Risk Factors for Mortality among HIV-Infected Patients with Disseminated Histoplasmosis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Study Conduct
2.4. Statistical Analysis
2.5. Ethical and Regulatory Aspects
3. Results
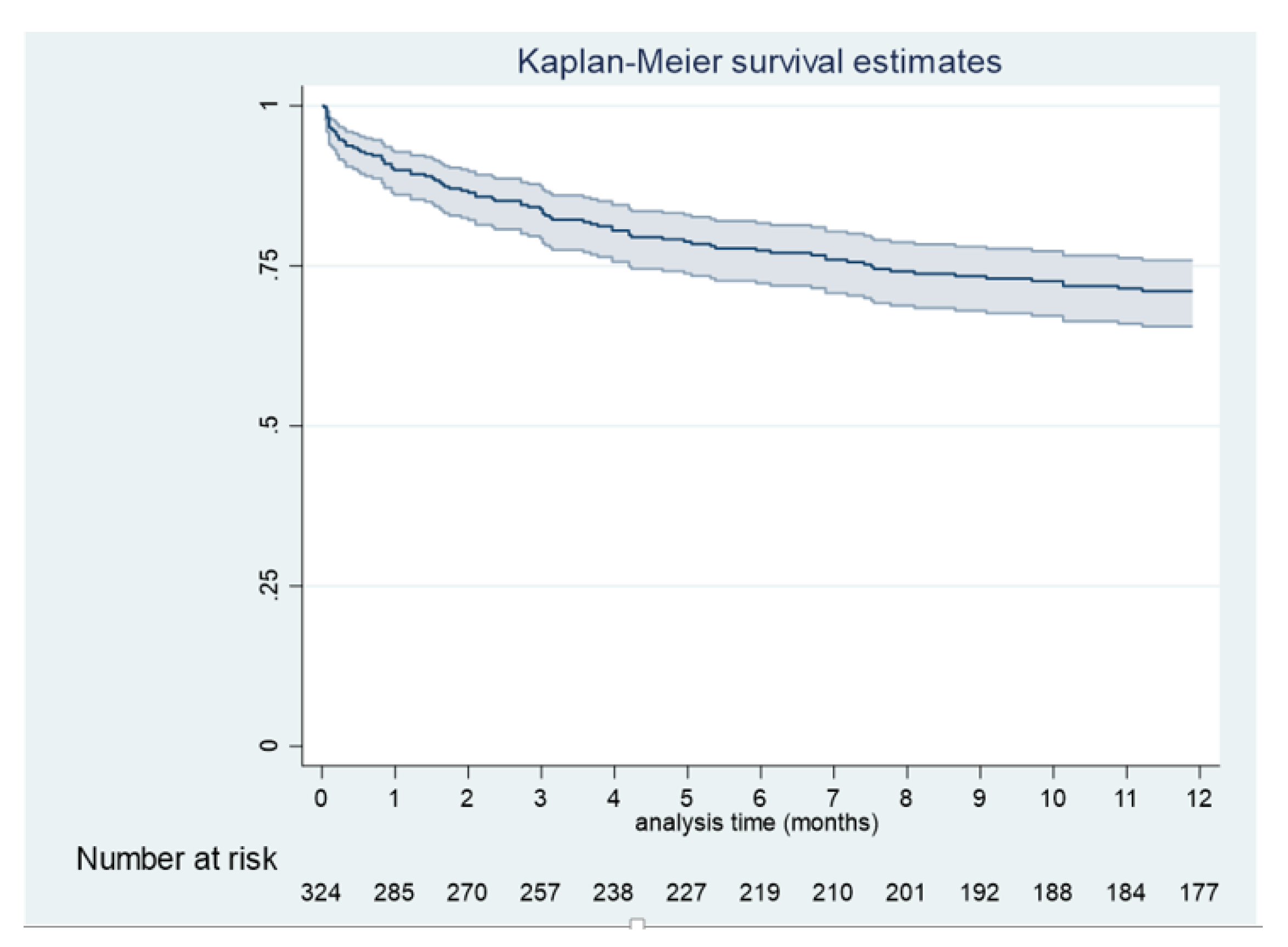
3.1. General Results
3.2. Univariate Analysis
3.3. Multivariate Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nacher, M.; Adriouch, L.; Huber, F.; Vantilcke, V.; Djossou, F.; Elenga, N.; Adenis, A.; Couppié, P. Modeling of the HIV epidemic and continuum of care in French Guiana. PLoS ONE 2018, 13, e0197990. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M.; Adenis, A.; Adriouch, L.; Dufour, J.; Papot, E.; Hanf, M.; Vantilcke, V.; Calvez, M.; Aznar, C.; Carme, B.; et al. What is AIDS in the Amazon and the Guianas? Establishing the burden of disseminated histoplasmosis. Am. J. Trop. Med. Hyg. 2011, 84, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M.; Adenis, A.; Guarmit, B.; Lucarelli, A.; Blanchet, D.; Demar, M.; Djossou, F.; Abboud, P.; Epelboin, L.; Couppié, P. What is AIDS in the Amazon and the Guianas in the 90-90-90 era? bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M.; Huber, F.; Adriouch, L.; Djossou, F.; Adenis, A.; Couppié, P. Temporal trend of the proportion of patients presenting with advanced HIV in French Guiana: Stuck on the asymptote? BMC Res. Notes 2018, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for Diagnosing and Managing Disseminated Histoplasmosis among People Living with HIV (Sólo en Inglés)—PAHO/WHO | Pan American Health Organization. Available online: https://www.paho.org/en/node/71472 (accessed on 6 August 2020).
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Couppie, P.; Aznar, C.; Carme, B.; Nacher, M. American histoplasmosis in developing countries with a special focus on patients with HIV: Diagnosis, treatment, and prognosis. Curr. Opin. Infect. Dis. 2006, 19, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Couppie, P.; Sobesky, M.; Aznar, C.; Bichat, S.; Clyti, E.; Bissuel, F.; El Guedj, M.; Alvarez, F.; Demar, M.; Louvel, D.; et al. Histoplasmosis and acquired