Cork Oak Endophytic Fungi as Potential Biocontrol Agents against Biscogniauxia mediterranea and Diplodia corticola
Abstract
1. Introduction
2. Materials and Methods
2.1. Cork Oak Forests Sampling and Endophyte Recovery
2.2. Endophytic Fungi Identification and Selection of Potential Antagonistic Fungi
2.3. Antagonistic Assay In Vitro by Dual-Plate and Categorization of Fungal Interactions
2.4. Antifungal Non-Volatile Compounds Assay
2.5. Antifungal Volatile Compounds Assay
3. Results and Discussion
3.1. Endophytic Fungal Community of Cork Oak
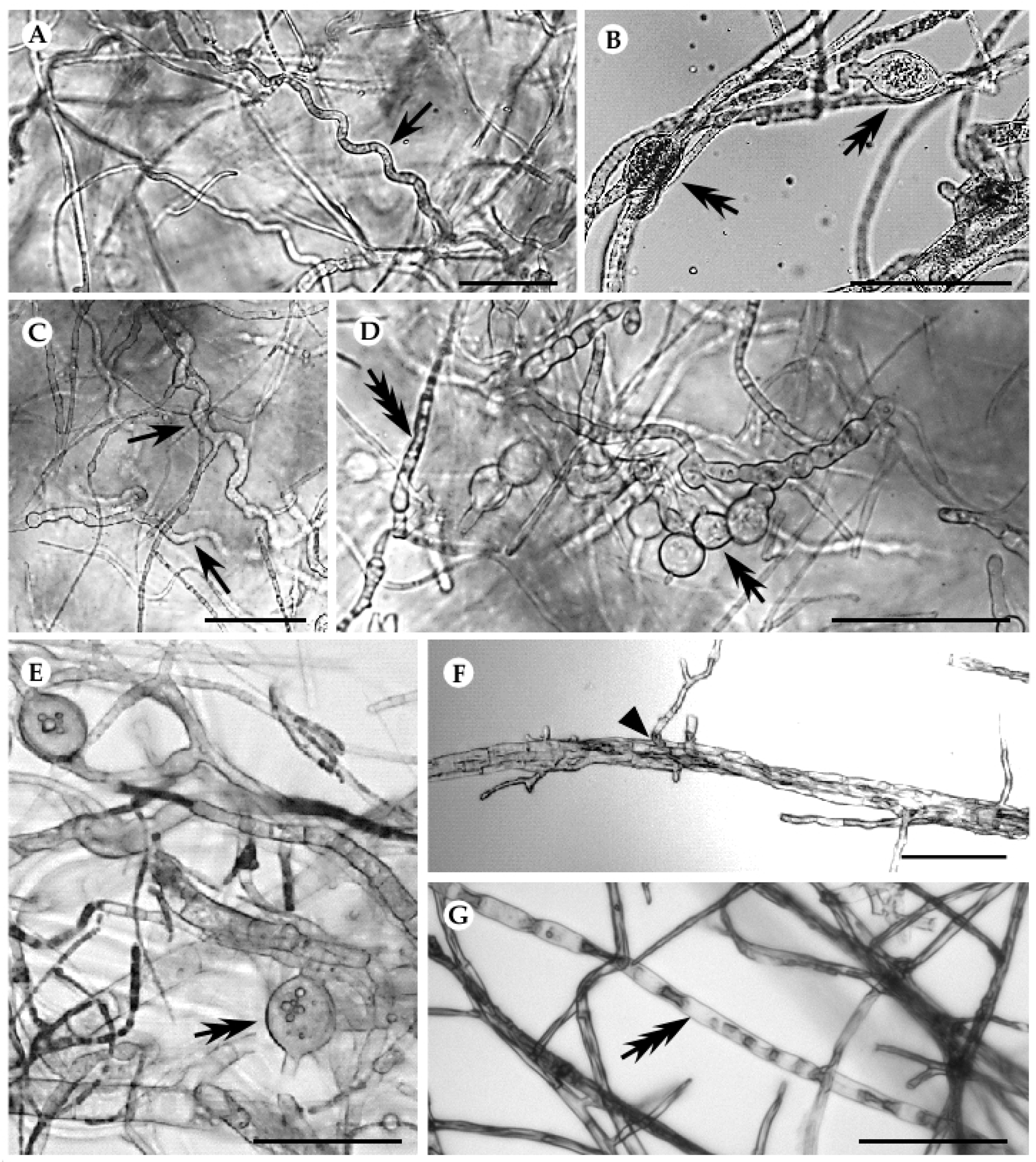
3.2. Interactions of Endophytes against B. mediterranea and D. corticola
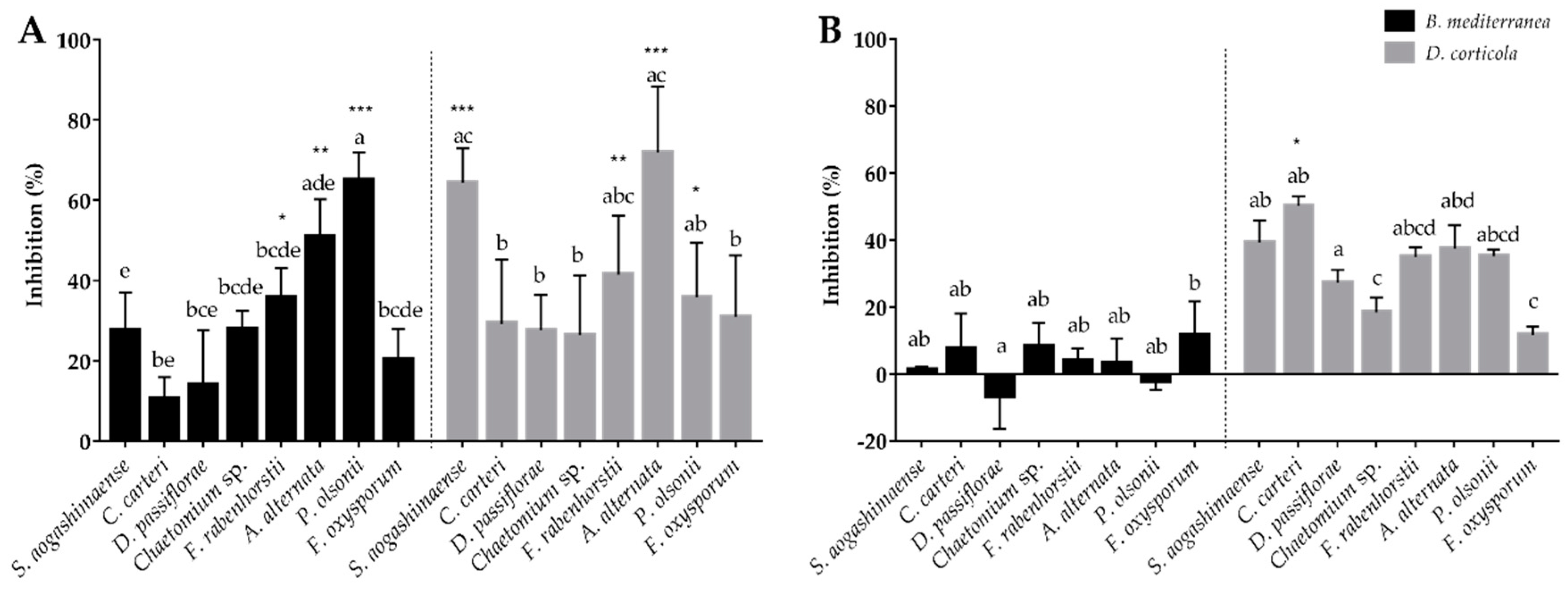
3.3. Fungal Inhibitors Production by Cork Oak Endophytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gauquelin, T.; Michon, G.; Joffre, R.; Duponnois, R.; Génin, D.; Fady, B.; Dagher-Kharrat, M.B.; Derridj, A.; Slimani, S.; Badri, W.; et al. Mediterranean forests, land use and climate change: A social-ecological perspective. Reg. Environ. Chang. 2018, 18, 623–636. [Google Scholar] [CrossRef]
- FAO and Plan Bleu. State of Mediterranean Forests 2018; Food and Agriculture Organization of the United Nations, Food and Agriculture Organization of the United Nations, Rome and Plan Bleu: Marseille, France, 2018. [Google Scholar]
- Costa, R.; Lourenço, A.; Oliveira, V.; Pereira, H. Chemical characterization of cork, phloem and wood from different Quercus suber provenances and trees. Heliyon 2019, 5, 02910. [Google Scholar] [CrossRef] [PubMed]
- APCOR. APCOR’s Cork Yearbook 2018/2019; Portuguese Cork Association: Santa Maria de Lamas, Portugal, 2019. [Google Scholar]
- Touhami, I.; Chirino, E.; Aouinti, H.; El Khorchani, A.; Elaieb, M.T.; Khaldi, A.; Nasr, Z. Decline and dieback of cork oak (Quercus suber L.) forests in the Mediterranean basin: A case study of Kroumirie, Northwest Tunisia. J. For. Res. 2020, 31, 1461–1477. [Google Scholar] [CrossRef]
- Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Rego, F.C.; Rocha, M.S. Climatic patterns in the Mediterranean region. Ecol. Mediterr. 2014, 40, 49–59. [Google Scholar] [CrossRef]
- Shaw, M.W.; Osborne, T.M. Geographic distribution of plant pathogens in response to climate change. Plant Pathol. 2011, 60, 31–43. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I. Climate Change Impacts on Plant Pathogens and Plant Diseases. J. Crop. Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Moricca, S.; Linaldeddu, B.T.; Ginetti, B.; Scanu, B.; Franceschini, A.; Ragazzi, A. Endemic and Emerging Pathogens Threatening Cork Oak Trees: Management Options for Conserving a Unique Forest Ecosystem. Plant Dis. 2016, 100, 2184–2193. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Sirca, C.; Spano, D.; Franceschini, A. Variation of endophytic cork oak-associated fungal communities in relation to plant health and water stress. For. Pathol. 2011, 41, 193–201. [Google Scholar] [CrossRef][Green Version]
- Linaldeddu, B.T.; Sirca, C.; Spano, D.; Franceschini, A. Physiological responses of cork oak and holm oak to infection by fungal pathogens involved in oak decline. For. Pathol. 2009, 39, 232–238. [Google Scholar] [CrossRef]
- Luque, J.; Pera, J.; Parladé, J. Evaluation of fungicides for the control of Botryosphaeria corticola on cork oak in Catalonia (NE Spain). For. Pathol. 2008, 38, 147–155. [Google Scholar] [CrossRef]
- Serrano, M.S.; Romero, M.A.; Jiménez, J.J.; De Vita, P.; Ávila, A.; Trapero, A.; Sánchez, M.E. Preventive control of Botryosphaeria canker affecting Quercus suber in southern Spain. Forestry 2015, 88, 500–507. [Google Scholar] [CrossRef]
- Terhonen, E.; Kovalchuk, A.; Zarsav, A.; Asiegbu, F.O. Biocontrol potential of forest tree endophytes. In Endophytes of Forest Trees; Springer: Cham, Switzerland, 2018; pp. 283–318. [Google Scholar] [CrossRef]
- Martín-García, J.; Zas, R.; Solla, A.; Woodward, S.; Hantula, J.; Vainio, E.J.; Mullett, M.; Morales-Rodríguez, C.; Vannini, A.; Martínez-Álvarez, P.; et al. Environmentally friendly methods for controlling pine pitch canker. Plant Pathol. 2019, 68, 843–860. [Google Scholar] [CrossRef]
- Campanile, G.; Ruscelli, A.; Luisi, N. Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta tests. Eur. J. Plant Pathol. 2007, 117, 237–246. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Maddau, L.; Franceschini, A. Preliminary in vitro investigation on the interactions among endophytic fungi isolated from Quercus spp. IOBC WPRS Bull. 2005, 28, 101. [Google Scholar]
- Maddau, L.; Cabras, A.; Franceschini, A.; Linaldeddu, B.T.; Crobu, S.; Roggio, T.; Pagnozzi, D. Occurrence and characterization of peptaibols from Trichoderma citrinoviride, an endophytic fungus of cork oak, using electrospray ionization quadrupole time-of-flight mass spectrometry. Microbiology 2009, 155, 3371–3381. [Google Scholar] [CrossRef]
