Abstract
Plants belonging to the genus Copaifera are widely used in Brazil due to their antimicrobial properties, among others. The re-emergence of classic fungal diseases as a consequence of antifungal resistance to available drugs has stimulated the search for plant-based compounds with antifungal activity, especially against Candida. The Candida-infected Caenorhabditis elegans model was used to evaluate the in vitro antifungal potential of Copaifera leaf extracts and trunk oleoresins against Candida species. The Copaifera leaf extracts exhibited good antifungal activity against all Candida species, with MIC values ranging from 5.86 to 93.75 µg/mL. Both the Copaifera paupera and Copaifera reticulata leaf extracts at 46.87 µg/mL inhibited Candida glabrata biofilm formation and showed no toxicity to C. elegans. The survival of C. glabrata-infected nematodes increased at all the tested extract concentrations. Exposure to Copaifera leaf extracts markedly increased C. glabrata cell vacuolization and cell membrane damage. Therefore, Copaifera leaf extracts are potential candidates for the development of new and safe antifungal agents.
1. Introduction
Although modern medicine is well developed in most parts of the world, WHO recognizes that a large part of the population in developing countries depends on traditional medicine for primary care [1]. In this regard, Brazil is the most biodiverse country on the planet and holds valuable traditional knowledge associated with the use of medicinal plants [2].
Copaifera (Leguminosae Juss., subfamily Caesalpinoideae Kunth) is a genus of large trees that comprises more than 72 species occurring in Latin America, India, and West Africa. Sixteen of these species are found only in Brazil, mainly in the northern and northeastern regions, in the states of Amazonas, Pará, and Ceará [3,4]. Plant derivatives such as oleoresins and essential oils are widely employed in folk medicine; the use of leaf extracts is less common, but some reports have demonstrated their antiurolithiac, antiedematogenic, and gastroprotective potential, which were mostly related to the presence of galloylquinic acid derivatives and flavonoids [5,6,7].
In the last three decades, antifungal resistance has caused great concern and stimulated studies for the discovery of new antifungal agents, especially against Candida spp., given that classical fungal diseases have re-emerged due to the development of antifungal resistance. Candida albicans is the most prevalent species among Candida infections, but other species such as C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei (Pichia kudriavzevii/Issatchenkia orientalis) also underlie human infections [8,9]. Even though C. parapsilosis currently consists of a complex of three related species, namely, C. parapsilosis sensu lato, C. orthopsilosis, and C. metapsilosis, Brazilian isolates of the latter species have been reported at a lower frequency [10]. In addition, some Candida species, like C. glabrata and C. krusei, have different degrees of susceptibility and may be resistant to antifungals that are employed in clinical practice [11].
However, the biocompatibility of new products or products of unknown action should be analyzed by in vitro cell culture studies and animal experiments before such products are applied to humans. Studies on invertebrates have been conducted more frequently, and the use of the nematode Caenorhabditis elegans to investigate infections, virulence, toxicity, and antifungal drug activity [12] is noteworthy.
In view of the emergence of resistant strains and the expansion of fungal virulence mechanisms, we evaluate the Candida species susceptibility profile against Copaifera species extracts and oleoresins by determining their minimal inhibitory concentration values, their ability to inhibit biofilm formation, and their effect when associated with amphotericin B. In addition, the potential cellular target, as well as the toxicity of these plant-derived products, was assessed.
2. Materials and Methods
2.1. Plant Material
The Copaifera oleoresins and leaf extracts were obtained and analyzed for their chemical compositions, as previously described by our group [5]. Briefly, the C. duckei, C. langsdorffii, C. paupera, C. reticulata, C. trapezifolia, and C. pubiflora oleoresins were collected from holes made in the center of the tree trunks. The leaf extracts were prepared by macerating the ground air-dried C. duckei, C. langsdorffii, C. lucens, C. multijuga, C. oblongifolia, C. paupera, C. reticulata, and C. trapezifolia leaves in 7:3 ethanol/water at room temperature for 48 h. The extracts were concentrated under vacuum and lyophilized. Then, the plant-derived products were chemically characterized by UPLC–MS/MS (oleoresins) and HPLC (leaf extracts), as previously published by our group [5].