immunodeficiency syndrome: A study of prognostic factors. Clin. Infect. Dis. 2004, 38, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Chetchotisakd, P.; Williams, B.; Connolly, P.; Shutt, K.; Hajjeh, R. Factors associated with severe manifestations of histoplasmosis in AIDS. Clin. Infect. Dis. 2000, 30, 877–881. [Google Scholar] [CrossRef] [PubMed]
- De Francesco Daher, E.; de Sousa Barros, F.A.; da Silva Junior, G.B.; Takeda, C.F.; Mota, R.M.; Ferreira, M.T.; Martins, J.C.; Oliveira, S.A.; Gutierrez-Adrianzen, O.A. Risk factors for death in acquired immunodeficiency syndrome-associated disseminated histoplasmosis. Am. J. Trop. Med. Hyg. 2006, 74, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.A.; Kaplan, J.E.; Masur, H.; Pau, A.; Holmes, K.K.; CDC; National Institutes of Health; Infectious Diseases Society of America. Treating opportunistic infections among HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm. Rep. 2004, 53, 1–112. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Wheat, L.J.; Cloud, G.A.; Goldman, M.; Lancaster, D.; Bamberger, D.M.; Powderly, W.G.; Hafner, R.; Kauffman, C.A.; Dismukes, W.E.; et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann. Intern. Med. 2002, 137, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Corzo-León, D.E.; Perales-Martínez, D.; Martin-Onraet, A.; Rivera-Martínez, N.; Camacho-Ortiz, A.; Villanueva-Lozano, H. Monetary costs and hospital burden associated with the management of invasive fungal infections in Mexico: A multicenter study. Br. J. Infect. Dis. 2018, 22, 360–370. [Google Scholar] [CrossRef] [PubMed]
| Variable | Time at Risk (People/Year) | Number of Subjects | Incidence Rate per 100 People/Year | Crude Hazard Ratio (IC95%) |
|---|---|---|---|---|
| Time period | ||||
| <1998 | 135.1 | 42 | 22.9 | 1 |
| 1998–2003 | 318.4 | 99 | 16.9 | 0.6 (0.39–0.95) |
| 2004–2009 | 294.3 | 103 | 9.8 | 0.28 (0.16–0.46) |
| 2010–2014 | 102.1 | 86 | 11.7 | 0.19 (0.10–0.38) |
| Age category (years) | ||||
| <40 | 460.2 | 187 | 15.6 | 1 |
| 40–60 | 371.1 | 133 | 13.4 | 0.90 (0.63–1.30) |
| >60 | 18.5 | 10 | 21.5 | 1.15 (0.42–3.16) |
| Sex | ||||