- Karami, J.; Kavosi, M.R.; Babanezhad, M.; Kiapasha, K. Integrated management of the charcoal disease by silviculture, chemical and biological methods in forest parks. J. Sustain. For. 2018, 37, 429–444. [Google Scholar] [CrossRef]
- Martins, F.; Pereira, J.A.; Bota, P.; Bento, A.; Baptista, P. Fungal endophyte communities in above- and belowground olive tree organs and the effect of season and geographic location on their structures. Fungal Ecol. 2016, 20, 193–201. [Google Scholar] [CrossRef]
- Costa, D.; Tavares, R.; Baptista, P.; Lino-Neto, T. Diversity of fungal endophytic community in Quercus suber L. under different climate scenarios. Rev. Ciências Agrárias 2018, 41, 41–50. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee SJ, W.T.; Taylor, J.L. Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. PCR Protoc. Guid. Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Pereira, J.A.; Lino-Neto, T.; Bennett, A.E.; Baptista, P. Bacterial disease induced changes in fungal communities of olive tree twigs depend on host genotype. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Tuininga, A. Interspecific interaction terminology: From mycology to general ecology. In The Fungal Community: Its Organization and Role in the Ecosystem; Dighton, J., White, J.P.O., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 274–280. [Google Scholar]
- Badalyan, S.M.; Innocenti, G.; Garibyan, N.G. Antagonistic Activity of Xylotrophic Mushrooms against Pathogenic Fungi of Cereals in Dual Culture. Phytopathol. Mediterr. 2002, 41, 220–225. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma. Trans. Br. Mycol. Soc. 1971, 57, 41–48. [Google Scholar] [CrossRef]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Purahong, W.; Wubet, T.; Hyde, K.D.; Zhang, W.; Xu, H.; Zhang, G.; Fu, C.; Liu, M.; Xing, Q.; et al. Direct comparison of culture-dependent and culture-independent molecular approaches reveal the diversity of fungal endophytic communities in stems of grapevine (Vitis vinifera). Fungal Divers. 2018, 90, 85–107. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, J.A.; Benhadi, J.; Lino-Neto, T.; Baptista, P. Endophytic and Epiphytic Phyllosphere Fungal Communities Are Shaped by Different Environmental Factors in a Mediterranean Ecosystem. Microb. Ecol. 2018, 76, 668–679. [Google Scholar] [CrossRef]
- Bragança, H.; Machado, H.; Inácio, L.; Henriques, J.; Diogo, E.; Moreira, C. Detecção de agentes potencialmente patogénicos em sobreiro e azinheira. In Abstracts of the Congresso Florestal Nacional; Vila Real/Bragança: Bragança, Portugal, 2013. [Google Scholar]
- Smahi, H.; Belhoucine-Guezouli, L.; Bouhraoua, R.T.; Franceschini, A.; Linaldeddu, B.T. First Report of Branch Canker and Dieback Caused by Cryphonectria naterciae on Quercus suber in Algeria. Plant Dis. 2018, 102, 251. [Google Scholar] [CrossRef]
- Luque, J.; Parladé, J.; Pera, J. Pathogenicity of fungi isolated from Quercus suber in Catalonia (NE Spain). For. Pathol. 2000, 30, 247–263. [Google Scholar] [CrossRef]
- Bragança, H.; Neno, J.; Henriques, J.; Diogo, E.; Alves, A. First Report of Diplodia quercivora Causing Dieback on Quercus suber and in Europe. Plant Dis. 2016, 100, 2166. [Google Scholar] [CrossRef]
- Ragazzi, A.; Turco, E.; Marianelli, L.; Dellavalle, I.; Moricca, S. Disease gradient of the anthracnose agent Apiognomonia quercina in a natural oak stand. Phytopathol. Mediterr. 2007, 46, 295–303. [Google Scholar] [CrossRef]
- Franceschini, A.; Linaldeddu, B.T.; Marras, F. Occurrence and distribution of fungal endophytes in declining cork oak forests in Sardinia (Italy). IOBC WPRS Bull. 2005, 28, 67–74. [Google Scholar]
- Ferreira, S.L.; Stauder, C.M.; Martin, D.; Kasson, M.T. Morphological and Phylogenetic Resolution of Diplodia corticola and D. quercivora, Emerging Canker Pathogens of Oak (Quercus spp.), in the United States. Plant Dis. 2020. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Franceschini, A.; Alves, A.; Phillips, A.J.L. Diplodia quercivora sp. nov.: A new species of Diplodia found on declining Quercus canariensis trees in Tunisia. Mycologia 2013, 105, 1266–1274. [Google Scholar] [CrossRef]
- Bakker, P.A.; Pieterse, C.M.; De Jonge, R.; Berendsen, R.L. The Soil-Borne Legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef]
- Liu, H.; Macdonald, C.A.; Cook, J.; Anderson, I.C.; Singh, B.K. An Ecological Loop: Host Microbiomes across Multitrophic Interactions. Trends Ecol. Evol. 2019, 34, 1118–1130. [Google Scholar] [CrossRef]
- Lombardi, N.; Vitale, S.; Turrà, D.; Reverberi, M.; Fanelli, C.; Vinale, F.; Marra, R.; Ruocco, M.; Pascale, A.; D’Errico, G.; et al. Root Exudates of Stressed Plants Stimulate and Attract Trichoderma Soil Fungi. Mol. Plant-Microbe Interact. 2018, 31, 982–994. [Google Scholar] [CrossRef]
