E.; Nelson, C.R.; Maxwell, C. Assimilation of cholesterol by Lactobacillus acidophilus. Appl. Environ. Microbiol. 1985, 49, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Hill, C.; Gahan, C.G.M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.M.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kinoshita, H.; Ishihara, S.; Sakai, K.; Nagai, S.; Nihira, T. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2005, 71, 3453–3457. [Google Scholar] [CrossRef] [PubMed]
- Patrovsky, M.; Sinovska, K.; Branska, B.; Patakova, P. Effect of initial pH, different nitrogen sources, and cultivation time on the production of yellow or orange Monascus purpureus pigments and the mycotoxin citrinin. Food Sci. Nutr. 2019, 7, 3494–3500. [Google Scholar] [CrossRef]
- Hajjaj, H.; Blanc, P.; Groussac, E.; Uribelarrea, J.L.; Goma, G.; Loubiere, P. Kinetic analysis of red pigment and citrinin production by Monascus ruber as a function of organic acid accumulation. Enzyme Microb. Technol. 2000, 27, 619–625. [Google Scholar] [CrossRef]
- Hajjaj, H.; Klaébé, A.; Goma, G.; Blanc, P.J.; Barbier, E.; François, J. Medium-chain fatty acids affect citrinin production in the filamentous fungus Monascus ruber. Appl. Environ. Microbiol. 2000, 66, 1120–1125. [Google Scholar] [CrossRef]
- Rasheva, T.V.; Nedeva, T.S.; Hallet, J.N.; Kujumdzieva, A.V. Characterization of a non-pigment producing Monascus purpureus mutant strain. Antonie Leeuwenhoek 2003, 83, 333–340. [Google Scholar] [CrossRef]
- Pisareva, E.; Savov, V.; Kujumdzieva, A. Pigments and citrinin biosynthesis by fungi belonging to genus Monascus. Z. fur Naturforsch. C 2005, 60, 116–120. [Google Scholar] [CrossRef]
- Fabre, C.E.; Santerre, A.L.; Loret, M.O.; Baberian, R.; Pareilleux, A.; Goma, G.; Blanc, P.J. Production and Food Applications of the Red Pigments of Monascus ruber. J. Food Sci. 1993, 58, 1099–1102. [Google Scholar] [CrossRef]