| Male | 546.8 | 215 | 13.8 | 1 |
| Female | 303 | 115 | 16.4 | 1.16 (0.81–1.66) |
| Nationality | ||||
| French | 325.4 | 113 | 17.2 | 1 |
| Non French | 523.8 | 215 | 13.3 | 0.67 (0.47–0.96) |
| Dyspnea | ||||
| Yes | 74.2 | 47 | 40.4 | 3.32 (1.01–5.50) |
| No | 256.1 | 103 | 12.1 | 1 |
| Missing | 519.6 | 180 | 12.5 | 1.16 (0.75–1.78) |
| Systolic blood pressure (mm Hg) | ||||
| <90 | 47.9 | 29 | 22.9 | 2.02 (1.01–4.05) |
| ≥90 | 304.8 | 136 | 9.5 | 1 |
| Missing | 497 | 165 | 17.3 | 2.47 (1.62–3.78) |
| Maximum temperature (°C) | ||||
| ≥39.8 | 127 | 55 | 8.6 | 0.71 (0.35–1.43) |
| <39.8 | 248.5 | 107 | 11.6 | 1 |
| Missing | 474.3 | 168 | 18.1 | 1.97 (1.29–3.01) |
| Renal failure (creatinine > 120 µmol/L) | ||||
| Yes | 86.2 | 46 | 28.9 | 1.81 (1.16–2.82) |
| No | 743.9 | 276 | 13.1 | 1 |
| WHO performance score > 2 | ||||
| Yes | 272.3 | 149 | 25.3 | 2.10 (1.48–2.99) |
| No | 577.1 | 180 | 9.8 | 1 |
| Liposomal amphotericin B | ||||
| Yes | 172.9 | 92 | 11.5 | 0.54 (0.33–0.88) |
| No | 663.9 | 228 | 15.3 | 1 |
| Deoxycholate amphotericin B | ||||
| Yes | 133 | 44 | 18.7 | 1.82 (1.16–2.84) |
| No | 701.6 | 275 | 13.8 | 1 |
| Itraconazole | ||||
| Yes | 673.8 | 260 | 13.6 | 0.61 (0.41–0.93) |
| No | 168.37 | 63 | 18.4 | 1 |
| CD4 count < 50 | ||||
| Yes | 486.5 | 213 | 17 | 1.13 (0.90–1.93) |
| No | 359.2 | 112 | 11.1 | 1 |
| Serum protein (quartiles) g/L | ||||
| <60 | 26.1 | 13 | 15.3 | 1.72 (0.53–5.61) |
| (60–80) | 178.2 | 89 | 12.3 | 1.26 (0.58–2.74) |
| (80–90) | 125.2 | 49 | 9.5 | 1.11 (0.47–2.64) |
| >90 | 101.9 | 37 | 8.8 | 1 |
| Missing | 418.3 | 142 | 18.8 | 2.83 (1.41–5.65) |
| Neutrophils (quartiles) per mm3 | ||||
| <800 | 135.9 | 83 | 16.9 | 0.92 (0.53–1.60) |
| (800–1780) | 246.5 | 77 | 10.9 | 0.87 (0.52–1.48) |
| (1781–2820) | 238 | 78 | 12.6 | 1 |
| >2820 | 211.7 | 77 | 17.4 | 1.35 (0.83–2.19) |
| Missing | 17.5 | 15 | 51.2 | 2.73 (1.29–5.77) |
| Platelets (quartiles) per mm3 | ||||
| <38,000 | 118.1 | 79 | 19.4 | 1.00 (0.59–1.72) |
| (38,000–144,000) | 219.2 | 78 | 14.1 | 1.07 (0.65–1.74) |
| (144,001–240,000) | 245.9 | 79 | 12.6 | 0.97 (0.59–1.59) |
| >240,000 | 249.1 | 80 | 13.2 | 1 |