- Crous, P.; Wingfield, M.J.; Guarro, J.; Cheewangkoon, R.; Van Der Bank, M.; Swart, W.J.; Stchigel, A.M.; Cano-Lira, J.F.; Roux, J.; Madrid, H.; et al. Fungal Planet description sheets: 154–213. Persoonia Mol. Phylogeny Evol. Fungi 2013, 31, 188–296. [Google Scholar] [CrossRef] [PubMed]
- Rahi, P.; Vyas, P.; Sharma, S.; Gulati, A.; Gulati, A. Plant growth promoting potential of the fungus Discosia sp. FIHB 571 from tea rhizosphere tested on chickpea, maize and pea. Indian J. Microbiol. 2009, 49, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Szink, I.; Davis, E.L.; Ricks, K.D.; Koide, R.T. New evidence for broad trophic status of leaf endophytic fungi of Quercus gambelii. Fungal Ecol. 2016, 22, 2–9. [Google Scholar] [CrossRef]
- Smahi, H.; Belhoucine-Guezouli, L.; Berraf-Tebbal, A.; Chouih, S.; Arkam, M.; Franceschini, A.; Linaldeddu, B.T.; Phillips, A.J.L. Molecular characterization and pathogenicity of Diplodia corticola and other Botryosphaeriaceae species associated with canker and dieback of Quercus suber in Algeria. Mycosphere 2017, 8, 1261–1272. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Ruan, G.; Yu, Y.; Liu, X. Asymmetric reduction of acetophenone into R-(+)-1-phenylethanol by endophytic fungus Neofusicoccum parvum BYEF07 isolated from Illicium verum. Biochem. Biophys. Res. Commun. 2016, 473, 874–878. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Rehn, K.G.; Menezes, M.; Mariano, R.D.L.R. Antagonism of Trichoderma species on Cladosporium herbarum and their enzimatic characterization. Braz. J. Microbiol. 2001, 32, 98–104. [Google Scholar] [CrossRef]
- Larran, S.; Perelló, A.; Simón, M.R.; Moreno, V. The endophytic fungi from wheat (Triticum aestivum L.). World J. Microbiol. Biotechnol. 2007, 23, 565–572. [Google Scholar] [CrossRef]
- Oliveira, R.R.; Aguiar, R.L.; Tessmann, D.J.; Nunes, W.M.C.; Santos, A.F.; Vida, J.B. First Report of Leaf Spot Caused by Cladosporium perangustum on Syagrus oleracea in Brazil. Plant Dis. 2014, 98, 280. [Google Scholar] [CrossRef]
- Ashkezari, S.J.; Fotouhifar, K.-B. Diversity of endophytic fungi of common yew (Taxus baccata L.) in Iran. Mycol. Prog. 2017, 16, 247–256. [Google Scholar] [CrossRef]
- Jones, D.A.; Thomas, C.M.; Hammond-Kosack, K.E.; Balint-Kurti, P.J.; Jones, J.D.G. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 1994, 266, 789–793. [Google Scholar] [CrossRef]
- Wang, X.; Gul, W.; Taráwneh, A.H.; Gao, J.; Wedge, D.E.; Rosa, L.H.; Cutler, H.G.; Cutler, S.J. Antifungal Activity against Plant Pathogens of Metabolites from the Endophytic Fungus Cladosporium cladosporioides. J. Agric. Food Chem. 2013, 61, 4551–4555. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Khan, S.A.; Ahmad, N.; Tang, D.-S.; Kang, S.-M.; Na, C.-I.; Sohn, E.-Y.; Hwang, Y.-H.; Shin, D.-H.; Lee, B.-H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Pascoe, I.; (Maher), P.M.; Smith, I.; Dinh, S.-Q.; Edwards, J. Caliciopsis pleomorpha sp. nov. (Ascomycota: Coryneliales) causing a severe canker disease of Eucalyptus cladocalyx and other eucalypt species in Australia. Fungal Syst. Evol. 2018, 2, 45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panteleev, S.V.; Baranov, O.Y.; Rubel, I.E.; Yarmolovich, V.A.; Dishuk, N.G.; Seredich, M.O. Diseases of Container-Grown Conifers in the Nurseries of Mogilev Area According to Molecular Phytopathological Survey. Proceedings of BSTU 2016, 1, 95–97. [Google Scholar]
- Alidadi, A.; Kowsari, M.; Javan-Nikkhah, M.; Jouzani, G.R.S.; Rastaghi, M.E. New pathogenic and endophytic fungal species associated with Persian oak in Iran. Eur. J. Plant Pathol. 2019, 155, 1017–1032. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Pouzoulet, J.; Rolshausen, P.E.; Wilcox, W.F.; Baumgartner, K. Characterization of Cytospora isolates from wood cankers of declining grapevine in North America, with the descriptions of two newCytosporaspecies. Plant Pathol. 2016, 66, 713–725. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, X.-L.; Crous, P.W.; Tian, C. Species of Dendrostoma (Erythrogloeaceae, Diaporthales) associated with chestnut and oak canker diseases in China. MycoKeys 2019, 48, 67–96. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Tang, W.; Miao, M.; Liu, Y. First Report of Diaporthe passiflorae and Diaporthe nobilis Causing a Postharvest Kiwifruit Rot in Sichuan Province, China. Plant Dis. 2019, 103, 771. [Google Scholar] [CrossRef]
- Elfar, K.; Torres, R.; Díaz, G.A.; Latorre, B.A. Characterization of Diaporthe australafricana and Diaporthe spp. Associated with Stem Canker of Blueberry in Chile. Plant Dis. 2013, 97, 1042–1050. [Google Scholar] [CrossRef]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Pers. Mol. Phylogeny Evol. Fungi 2013, 31, 1–41. [Google Scholar] [CrossRef]