- Kang, B.; Zhang, X.; Wu, Z.; Wang, Z.; Park, S. Production of citrinin-free Monascus pigments by submerged culture at low pH. Enzyme Microb. Technol. 2014, 55, 50–57. [Google Scholar] [CrossRef]
- Feng, Y.; Shao, Y.; Zhou, Y.; Chen, F. Monacolin K production by citrinin-free Monascus pilosus MS-1 and fermentation process monitoring. Eng. Life Sci. 2014, 14, 538–545. [Google Scholar] [CrossRef]
- Xu, M.J.; Yang, Z.L.; Liang, Z.Z.; Zhou, S.N. Construction of a Monascus purpureus mutant showing lower citrinin and higher pigment production by replacement of ctnA with pks1 without using vector and resistance gene. J. Agric. Food Chem. 2009, 57, 9764–9768. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.Q.; Xu, Z.N.; Zhou, L.P.; Sung, C.K. Elimination of the mycotoxin citrinin production in the industrial important strain Monascus purpureus SM001. Metab. Eng. 2010, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; He, Y.; Zhou, Y.; Shao, Y.; Feng, Y.; Li, M.; Chen, F. Edible Filamentous Fungi from the Species Monascus: Early Traditional Fermentations, Modern Molecular Biology, and Future Genomics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 555–567. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef]
- Cohen, D.E. Balancing cholesterol synthesis and absorption in the gastrointestinal tract. J. Clin. Lipidol. 2008, 2, 1–5. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Dufossé, L. Red colourants from filamentous fungi: Are they ready for the food industry? J. Food Compos. Anal. 2018, 69, 156–161. [Google Scholar] [CrossRef]
- Tsukahara, M.; Shinzato, N.; Tamaki, Y.; Namihira, T.; Matsui, T. Red yeast rice fermentation by selected Monascus sp. with deep-red color, lovastatin production but no citrinin, and effect of temperature-shift cultivation on lovastatin production. Appl. Biochem. Biotechnol. 2009, 158, 476–482. [Google Scholar] [CrossRef] [PubMed]