| Missing | 17.4 | 14 | 45.7 | 2.42 (1.11–5.26) |
| Hemoglobin (quartiles) g/dL | ||||
| <7.6 | 155.6 | 79 | 25.1 | 2.78 (1.60–4.83) |
| (7.6–8.9) | 240.1 | 85 | 13.3 | 1.77 (1.00–3.13) |
| (8.9–10.4) | 258.2 | 77 | 7.3 | 1 |
| >10.4 | 178.5 | 76 | 15 | 1.74 (0.96–3.13) |
| Missing | 17.4 | 13 | 51 | 4.87 (2.19–10.80) |
| LDH (median) (U/L) | ||||
| <421 | 435.9 | 147 | 8.2 | 1 |
| 421-max | 341.7 | 142 | 20.7 | 2.48 (1.66–3.70) |
| Missing | 72.2 | 41 | 26.3 | 2.63 (1.50–4.59) |
| GOT (median) (IU) | ||||
| <55 | 422.6 | 161 | 13.9 | 1 |
| 55/max | 406 | 153 | 14.2 | 1.03 (0.71–1.48) |
| Missing | 21.3 | 16 | 0.4 | 2.3 (1.14–4.66) |
| Prothrombin time | ||||
| <70% | 126.8 | 51 | 13.3 | 1.36 (0.74–2.50) |
| ≥70% | 247.8 | 119 | 11.2 | 1 |
| Missing | 475.1 | 160 | 17 | 2.11 (1.37–3.25) |
| Concomitant opportunistic infection | ||||
| Yes | 314.8 | 132 | 15.2 | 1.00 (0.70–1.44) |
| No | 535.1 | 198 | 14.6 | 1 |
| Variable | Incidence Rate per 100 Person-Years | Adjusted Hazard Ratio (95% Confidence Interval) | p |
|---|---|---|---|
| Time period | |||
| <1998 | 22.9 | 1 | |
| 1998–2003 | 16.9 | 1.09 (0.62–1.92) | 0.75 |
| 2004–2009 | 9.8 | 0.46 (0.23–0.89) | 0.02 |
| 2010–2014 | 11.7 | 0.41 (0.29–0.58) | <0.001 |
| Age category (years) | |||
| <40 | 15.6 | 1 | |
| 40–60 | 13.4 | 0.99 (0.45–2.15) | 0.98 |
| >60 | 21.5 | 1.70 (0.87–3.31) | 0.11 |
| Dyspnea (main effect) | |||
| Yes | 40.4 | 11.74 (1.54–89.10) | 0.01 |
| No | 12.1 | 1 | |
| Missing | 12.5 | 6.78 (2.07–22.20) | 0.002 |
| Renal failure (creatinine >120 µmol/L) (main effect) | |||
| Yes | 28.9 | 0.51 (0.07–3.57) | 0.5 |
| No | 13.1 | 1 | |
| WHO performance score > 2 | |||
| Yes | 25.3 | 1.95 (1.25–3.03) | 0.003 |
| No | 9.8 | 1 | |
| Liposomal amphotericin B (main effect) | |||
| Yes | 11.5 | 0.62 (0.38–1.01) | 0.05 |
| No | 15.3 | 1 | |
| Deoxycholate amphotericin B | |||
| Yes | 18.7 | 0.30 (0.05–1.80) | 0.19 |
| No | 13.8 | 1 | |
| Itraconazole | |||
| Yes | 13.6 | 0.63 (0.28–1.40) | 0.26 |
| No | 18.4 | 1 | |
| CD4 count < 50 | |||
| Yes | 17 | 0.94 (0.62–1.43) | 0.78 |
| No | 11.1 | 1 | |
| Serum protein (quartiles) g/L | |||