- Venkatasubbaiah, P.; Chilton, W.S. Toxins Produced by the Dogwood Anthracnose Fungus Discula sp. J. Nat. Prod. 1991, 54, 1293–1297. [Google Scholar] [CrossRef]
- Ganley, R.J.; Brunsfeld, S.J.; Newcombe, G. A community of unknown, endophytic fungi in western white pine. Proc. Natl. Acad. Sci. USA 2004, 101, 10107–10112. [Google Scholar] [CrossRef] [PubMed]
- Castoria, R.; De Curtis, F.; Lima, G.; Caputo, L.; Pacifico, S.; De Cicco, V. Aureobasidium pullulans (LS-30) an antagonist of postharvest pathogens of fruits: Study on its modes of action. Postharvest Biol. Technol. 2001, 22, 7–17. [Google Scholar] [CrossRef]
- Wachowska, U.; Głowacka, K. Antagonistic interactions between Aureobasidium pullulans and Fusarium culmorum, a fungal pathogen of winter wheat. BioControl 2014, 59, 635–645. [Google Scholar] [CrossRef]
- Bardas, G.A.; Tzelepis, G.D.; Lotos, L.; Karaoglanidis, G.S. First Report of Penicillium glabrum Causing Fruit Rot of Pomegranate (Punica granatum) in Greece. Plant Dis. 2009, 93, 1347. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, L.; Wray, V.; Lin, W.; Kamilova, E.; Proksch, P.; Aly, A.H. New styrylpyrones from the fungal endophyte Penicillium glabrum isolated from Punica granatum. Phytochem. Lett. 2012, 5, 600–603. [Google Scholar] [CrossRef]
- Demirci, E.; Dane, E.; Eken, C. In vitro antagonistic activity of fungi isolated from sclerotia on potato tubers against Rhizoctonia solani. Turk. J. Biol. 2011, 35, 457–462. [Google Scholar] [CrossRef]
- Vega, F.E.; Posada, F.; Peterson, S.W.; Gianfagna, T.J.; Chaves, F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia 2006, 98, 31–42. [Google Scholar] [CrossRef]
- Stierle, A.A.; Stierle, D.B. Bioactive Compounds from four Endophytic Penicillium sp. of a Northwest Pacific Yew Tree. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2000; Volume 24, pp. 933–977. [Google Scholar]
- Whetzel, H.H.; Wolf, F.A. The Cup Fungus, Ciboria carunculoides, Pathogenic on Mulberry Fruits. Mycologia 1945, 37, 476. [Google Scholar] [CrossRef]
- Kehr, R.D. Pezicula canker of Quercus rubra L., caused by Pezicula cinnamomea (DC.) Sacc. I. Symptoms and pathogenesis. Eur. J. For. Pathol. 1991, 21, 218–233. [Google Scholar] [CrossRef]
- Bissegger, M.; Sieber, T.N. Assemblages of Endophytic Fungi in Coppice Shoots of Castanea sativa. Mycologia 1994, 86, 648. [Google Scholar] [CrossRef]
- Chen, C.; Verkley, G.J.M.; Sun, G.; Groenewald, J.Z.; Crous, P.W. Redefining common endophytes and plant pathogens in Neofabraea, Pezicula, and related genera. Fungal Biol. 2016, 120, 1291–1322. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Verkley, G.J.M. Pezicula neosporulosa sp. nov. (Helotiales, Ascomycota), an endophytic fungus associated with Abies spp. in China and Europe. Mycoscience 2015, 56, 205–213. [Google Scholar] [CrossRef]
- McMullin, D.R.; Green, B.D.; Prince, N.C.; Tanney, J.B.; Miller, J.D. Natural Products of Picea Endophytes from the Acadian Forest. J. Nat. Prod. 2017, 80, 1475–1483. [Google Scholar] [CrossRef]
- Liu, K.H.; Ding, X.; Deng, B.W.; Chen, W. Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis. J. Ind. Microbiol. Biotechnol. 2009, 36, 1171–1177. [Google Scholar] [CrossRef]
- Schulz, B.; Sucker, J.; Aust, H.J.; Krohn, K.; Ludewig, K.; Jones, P.G.; Döring, D. Biologically active secondary metabolites of endophytic Pezicula species. Mycol. Res. 1995, 99, 1007–1015. [Google Scholar] [CrossRef]
- Xue, A.G. Biological Control of Pathogens Causing Root Rot Complex in Field Pea Using Clonostachys rosea Strain ACM941. Phytopathology 2003, 93, 329–335. [Google Scholar] [CrossRef]
- Cannon, P.F.; Simmons, C.M. Diversity and host preference of leaf endophytic fungi in the Iwokrama Forest Reserve, Guyana. Mycologia 2002, 94, 210–220. [Google Scholar] [CrossRef]
- Madar, Z.; Kimchi, M.; Solel, Z. Fusarium canker of Italian cypress. Eur. J. For. Pathol. 1996, 26, 107–112. [Google Scholar] [CrossRef]
- Manici, L.M.; Kelderer, M.; Franke-Whittle, I.H.; Rühmer, T.; Baab, G.; Nicoletti, F.; Caputo, F.; Topp, A.; Insam, H.; Naef, A. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl. Soil Ecol. 2013, 72, 207–214. [Google Scholar] [CrossRef]
- Di Pietro, A.; García-Maceira, F.I.; Méglecz, E.; Roncero, M.I.G. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 2001, 39, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Bolwerk, A.; Lagopodi, A.L.; Lugtenberg, B.J.J.; Bloemberg, G.V. Visualization of Interactions Between a Pathogenic and a Beneficial Fusarium Strain During Biocontrol of Tomato Foot and Root Rot. Mol. Plant-Microbe Interact. 2005, 18, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Kour, A.; Shawl, A.S.; Rehman, S.; Sultan, P.; Qazi, P.H.; Suden, P.; Khajuria, R.K.; Verma, V. Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J. Microbiol. Biotechnol. 2008, 24, 1115–1121. [Google Scholar] [CrossRef]