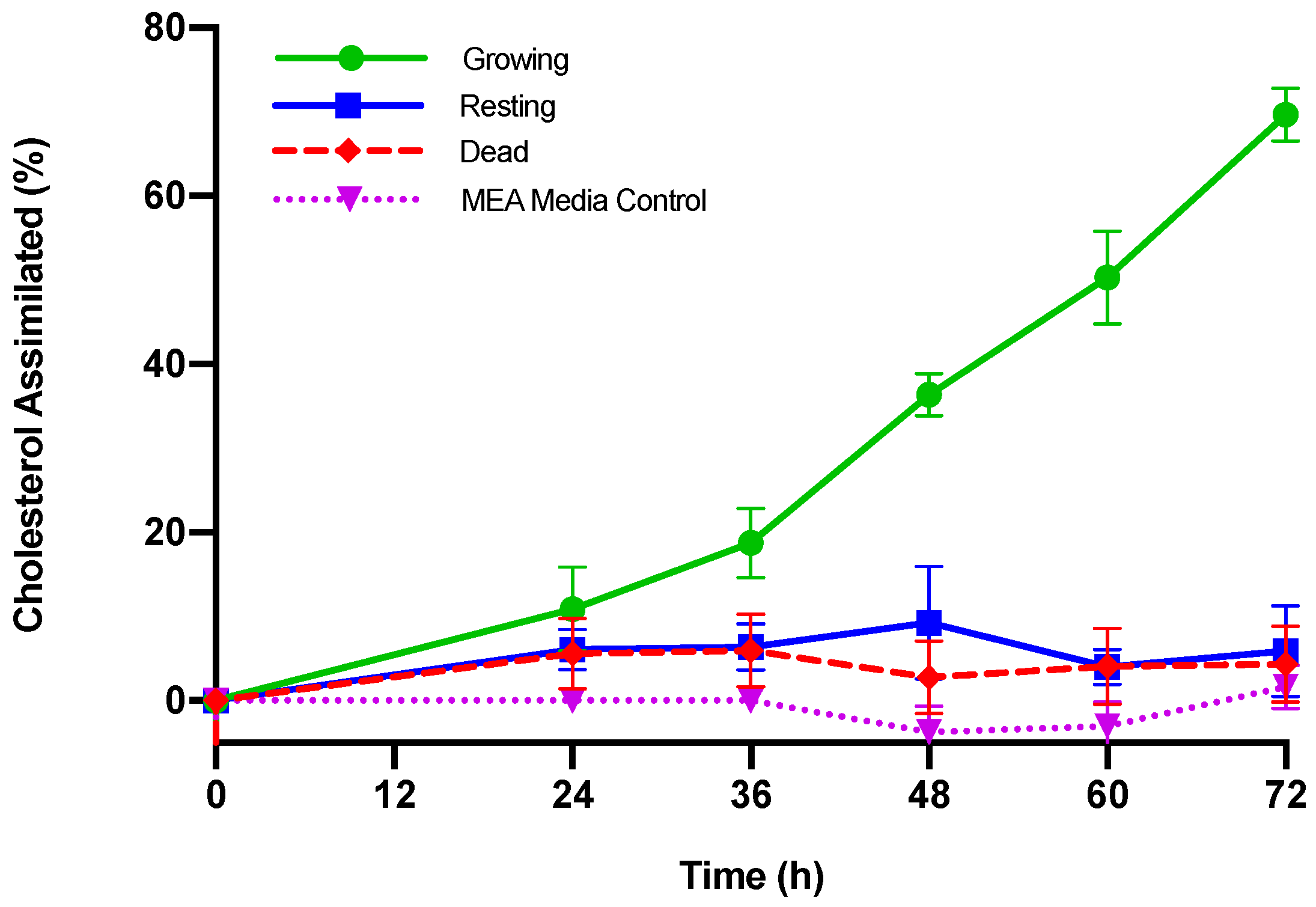
| Cholesterol Assimilated | ||||||
|---|---|---|---|---|---|---|
| Time (h) | Growing M. purpureus | Resting M. purpureus | Dead M. purpureus | |||
| (µg/mL) | (%) | (µg/mL) | (%) | (µg/mL) | (%) | |
| 0 | -- | -- | -- | -- | -- | -- |
| 24 | 10.89 ± 5.62 + | 10.91 ± 7.47 | 6.78 ± 2.79 | 6.07 ± 6.97 | 5.68 ± 4.40 | 5.59 ± 4.95 |
| 36 | 18.69 ± 4.53 a,+ | 18.78 ± 7.03 a,+ | 7.20 ± 3.33 b | 6.40 ± 6.02 b | 6.04 ± 4.54 b | 5.94 ± 5.02 b |
| 48 | 36.18 ± 3.84 a,+ | 36.38 ± 5.38 a,+ | 10.53 ± 7.94 b,+ | 9.29 ± 7.25 b | 2.88 ± 4.45 c | 2.75 ± 4.69 b |
| 60 | 49.86 ± 5.30 a,+ | 50.27 ± 13.5 a,+ | 4.54 ± 2.48 b | 4.03 ± 6.03 b | 4.21 ± 4.63 b | 4.08 ± 4.71 b |
| 72 | 69.13 ± 3.95 a,+ | 69.65 ± 12.5 a,+ | 6.70 ± 6.33 b | 5.91 ± 7.23 b | 4.44 ± 4.67 b | 4.33 ± 5.16 b |
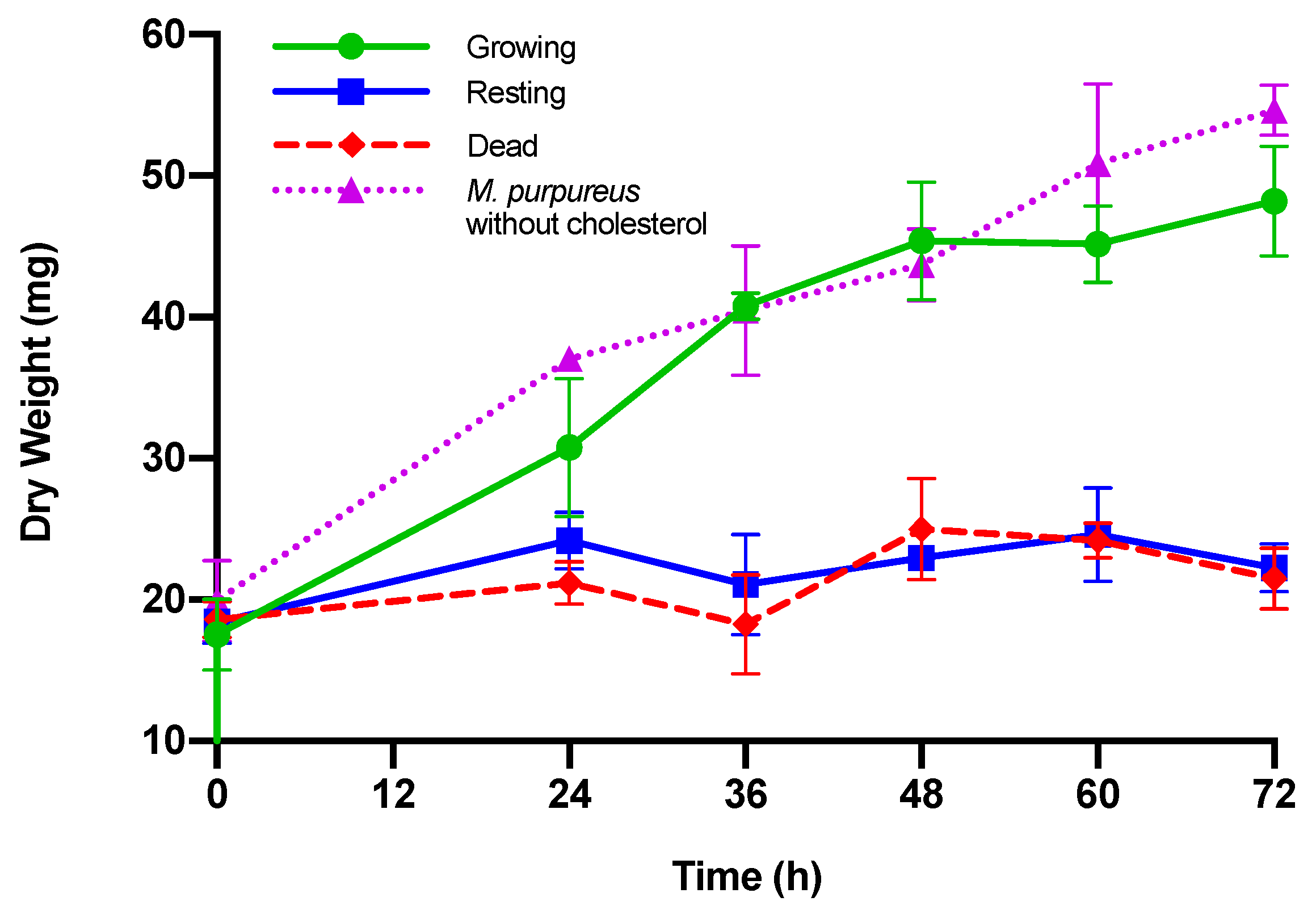
| Dry Weight (mg) | ||||
|---|---|---|---|---|
| Time (h) | Growing M. purpureus | Resting M. purpureus | Dead M. purpureus | M. purpureus without Cholesterol |
| 0 | 17.54 ± 2.5 | 18.40 ± 1.5 | 18.60 ± 1.3 | 19.91 ± 2.9 |
| 24 | 30.77 ± 4.9 a,+ | 24.17 ± 2.0 a | 21.17 ± 1.5 b | 37.05 ± 0.5 c,+ |
| 36 | 40.77 ± 0.9 a,+ | 21.07 ± 3.5b | 16.93 ± 5.6 b | 40.45 ± 4.6 a,+ |
| 48 | 45.40 ± 4.2 a,+ | 22.93 ± 0.5 b | 25.00 ± 3.6 b,+ | 43.72 ± 2.5 a,+ |
| 60 | 45.17 ± 2.7 a,+ | 24.60 ± 3.3 b | 24.17 ± 1.3 b | 50.87 ± 5.6 a,+ |
| 72 | 48.20 ± 3.9 a,+ | 22.27 ± 1.7 b | 21.50 ± 2.2 b | 54.63 ± 1.8 a,+ |
| PBS 1 | Day 4 Incubation in MEA 1 | Day 7 Incubation in MEA 1 | Day 7 Incubation in MEA 1 | ||
|---|---|---|---|---|---|
| Time (h) | Cholesterol Content (µg/mL) | Time 2 (h) | Cholesterol Content (µg/mL) | Cholesterol Content (µg/mL) | Dry Weight (mg) |
| 24 | 114.46 ± 3.73 a | 24 | 97.73 ± 2.66 b | 38.83 ± 9.95 c,+ | 32.87 ± 2.9 ++ |
| 48 | 118.30 ± 3.12 a | 48 | 97.12 ± 4.40 b | 40.88 ± 9.51 c,+ | 31.70 ± 2.7 ++ |