2.2. Microorganisms
The Candida strains were acquired from the American Type Culture Collection (ATCC) and included C. albicans ATCC 5314, C. glabrata ATCC 2001, C. parapsilosis ATCC 22019, C. krusei ATCC 6258, and C. tropicalis ATCC 13803. The strains were cryopreserved at −80 °C. At the time of the assays, they were inoculated in Sabouraud dextrose agar (SBA, Difco Laboratories, Detroit, MI, USA) and Chromagar Candida (Becton Dickinson and Company, Sparks, MD, USA) at 37 °C for 48 h in order to evaluate their purity and viability.
2.3. Antifungal Activity Evaluation
The methodology was based on the studies published by Santos et al. [13] and the Clinical Laboratory Standards Institute (CLSI) protocols M27-A2 S4 [14] and M60 [15]. The leaf extracts or oleoresins were solubilized in dimethyl sulfoxide (2% DMSO, v/v) and diluted in Roswell Park Memorial Institute (RPMI) 1640 medium. The tested concentrations ranged from 1.46 to 750 μg/mL. Yeast-containing cell suspensions were prepared and diluted in RPMI to reach a final concentration of 0.5 × 103 to 2.5 × 103 CFU/mL. Each yeast cell suspension was inoculated into 96-well microplates that had previously been prepared with the dilutions of the Copaifera samples, which was followed by incubation at 37 °C for 48 h. The wells containing only inoculum and culture medium were considered as 100% organism viability. The addition of 0.015% aqueous rezasurin solution and further incubation at 37 °C for 3 h helped to visualize yeast viability [16]. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of antifungal agent that maintained blue staining in the supernatant medium. Controls consisting of 2% DMSO (v/v), RPMI medium without inoculum, and plant extracts diluted in RPMI were also included. The C. krusei ATCC 6258 and C. parapsilosis ATCC 22,019 strains and amphotericin B (AMB) at concentrations ranging from 0.015 to 8 μg/mL were employed as test quality controls [14]. Plant-derived products are considered good, moderate, weak, and inactive antimicrobials when they display MIC values lower than 100 µg/mL, from 100 to 500 µg/mL, from 500 to 1000 µg/mL, and above 1000 µg/mL, respectively [17,18]. The tests were performed in triplicate, and the mean value was used as a result. These tests allowed us to select the most active Copaifera species and the most susceptible Candida species.
2.4. Checkerboard Assay
In clinical practice, AMB has been the standard fungicidal in the treatment of severe fungal infections. Therefore, we evaluated the effect of AMB combined with the Copaifera leaf extracts by the checkerboard method [19]. The tested AMB final concentrations and Copaifera sample concentrations ranged from 0.03 to 16 µg/mL [14] and from 3.90 to 500 µg/mL, respectively. The assay was carried out with a final inoculum of 2 × 103 CFU/mL [20], and the plates were incubated at 37 °C for 48 h. A 0.015% aqueous rezasurin solution was used to assess fungal cell viability. The Fractional Inhibitory Concentration Index (FICI), which is defined as the sum of MIC of each drug in combination divided by MIC of the drug alone, was calculated. Synergy, additivity, indifference, and antagonism were defined as FICI ≤ 0.5, 0.5 < FICI < 1, 1 ≤ FICI < 4, and FICI ≥ 4, respectively [19]. The tests were conducted in triplicate.
2.5. Copaifera Species Effect on the Inhibition of Candida Biofilm Formation
For these assays, only the most active Copaifera leaf extracts were selected based on the results obtained in the MIC and FICI assays. A suspension prepared by equitable mixing of the standardized Candida cell suspension (106 CFU/mL) and Copaifera extract, which had previously been diluted in RPMI at concentrations ranging from 2.78 to 750 μg/mL, were inoculated into 96-well microplates. Then, the plates were statically incubated at 37 °C for 48 h. After incubation, the supernatant was aspirated, the wells were washed with PBS, and viable cells were determined by the tetrazolium salt (sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) hydroxylated benzene sulfonic acid—XTT) reduction assay [21]. The effective Copaifera extract concentration that was able to inhibit biofilm formation was determined by the reduction of approximately 80% (MIBC80) of the optical density as compared to the control that was free of the chemical substance (100% of survivors).