- Fernández-Silva, F.; Capilla, J.; Mayayo, E.; Sutton, D.; Guarro, J. In VitroEvaluation of Antifungal Drug Combinations against Sarocladium (Acremonium) kiliense, an Opportunistic Emergent Fungus Resistant to Antifungal Therapies. Antimicrob. Agents Chemother. 2014, 58, 1259–1260. [Google Scholar] [CrossRef][Green Version]
- Campos, L.A. Caracterização de Leveduras Promotoras do Crescimento de Plantas; RIUFSC: Florianópolis, Brazil, 2017. [Google Scholar]
- Yuan, W.H.; Jiang, N.; Dong, C.H.; Wei, Z.W.; Wu, H.K.; Chen, C.F.; Zhao, Y.X.; Zhou, S.L.; Zhang, M.M.; Zheng, W.F. Lasiodiplodin analogues from the endophytic fungus Sarocladium kiliense. Chem. Pharm. Bull. 2013, 61, 363–365. [Google Scholar] [CrossRef]
- Tschen, J.S.M.; Chen, L.L.; Hsieh, S.T.; Wu, T.S. Isolation and phytotoxic effects of helvolic acid from plant pathogenic fungus Sarocladium oryzae. Bot. Bull. Acad. Sin. 1997, 38, 251–256. [Google Scholar]
- Potshangbam, M.; Indira, S.; Sahoo, D.; Strobel, G.A. Functional Characterization of Endophytic Fungal Community Associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef]
- Gomes, A.A.M.; Pinho, D.B.; Cardeal, Z.D.L.; Menezes, H.C.; De Queiroz, M.V.; Pereira, O.L. Simplicillium coffeanum, a new endophytic species from Brazilian coffee plants, emitting antimicrobial volatiles. Phytotaxa 2018, 333, 188–198. [Google Scholar] [CrossRef]
- Herrero, N.; Zabalgogeazcoa, I. Mycoviruses infecting the endophytic and entomopathogenic fungus Tolypocladium cylindrosporum. Virus Res. 2011, 160, 409–413. [Google Scholar] [CrossRef]
- Hanada, R.E.; Pomella, A.W.V.; Costa, H.S.; Bezerra, J.L.; Loguercio, L.L.; Pereira, J.O. Endophytic fungal diversity in Theobroma cacao (cacao) and T. grandiflorum (cupuaçu) trees and their potential for growth promotion and biocontrol of black-pod disease. Fungal Biol. 2010, 114, 901–910. [Google Scholar] [CrossRef]
- Deng, Z.; Li, C.; Luo, D.; Teng, P.; Guo, Z.; Tu, X.; Zou, K.; Gong, D. A new cinnamic acid derivative from plant-derived endophytic fungus Pyronema sp. Nat. Prod. Res. 2017, 31, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Botella, L.; Diez, J.J. Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis. Fungal Divers. 2011, 47, 9–18. [Google Scholar] [CrossRef]
- Ferreira, A.B.M.; Leite, L.G.; Hernandes, J.L.; Harakava, R.; Padovani, C.R.; Bueno, C.J. Colonization of vines by Petri disease fungi, susceptibility of rootstocks to Phaeomoniella chlamydospora and their disinfection. Arquivos Instituto Biológico 2018, 85. [Google Scholar] [CrossRef]
- Lacerda, L.T.; Gusmão, L.F.P.; Rodrigues, A. Diversity of endophytic fungi in Eucalyptus microcorys assessed by complementary isolation methods. Mycol. Prog. 2018, 17, 719–727. [Google Scholar] [CrossRef]
- Peever, T.L.; Ibañez, A.; Akimitsu, K.; Timmer, L.W. Worldwide Phylogeography of the Citrus Brown Spot Pathogen, Alternaria alternata. Phytopathology 2002, 92, 794–802. [Google Scholar] [CrossRef]
- Soltani, J.; Moghaddam, M.S.H. Antiproliferative, Antifungal, and Antibacterial Activities of Endophytic Alternaria Species from Cupressaceae. Curr. Microbiol. 2014, 69, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Jinu, M.; Jayabaskaran, C. Diversity and anticancer activity of endophytic fungi associated with the medicinal plant Saraca asoca. Curr. Res. Environ. Appl. Mycol. 2015, 5, 169–179. [Google Scholar] [CrossRef]
- Qadri, M.; Rajput, R.; Abdin, M.Z.; Vishwakarma, R.A.; Riyaz-Ul-Hassan, S. Diversity, Molecular Phylogeny, and Bioactive Potential of Fungal Endophytes Associated with the Himalayan Blue Pine (Pinus wallichiana). Microb. Ecol. 2014, 67, 877–887. [Google Scholar] [CrossRef]
- Ibrahim, A.; Sørensen, D.; Jenkins, H.A.; Ejim, L.; Capretta, A.; Sumarah, M.W. Epoxynemanione A, nemanifuranones A–F, and nemanilactones A–C, from Nemania serpens, an endophytic fungus isolated from Riesling grapevines. Phytochemistry 2017, 140, 16–26. [Google Scholar] [CrossRef]
- Abed-Ashtiani, F.; Narmani, A.; Arzanlou, M. Analysis of Kalmusia variispora associated with grapevine decline in Iran. Eur. J. Plant Pathol. 2019, 154, 787–799. [Google Scholar] [CrossRef]
- Ghobad-Nejhad, M.; Asgari, B.; Dokhaharani, S.C. Notes on some endophytic fungi isolated from Quercus brantii in Dena Region of Kohgiluyeh and Boyer-Ahmad Province. Iran. Mycol. Iran. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Kwaśna, H.; Szewczyk, W.; Behnke-Borowczyk, J. Fungal root endophytes of Quercus robur subjected to flooding. For. Pathol. 2015, 46, 35–46. [Google Scholar] [CrossRef]
- Strobel, G.; Singh, S.K.; Riyaz-Ul-Hassan, S.; Mitchell, A.; Geary, B.; Sears, J. An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol. Lett. 2011, 320, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.A.; Gloer, J.B. The preussomerins: Novel antifungal metabolites from the coprophilous fungus Preussia isomera Cain. J. Org. Chem. 1991, 56, 4355–4360. [Google Scholar] [CrossRef]