| <60 | 15.3 | 3.57 (1.69–7.54) | 0.001 |
| (60–80) | 12.3 | 2.38 (0.84–6.75) | 0.1 |
| (80–90) | 9.5 | 2.37 (0.69–8.09) | 0.16 |
| >90 | 8.8 | 1 | 1 |
| Missing | 18.8 | 2.92 (0.88–9.64) | 0.07 |
| Hemoglobin (quartiles) g/dL (main effect) | |||
| <7.6 | 25.1 | 14.58 (5.07–41.95) | <0.001 |
| (7.6–8.9) | 13.3 | 6.21 (1.27–30.32) | 0.02 |
| (8.9–10.4) | 7.3 | 1 | |
| >10.4 | 15 | 16.30 (1.69–157.30) | 0.01 |
| Missing | 51 | 25.45 (5.02–129.01) | <0.001 |
| LDH (median) (U/L) (main effect) | |||
| <421 | 8.2 | 1 | |
| 421-max | 20.7 | 1.50 (0.61–3.67) | 0.36 |
| Missing | 26.3 | 1.83 (0.77–4.35) | 0.16 |
| Interaction terms | |||
| Renalfailure#LDH | - | 8.75 (1.78–43.02) | 0.008 |
| Renal failure#Liposomal amphotericin B | - | 0.14 (0.06–0.32) | <0.001 |
| Dyspnea#Hemoglobin in first quartile (<7.6 g/dL) | - | 0.15 (0.05–0.49) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nacher, M.; Drak Alsibai, K.; Valdes, A.; Blaizot, R.; Abboud, P.; Demar, M.; Djossou, F.; Epelboin, L.; Misslin, C.; Ntab, B.; et al. Risk Factors for Mortality among HIV-Infected Patients with Disseminated Histoplasmosis. J. Fungi 2020, 6, 326. https://doi.org/10.3390/jof6040326
Nacher M, Drak Alsibai K, Valdes A, Blaizot R, Abboud P, Demar M, Djossou F, Epelboin L, Misslin C, Ntab B, et al. Risk Factors for Mortality among HIV-Infected Patients with Disseminated Histoplasmosis. Journal of Fungi. 2020; 6(4):326. https://doi.org/10.3390/jof6040326
Chicago/Turabian StyleNacher, Mathieu, Kinan Drak Alsibai, Audrey Valdes, Romain Blaizot, Philippe Abboud, Magalie Demar, Félix Djossou, Loïc Epelboin, Caroline Misslin, Balthazar Ntab, and et al. 2020. "Risk Factors for Mortality among HIV-Infected Patients with Disseminated Histoplasmosis" Journal of Fungi 6, no. 4: 326. https://doi.org/10.3390/jof6040326
APA StyleNacher, M., Drak Alsibai, K., Valdes, A., Blaizot, R., Abboud, P., Demar, M., Djossou, F., Epelboin, L., Misslin, C., Ntab, B., Adenis, A., & Couppié, P. (2020). Risk Factors for Mortality among HIV-Infected Patients with Disseminated Histoplasmosis. Journal of Fungi, 6(4), 326. https://doi.org/10.3390/jof6040326