| 72 | 117.36 ± 6.84 a | 72 | 98.36 ± 2.10 b | 37.24 ± 11.47 c,+ | 31.60 ± 4.9 ++ |
| 96 | 114.81 ± 3.86 a | 96 | 98.63 ± 8.19 b | 52.23 ± 5.30 c | 25.33 ± 2.0 |
| Cholesterol Content of M. purpureus Incubated in PBS with 0.3% Oxgall (µg/mL) | ||
|---|---|---|
| Time (h) | With Yeast Nitrogen Base without Amino Acids | With Ammonium Sulfate |
| 0 | 105.82 ± 2.20 | 105.46 ± 4.59 |
| 24 | 97.91 ± 2.03 | 89.64 ± 2.59 |
| 72 | 97.57 ± 1.60 | 93.45 ± 8.03 |
| Dry Weight of M. purpureus (mg) | ||||
|---|---|---|---|---|
| PBS + 0.3% Oxgall | Day 4 Incubation in MEA + 0.3% Oxgall | |||
| Time 1 (h) | With Yeast Nitrogen Base | With Ammonium Sulfate | With Yeast Nitrogen Base | With Ammonium Sulfate |
| 0 | 19.4 ± 3.1 * | 18.8 ± 3.1 * | ||
| 24 | 37.5 ± 2.8 + | 34.7 ± 3.3 + | ||
| 72 | 25.8 ± 5.3 | 23.3 ± 3.2 | 39.3 ± 0.4 + | 30.8 ± 1.8 + |
| Citrinin Production of M. purpureus (µg Citrinin/mL of Culture Broth) | |||
|---|---|---|---|
| Media Conditions | After 24 h 1 | After 72 h 1 | After 14 Days |
| MEA + 0.3% oxgall | ND | ND | 6.77 ± 1.02 |
| PBS + 0.3% oxgall | ND | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.P.T.; Garrahan, M.A.; Nance, S.A.; Seeger, C.E.; Wong, C. Assimilation of Cholesterol by Monascus purpureus. J. Fungi 2020, 6, 352. https://doi.org/10.3390/jof6040352
Nguyen TPT, Garrahan MA, Nance SA, Seeger CE, Wong C. Assimilation of Cholesterol by Monascus purpureus. Journal of Fungi. 2020; 6(4):352. https://doi.org/10.3390/jof6040352
Chicago/Turabian StyleNguyen, Theresa P. T., Margaret A. Garrahan, Sabrina A. Nance, Catherine E. Seeger, and Christian Wong. 2020. "Assimilation of Cholesterol by Monascus purpureus" Journal of Fungi 6, no. 4: 352. https://doi.org/10.3390/jof6040352
APA StyleNguyen, T. P. T., Garrahan, M. A., Nance, S. A., Seeger, C. E., & Wong, C. (2020). Assimilation of Cholesterol by Monascus purpureus. Journal of Fungi, 6(4), 352. https://doi.org/10.3390/jof6040352