- Pulina, M.A.; Linaldeddu, B.T.; Franceschini, A. Topoclimats et communautés des champignons endophytiques dans des bois de chênes-lièges dépéris et non dépéris en Sardaigne (Italie). Proceddings of XIXe COLLOQUE INTERNATIONAL DE CLIMATOLOGIE, Epernay, France, 6–9 September 2006; pp. 474–479. [Google Scholar]
- Summerell, B.A. Diseases of Proteaceae. In Handbook of Plant Disease Management; McGovern, R.E.W., Ed.; Springer: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar]
- Zhao, S.-S.; Zhang, Y.-Y.; Yan, W.; Cao, L.-L.; Xiao, Y.; Ye, Y.-H. Chaetomium globosum CDW7, a potential biological control strain and its antifungal metabolites. FEMS Microbiol. Lett. 2016. [Google Scholar] [CrossRef]
- Qin, C.; Tao, J.; Liu, T.; Liu, Y.; Xiao, N.; Li, T.; Gu, Y.; Yin, H.; Meng, D. Responses of phyllosphere microbiota and plant health to application of two different biocontrol agents. AMB Express 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Tveit, M.; Wood, R.K.S. The control of Fusarium blight in oat seedlings with antagonistic species of chaetomium. Ann. Appl. Biol. 1955, 43, 538–552. [Google Scholar] [CrossRef]
- Fisher, P.; Petrini, O.; Petrini, L. Endophytic ascomycetes and deuteromycetes in roots of Pinus sylvestris. Nov. Hedwig. 1991, 52, 11–15. [Google Scholar]
- Deng, L.; Niu, S.; Liu, X.; Che, Y.; Li, E. Coniochaetones E–I, new 4H-chromen-4-one derivatives from the Cordyceps-colonizing fungus Fimetariella sp. Fitoterapia 2013, 89, 8–14. [Google Scholar] [CrossRef]
- Bashiri, S.; Abdollahzadeh, J.; Di Lecce, R.; Alioto, D.; Górecki, M.; Pescitelli, G.; Masi, M.; Evidente, A. Rabenchromenone and Rabenzophenone, Phytotoxic Tetrasubstituted Chromenone and Hexasubstituted Benzophenone Constituents Produced by the Oak-Decline-Associated Fungus Fimetariella rabenhorstii. J. Nat. Prod. 2020, 83, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.H.; Li, D.-L.; Zhang, W.-M.; Tan, J.-W.; Wei, X.-Y. [Study on the chemical constituents of endophytic fungus Fimetariella rabenhorstii isolated from Aquilaria sinensis]. Zhong Yao Cai 2011, 34, 221–223. [Google Scholar] [PubMed]
- Liarzi, O.; Bar, E.; Lewinsohn, E.; Ezra, D. Use of the Endophytic Fungus Daldinia cf. concentrica and Its Volatiles as Bio-Control Agents. PLoS ONE 2016, 11, e0168242. [Google Scholar] [CrossRef]
- Higginbotham, S.J.; Arnold, A.E.; Ibañez, A.; Spadafora, C.; Coley, P.D.; Kursar, T.A. Bioactivity of Fungal Endophytes as a Function of Endophyte Taxonomy and the Taxonomy and Distribution of Their Host Plants. PLoS ONE 2013, 8, e73192. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.H.; Kunimoto, R.K. Quick decline of macadamia trees: Association with Xylaria arbuscula. Plant Pathol. 1991, 40, 643–644. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, G.J.; Lee, H.B.; Kim, K.M.; Jung, H.S.; Lee, S.W.; Jang, K.S.; Cho, K.Y.; Kim, J.C. Griseofulvin from Xylaria sp. Strain F0010, an endophytic fungus of Abies holophylla and its antifungal activity against plant pathogenic fungi. J. Microbiol. Biotechnol. 2005, 15, 112–117. [Google Scholar]
- Liu, X.; Dong, M.; Chen, X.; Jiang, M.; Lv, X.; Yan, G. Antioxidant activity and phenolics of an endophytic Xylaria sp. from Ginkgo biloba. Food Chem. 2007, 105, 548–554. [Google Scholar] [CrossRef]
- Adams, G.C.; Kropp, B.R. Athelia arachnoidea, the sexual state of Rhizoctonia carotae, a pathogen of carrot in cold storage. Mycologia 1996, 88, 459–472. [Google Scholar] [CrossRef]
- Kotasthane, A.S.; Agrawal, T.; Kushwah, R.; Rahatkar, O.V. In-vitro antagonism of Trichoderma spp. against Sclerotium rolfsii and Rhizoctonia solani and their response towards growth of cucumber, bottle gourd and bitter gourd. Eur. J. Plant Pathol. 2014, 141, 523–543. [Google Scholar] [CrossRef]
- Ujor, V.C.; Adukwu, E.C.; Okonkwo, C.C. Fungal wars: The underlying molecular repertoires of combating mycelia. Fungal Biol. 2018, 122, 191–202. [Google Scholar] [CrossRef]
- Escano-Calderón, C.; Rotem, N.; Harris, R.; Vela-Corcía, D.; Levy, M. Pseudozyma aphidis activates reactive oxygen species production, programmed cell death and morphological alterations in the necrotrophic fungusBotrytis cinerea. Mol. Plant Pathol. 2019, 20, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Muhammad, S.A.; Khan, I.; Qazi, M.A.; Shahzadi, I.; Mumtaz, A.; Hashmi, M.A.; Khan, A.K.; Ismail, T. Chaetomium endophytes: A repository of pharmacologically active metabolites. Acta Physiol. Plant. 2016, 38, 1–18. [Google Scholar] [CrossRef]
- Yang, N. Secondary Metabolites Isolated from Coniothyrium Species. Nat. Prod. J. 2017, 7, 248–254. [Google Scholar] [CrossRef]