2.6. Copaifera Species Effect against Preformed C. glabrata Biofilms
For these tests, only the most active extracts (C. paupera and C. reticulata) and the most sensitive Candida species (C. glabrata) were used. The biofilms were formed according to a previously published protocol [21]. After incubation at 37 °C for 24 h, the biofilms were washed with PBS (3×), and the Copaifera extract was added at concentrations ranging from 2.78 to 750 µg/mL, which was followed by incubation for additional 24 h at 37 °C. The biofilms were washed (PBS 3×), and the viability was measured by the XTT assay. The effective Copaifera leaf extract concentration that was able to reduce ≥80% OD, as compared to the control that was free of extract (100% of survivors), was considered as MBEC80. Each assayed experimental condition was performed in triplicate, and the arithmetic mean of the results was used.
2.7. Sample Preparation for Transmission Electron Microscopy
The cells were prepared for observation by transmission electron microscopy (TEM) in agreement with previously published protocol [22]. Briefly, the C. glabrata cells were grown in SDA agar for 24 h. Two cultivar loops were transferred to Falcon-type conical tubes containing 20 mL of YPD broth (yeast peptone dextrose: 1% yeast extract, 2% peptone, 2% dextrose) added with the Copaifera spp leaf extract at the determined 0.5 × MIC and 1 × MIC concentrations. The tubes were placed in an orbital shaker (150–180 rpm) at 30 °C for 18 to 24 h. The cells were centrifuged, and the pellet was fixed with 2.5% (v/v) glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). Secondary fixation was carried out with 1% osmium tetroxide aqueous solution, and the cells were treated with 2% uranyl acetate for embedding. After dehydration with gradients of ethanol (50%, 75%, 95%, and 100%) and propylene oxide, the cells were included in Spurr resin before being subjected to ultrathin microtome cuts. The samples were contrasted with uranyl acetate and examined under an electron transmission microscope (Zeiss Libra 120, Oberkochen, Germany, acquisition voltage: 60 kv, emission: 10 µA). AMB at 2 µg/mL was employed as a control drug.
2.8. Experiments with the Animal Model Caenorhabditis Elegans
The C. elegans AU37 (glp-4 (bn2) I; sek-1 (km4) X) mutant strain, obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota), was used in all the experiments. The strain was grown on agar plates seeded with Escherichia coli OP50, which were incubated at 15 °C [12,22]. The tested Candida strain was grown in SDA broth (Difco) at 35 °C under shaking. A 100 μL aliquot of this culture was inoculated into a solid BHI medium (Difco) containing kanamycin (90 mg/mL) and ampicillin (200 mg/mL), which was followed by incubation at 30 °C for 24 h. Synchronized nematodes in stage L4 were inoculated on the agar plates containing grown yeast strains and incubated at 25 °C for 3 h [23].
In parallel, the L4 worms were plated on agar plates containing grown E. coli OP50. After incubation for 3 h, the worms were washed with M9 buffer (3.0 g KH2 PO4, 6.0 g Na2HPO4, 0.5 g NaCl, 1.0 g NH4Cl, and H2O to complete 1 L). Next, they were transferred to 12-well plates containing 1 mL of a solution consisting of 60% M9 buffer, 40% BHI broth (Difco), 10 mg/mL cholesterol in ethanol, 200 mg/mL ampicillin, and 90 mg/mL kanamycin. Approximately 10–12 nematodes were placed in each well. To evaluate the antifungal efficacy, selected Copaifera extracts at selected concentrations (0.5 MIC, 1 × MIC, and 2 × MIC) and AMB (1 mg/mL) were added to the solution. For the toxicity tests, the nematodes in stage L4 were placed in contact with different extract concentrations. Three independent experiments were conducted for each treatment. The survival curves were analyzed with the software Graph-Pad Prism 5 (La Jolla, CA, USA). The Kaplan–Meier method and the level of significance calculated by the log-rank test (Mantel–Cox) were used. A p-value higher than 0.05 was considered significant [24].
3. Results and Discussion
Targeted research into the discovery of new drugs from natural products has proven to be an alternative of growing interest for scientists and the pharmaceutical industry. Products obtained from the Copaifera tree, such as oleoresins, essential oils, and leaf extracts, have shown relevant results in experiments related to antibacterial activity, but studies related to its antifungal activity are scarce [13,25,26,27,28].