- Tanapichatsakul, C.; Monggoot, S.; Gentekaki, E.; Pripdeevech, P. Antibacterial and Antioxidant Metabolites of Diaporthe spp. Isolated from Flowers of Melodorum fruticosum. Curr. Microbiol. 2018, 75, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Gandía, M.; Monge, A.; Garrigues, S.; Orozco, H.; Giner-Llorca, M.; Marcos, J.F.; Manzanares, P. Novel insights in the production, activity and protective effect of Penicillium expansum antifungal proteins. Int. J. Biol. Macromol. 2020, 164, 3922–3931. [Google Scholar] [CrossRef]
- Chen, R.-S.; Huang, C.-C.; Li, J.-C.; Tsay, J.-G. Evaluation of characteristics of Simplicillium lanosoniveum on pathogenicity to aphids and in vitro antifungal potency against plant pathogenic fungi. Int. J. Environ. Agric. Res. 2017, 3, 55–61. [Google Scholar]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Shin, T.S.; Yu, N.H.; Lee, J.; Choi, G.J.; Kim, J.-C.; Shin, C.S. Development of a Biofungicide Using a Mycoparasitic Fungus Simplicillium lamellicola BCP and Its Control Efficacy against Gray Mold Diseases of Tomato and Ginseng. Plant Pathol. J. 2017, 33, 337–344. [Google Scholar] [CrossRef]
- Fravel, D.; Olivain, C.; Alabouvette, C. Fusarium oxysporum and its biocontrol. New Phytol. 2003, 157, 493–502. [Google Scholar] [CrossRef]
- Gauthier, N.A.W.; Robertson, C.L.; Chanda, A.K.; Schneider, R.W. Effects of Simplicillium lanosoniveum on Phakopsora pachyrhizi, the Soybean Rust Pathogen, and Its Use as a Biological Control Agent. Phytopathology 2012, 102, 749–760. [Google Scholar] [CrossRef]
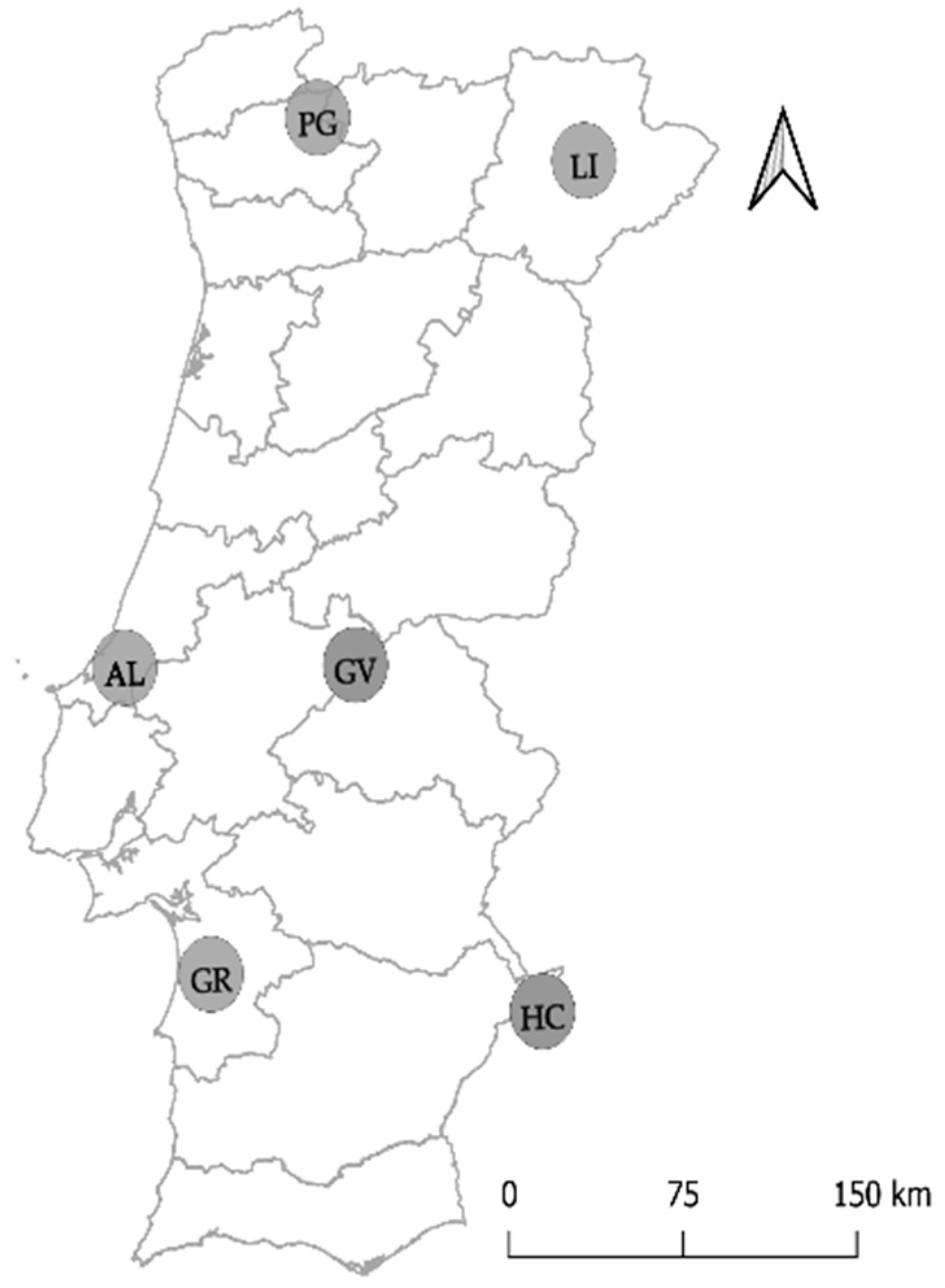
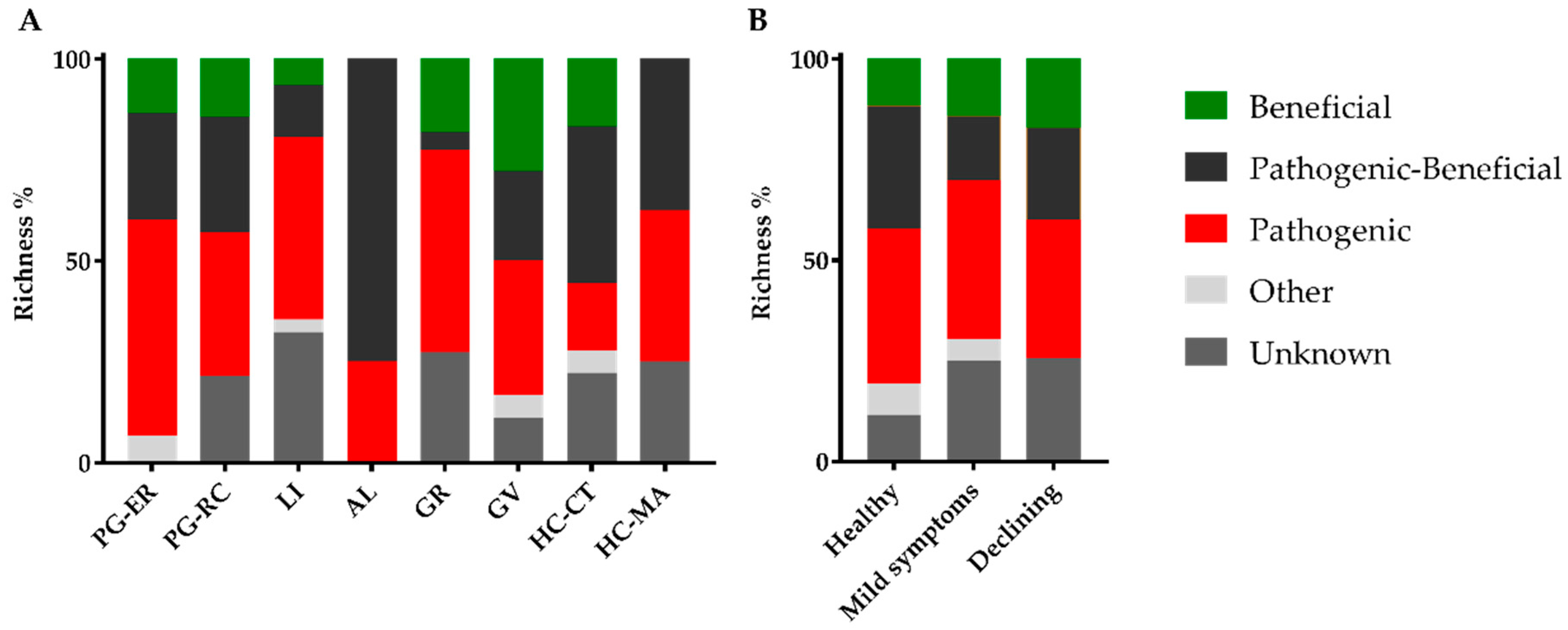
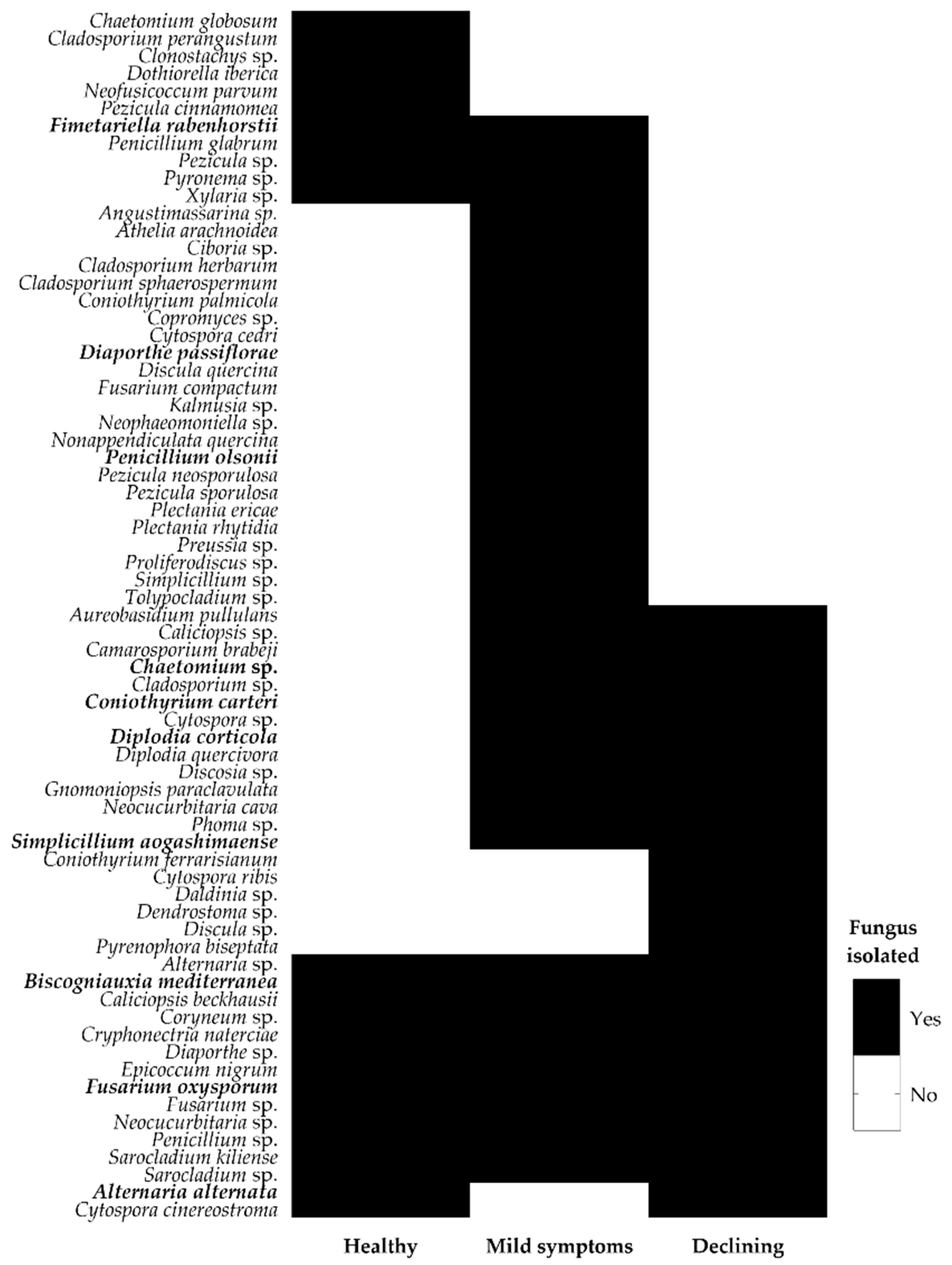
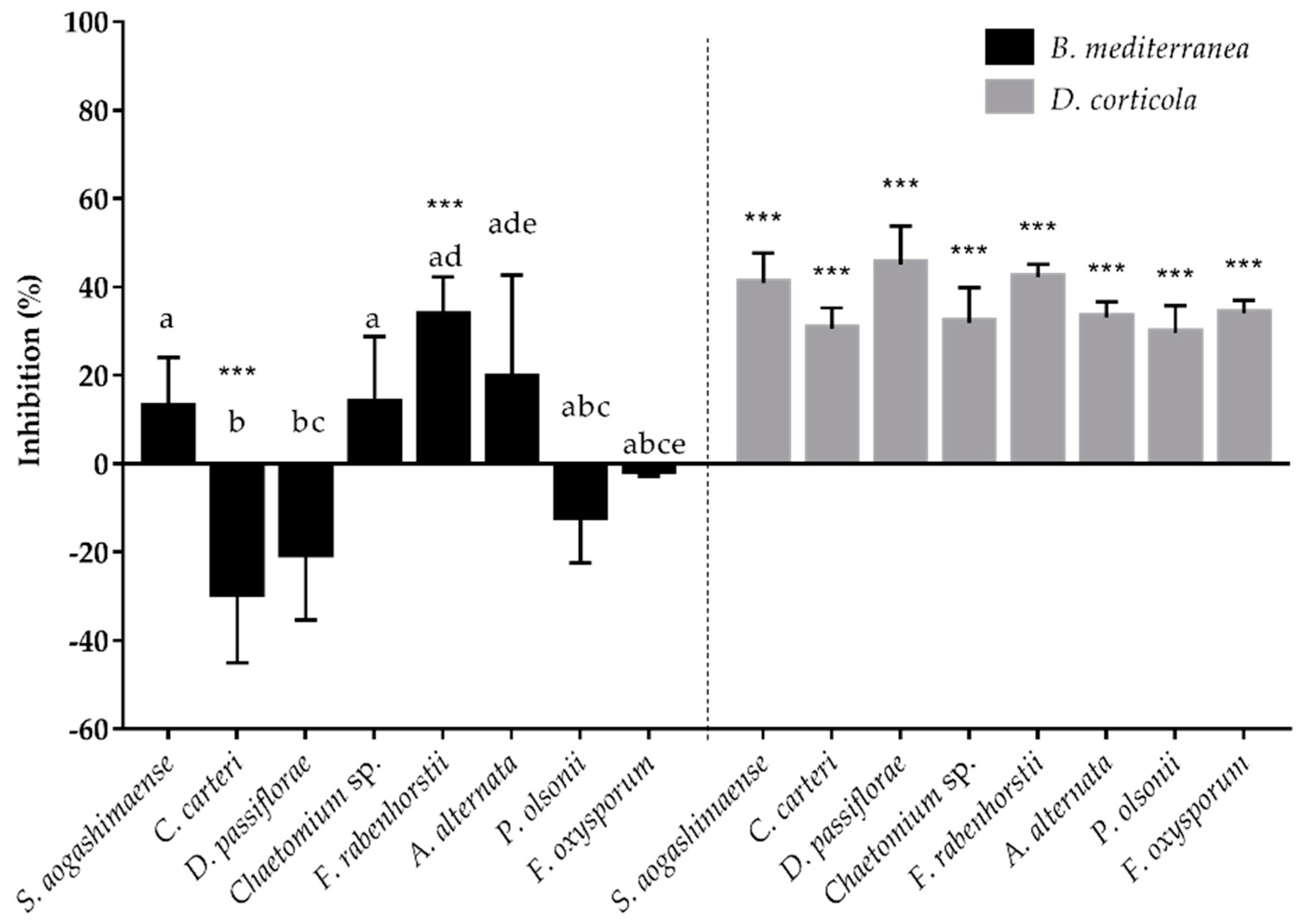
| Location | Cork Oak Stand | Collection Date | Disease Severity Level | ||
|---|---|---|---|---|---|
| Healthy | Mild Symptoms | Declining | |||
| Peneda-Gerês | PG-ER | May, 2017 | 2 | 3 | 0 |
| PG-RC | July, 2017 | 4 | 1 | 0 | |
| Limãos | LI | April, 2017 | 0 | 5 | 1 |
| Gavião | GV | July, 2017 | 0 | 4 | 2 |
| Alcobaça | AL | May, 2017 | 2 | 2 | 2 |
| Grândola | GR | May, 2017 | 0 | 3 | 3 |
| Herdade Contenda | HC-CT | October, 2017 | 2 | 2 | 2 |
| HC-MA | October, 2017 | 2 | 2 | 2 | |
| Taxonomic Classification | Closest Match GenBank | Cork Oak Forests | Functional Group | Identified Endophyte | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PG-ER | PG-RC | LI | AL | GV | GR | HC-CT | HC-MA | |||||
| Ascomycota | ||||||||||||
| Amphisphaeriales | Discosia sp. | KU325138.1 (100%) | x | x | P [46]/B [47] | [48] | ||||||
| Nonappendiculata quercina | MH554025.1 (98.78%) | x | U | - | ||||||||
| Botryosphaeriales | Diplodia corticola | MT015621.1 (100%) | x | P [37] | [40] | |||||||
| Diplodia quercivora | JX894205.1 (97.72%) | x | P[38] | [11] | ||||||||
| Dothiorella iberica | MT261024.1 (100%) | x | P[49] | [12] | ||||||||
| Neofusicoccum parvum | MT645697.1 (99.3%) | x | P[35] | [50] | ||||||||
| Capnodiales | Cladosporium herbarum | LT854669.1 (99.22%) | x | P [51] | [52] | |||||||
| Cladosporium perangustum | MK111614.1 (99.10%) | x | P [53] | [54] | ||||||||
| Cladosporium sp. | MN879328.1 (100%) | x | x | P [55]/B [56] | [40] | |||||||
| Cladosporium sphaerospermum | MT645920.1 (99.51%) | x | B [57] | [57] | ||||||||
| Coryneliales | Caliciopsis beckhausii | NR_132090.1 (99.57%) | x | x | U | - | ||||||
| Caliciopsis sp. | NR_132090.1 (91.91%) | x | x | P [58] | - | |||||||
| Diaporthales | Coryneum sp. | MH674330.1 (95.54%) | x | x | x | x | P [35] | [40] | ||||
| Cryphonectria naterciae | MT645942.1 (100%) | x | x | x | x | P [36] | - | |||||
| Cytospora cedri | MN871816.1 (100%) | x | x | x | P [59] | - | ||||||
| Cytospora cinereostroma | KY051964.1 (100%) | x | x | U | - | |||||||
| Cytospora ribis | KP641138.1 (100%) | x | U | [60] | ||||||||
| Cytospora sp. | MK656248.1 (100%) | x | x | P [61] | [40] | |||||||
| Dendrostoma sp. | MN447228.1 (99.66%) | x | P [62] | - | ||||||||
| Diaporthe passiflorae | NR_120155.1 (99.82%) | x | O [63]/P [64] | - | ||||||||
| Diaporthe sp. | MT561408.1 (99.48%) | x | x | x | P [65] | [65] | ||||||
| Discula quercina | MH758705.1 (99.18%) | x | P [39] | [40] | ||||||||
| Discula sp. | KY367498.2 (94.23%) | x | P [66] | [67] | ||||||||
| Gnomoniopsis paraclavulata | MH863162.1 (100%) | x | x | U | - | |||||||
| Dothideales | Aureobasidium pullulans | MT645930.1 (99.57%) | x | O [68]/B [69] | [40] | |||||||
| Eurotiales | Penicillium glabrum | MT582777.1 (100%) | x | x | x | P [70] | [71] | |||||
| Penicillium olsonii | MT582783.1 (100%) | x | B [72] | [73] | ||||||||
| Penicillium sp. | LN901128.1 (99.54%) | x | x | x | x | x | O [74]/P [70]/B [72] | [40] | ||||
| Helotiales | Ciboria sp. | KF545322.1 (94.59%) | x | P [75] | - | |||||||
| Pezicula cinnamomea | MK907714.1 (100%) | x | P [76] | [77] | ||||||||
| Pezicula neosporulosa | KR859231.1 (100%) | x | P [78] | [79] | ||||||||