According to the adopted criterion, all the Copaifera leaf extracts were active against all the tested Candida species (Table 1). Copaifera reticulata and C. paupera were the most effective and provided a MIC value of 5.86 μg/mL against C. glabrata, the most sensitive Candida species (Table 1). C. krusei and C. orthopsilosis followed as the most sensitive Candida species (Table 1). For both species, MIC values of 11.72 μg/mL were achieved with the Copaifera paupera and C. reticulata extracts against C. krusei and with the Copaifera duckei and C. lucens extracts against C. orthopsilosis.

Table 1.
Minimal inhibitory concentrations of the hydroalcoholic leaf extracts and oleoresins obtained from Copaifera species against Candida species (MIC values in µg/mL).
Candida glabrata and C. krusei have intrinsic resistance to fluconazole [29]. Therefore, the Copaifera species should be further investigated as an alternative treatment for these Candida infections.
Our research group has previously reported that the leaf extracts of Copaifera spp. are rich in galloylquinic acids and flavonoids (Figure 1) [5]. De Leo et al. [30] reported that phenolic compounds, mostly galloylquinic acid derivatives and flavonoids, were isolated from Baseonerna acuminatum P. Choux (Asclepiadaceae). According to these authors, 2-methoxy-5-(1′,2′,3′-trihydroxypropyl)-phenyl-1-O-(6″-galloyl)-beta-d-glucopyranoside, 2-methoxy-5-hydroxymethyl-phenyl-1-O-(6″-galloyl)-beta-d-glucopyranoside, benzyl 6′-O-galloyl-beta-d-glucopyranoside, and 1,6-di-O-galloyl-beta-d-glucopyranose were active against clinical and ATCC Candida albicans strains. Li et al. [31] reported that a flavonoid derivative known as mattucinol-7-O-[4″,6″-O-(S)-hexahydroxydiphenoyl]-beta-d-glucopyranoside inhibited the aspartic proteases (SAPs) secreted by Candida albicans, which are a major virulence factor in Candida infections. Another study showed that the C. glabrata species was the most sensitive to the Camellia sinensis leaf extract, which is rich in galloyl derivatives [32]. Other studies have also indicated the potential of polyphenolic-rich extracts [33,34,35] against pathogenic fungi, suggesting that the MIC values listed in Table 1 may be associated with the Copaifera leaf extract polyphenolic content.
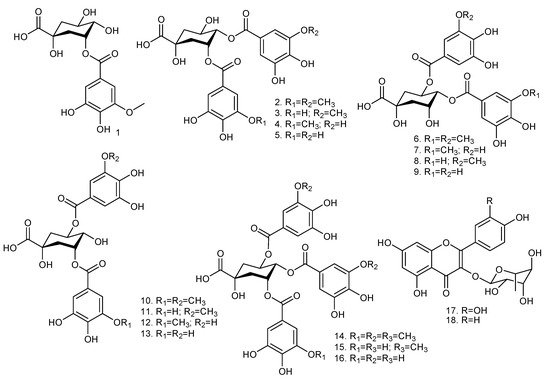
Figure 1.
Chemical structures of the galloylquinic acids and flavonoids present in Copaifera species: 3-O-(3-O-methyl galloyl) quinic acid (1); 3,4-di-O-(3-O-methyl galloyl) quinic acid (2); 3-O-(galloyl)-4-O-(3-O-methyl galloyl) quinic acid (3); 3-O-(3-O-methyl galloyl)-4-O-(galloyl) quinic acid (4); 3,4-di-O-(galloyl) quinic acid (5); 4,5-di-O-(3-O-methyl galloyl) quinic acid (6); 4-O-(3-O-methyl galloyl)-5-O-(galloyl) quinic acid (7); 4-O-(galloyl)-5-O-(3-O-methyl galloyl) quinic acid (8); 4,5-di-O-(galloyl) quinic acid (9); 3,5-di-O-(3-O-methyl galloyl) quinic acid (10); 3-O-(galloyl)-5-O-(3-O-methyl galloyl) quinic acid (11); 3-O-(3-O-methyl galloyl)-5-O-(galloyl) quinic acid (12); 3,5-di-O-(galloyl) quinic acid (13); 3,4,5-tri-O-(3-O-methyl galloyl) quinic acid (14); 3,5-di-O-(galloyl)-4-O-(methyl galloyl) quinic acid (15); 3,4,5-tri-O-(galloyl) quinic acid (16); quercetrin (17); afzelin (18). Adapted from Furtado et al. [5].