| Pezicula sporulosa | MH862573.1 (98.66%) | x | O[80] | [81] | ||||||||
| Pezicula sp. | MG098317.1 (100%) | x | x | O [82]/P [76] | [67] | |||||||
| Proliferodiscus sp. | MN901941.1 (95.50%) | x | U | - | ||||||||
| Hypocreales | Clonostachys sp. | MK789204.1 (91.84%) | x | B [83] | [84] | |||||||
| Fusarium compactum | KJ562364.1 (98.53%) | x | P [85] | [86] | ||||||||
| Fusarium oxysporum | MT530243.1 (100%) | x | x | x | x | P [87]/B [88] | [89] | |||||
| Fusarium sp. | MT645120.1 (100%) | x | x | x | x | x | x | P [87]/B [18] | [18] | |||
| Sarocladium kiliense | MK789203.1 (100%) | x | x | x | x | x | P [90]/B [91] | [92] | ||||
| Sarocladium sp. | MT645143.1 (99.36%) | x | x | x | x | P [93]/B [91] | [94] | |||||
| Simplicillium aogashimaense | MK685280.1 (99.82%) | x | x | U | - | |||||||
| Simplicillium sp. | MH859771.1 (99.12%) | x | x | x | B [95] | [95] | ||||||
| Tolypocladium sp. | KX034386.1 (100%) | x | O [96] | [97] | ||||||||
| Pezizales | Plectania rhytidia | MH003435.1 (98.99%) | x | x | U | [27] | ||||||
| Pseudoplectania ericae | MT498082.1 (99.65%) | x | U | - | ||||||||
| Pyronema sp. | MT556695.1 (100%) | x | x | O [98] | [99] | |||||||
| Phaeomoniellales | Neophaeomoniella sp. | MK646052.1 (96.14%) | x | P [100] | [101] | |||||||
| Pleosporales | Alternaria alternata | MT635274.1 (100%) | x | x | P [102]/B [103] | [40] | ||||||
| Alternaria sp. | MT557456.1 (100%) | x | x | x | x | P [104]/B [103] | [40] | |||||
| Angustimassarina sp. | MN963689.1 (100%) | x | U | - | ||||||||
| Camarosporium brabeji | LN714529.1 (97.76%) | x | x | U | [105] | |||||||
| Coniothyrium carteri | KX359604.1 (99.82%) | x | x | x | B [106] | [106] | ||||||
| Coniothyrium ferrarisianum | MH860854.1 (100%) | x | U | [107] | ||||||||
| Coniothyrium palmicola | JX681086.1 (99.53%) | x | U | - | ||||||||
| Epicoccum nigrum | MT548679.1 (100%) | x | x | x | B [27] | [40] | ||||||
| Kalmusia sp. | MK796143.1 (100%) | x | x | P [108] | [109] | |||||||
| Neocucurbitaria cava | MK796144.1 (100%) | x | x | x | U | [110] | ||||||
| Neocucurbitaria sp. | MH858303.1 (93.78%) | x | x | x | x | x | U | [110] | ||||
| Phoma sp. | KX815489.1 (100%) | x | x | P [111] | [40] | |||||||
| Preussia sp. | MN696547.1 (100%) | x | B [112] | [113] | ||||||||
| Pyrenophora biseptata | MH864748.1 (100%) | x | P [114] | - | ||||||||
| Sordariales | Chaetomium globosum | MT588864.1 (100%) | x | B [115] | [116] | |||||||
| Chaetomium sp. | MN153902.1 | x | x | x | B [117] | [118] | ||||||
| Copromyces sp. | (100%) | x | U | - | ||||||||
| Fimetariella rabenhorstii | MN555335.1 (100%) | x | x | x | O [119]/P [120] | [121] | ||||||
| Xylariales | Biscogniauxia mediterranea | MT862330.1 (100%) | x | x | x | x | x | x | x | x | P [12] | [40] |
| Daldinia sp. | MN341734.1 (97.83%) | x | B [122] | [123] | ||||||||
| Xylaria sp. | JQ761730.1 (99.78%) | x | x | P [124]/B [125] | [126] | |||||||
| Basidiomycota | ||||||||||||
| Atheliales | Athelia arachnoidea | MH860510.1 (100%) | x | P [127] | - | |||||||
| Endophyte | B. Mediterranea | D. Corticola | ||
|---|---|---|---|---|
| Type of Interaction | Mycelial Interaction | Type of Interaction | Mycelial Interaction | |
| S. aogashimaense | antagonism (0/-) | B | antagonism (0/-) | B |
| C. carteri | agonism (-/+) | CA2 | co-antagonism (-/-) | CA2 |
| D. passiflorae | agonism (-/+) | CB1 | co-antagonism (-/-) | CB1 |
| F. rabenhorstii | co-antagonism (-/-) | A | co-antagonism (-/-) | CA1 |
| F. oxysporum | commensalism (+/0) | CA1 | antagonism (0/-) | CA1 |
| Chaetomium sp. | agonism (+/-) | CA1 | co-antagonism (-/-) | CA1 |
| A. alternata | agonism (+/-) | CA1 | antagonism (0/-) | CA1 |
| P. olsonii | agonism (-/+) | CB1 | co-antagonism (-/-) | CA1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, D.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. Cork Oak Endophytic Fungi as Potential Biocontrol Agents against Biscogniauxia mediterranea and Diplodia corticola. J. Fungi 2020, 6, 287. https://doi.org/10.3390/jof6040287
Costa D, Tavares RM, Baptista P, Lino-Neto T. Cork Oak Endophytic Fungi as Potential Biocontrol Agents against Biscogniauxia mediterranea and Diplodia corticola. Journal of Fungi. 2020; 6(4):287. https://doi.org/10.3390/jof6040287
Chicago/Turabian StyleCosta, Daniela, Rui M. Tavares, Paula Baptista, and Teresa Lino-Neto. 2020. "Cork Oak Endophytic Fungi as Potential Biocontrol Agents against Biscogniauxia mediterranea and Diplodia corticola" Journal of Fungi 6, no. 4: 287. https://doi.org/10.3390/jof6040287
APA StyleCosta, D., Tavares, R. M., Baptista, P., & Lino-Neto, T. (2020). Cork Oak Endophytic Fungi as Potential Biocontrol Agents against Biscogniauxia mediterranea and Diplodia corticola. Journal of Fungi, 6(4), 287. https://doi.org/10.3390/jof6040287