As shown in Table 1, higher concentrations of the evaluated Copaifera oleoresins were required to inhibit Candida growth. Only the C. paupera oleoresin showed moderate antifungal activity against all the tested Candida species, and we achieved the best inhibition results with 187.5 μg/mL C. paupera oleoresin against C. glabrata and C. krusei. Santos et al. [13] reported similar moderate antifungal results for the C. paupera oleoresin, which displayed moderate activity against the dermatophyte fungi Trichophyton rubrum and Microsporum canis, with MIC values of 250 and 500 μg/mL, respectively.
By considering the best results obtained with the Copaifera leaf extracts, these derivatives were studied to understand other aspects of their antifungal activity. The Fractional Inhibitory Concentration Index (FICI) results revealed that the interaction between the investigated extracts and AMB was not promising because the FICI values ranged from 1.5 to 4 (Table 2). The extracts of some Copaifera species, like C. duckei, C. oblongifolia, and C. trapezifolia, presented antagonism when combined with AMB, that is, there was a decrease in AMB MIC when compared to the MIC value of AMB alone (Table 2). Combined with AMB, Copaifera paupera provided the lowest FICI (1.5) against all the tested Candida species (Table 2).

Table 2.
Interactions between Copaifera leaf extracts and amphotericin B against Candida spp. planktonic cells.
Microorganisms with a planktonic phenotype (that is, responsive to host defenses and therapeutics) can switch to a life form (biofilm) that is able to adapt in order to survive in an aggressive host tissue environment. This has assumed a specific meaning in the pathogenesis of infections and has been thoroughly studied by Costerton et al. [36]. Over the years, studies focusing on adhesion inhibition and biofilm formation have gained progressive importance [37].
In this context, it was investigated how the Copaifera extracts affected Candida biofilm formation inhibition. For most of the evaluated extracts, the MICB80 values did not reveal activity. The exceptions were the C. paupera and C. reticulata leaf extracts, which inhibited Candida glabrata biofilm formation at MICB80 = 46.87 µg/mL. Nevertheless, in the tested concentration range (2.78 to 750 µg/mL), the C. paupera and C. reticulata leaf extracts were not effective against preformed C. glabrata biofilms (MBEC80). Studies addressing Copaifera antifungal activity are scarce [13]. However, decreased biofilm sensitivity to antifungal compounds has been attributed to the properties of biofilm cells, including the expression of virulence genes, mRNA, and proteins and the production of an extracellular matrix that acts as a physical barrier to drug penetration and environmental adversities [38].
Infections caused by the opportunistic pathogen C. glabrata may be related to biofilm formation and resistance to commonly used antifungal agents [39,40]. The fact that both C. paupera and C. reticulata leaf extracts at 46.87 μg/mL inhibited C. glabrata biofilm formation (MBIC80) demonstrates an anti-C. glabrata effect with respect to surface adsorption. Attachment is the initial stage in biofilm formation. During this stage, most cells are still in the fluid phase and exhibit the same planktonic cell phenotype, so they are more susceptible to antimicrobial agents [41].
In addition, the position and quantity of surface cell wall mannoproteins and the presence of adhesin-like proteins contribute to the yeast hydrophobic state, allowing its initial attachment to solid surfaces or tissues and favoring its ability to form and maintain biofilms [42,43]. The C. glabrata cell walls, the only medically important species that does not form pseudohyphae, display more β-1,2-linked mannose residues [44] and adhesin-like cell wall proteins, which are incorporated in different stages of yeast growth [43]. It was inferred that the Copaifera extract may have interfered with the incorporation of these adhesins into the C. glabrata cell wall during biofilm development.
Transmission electron microscopy revealed a large increase in C. glabrata cell vacuolization, as well as cell membrane damage (Figure 2). Changes in conformation and rupture of the plasma membrane can contribute to cell death. The Copaifera plant has already been reported for its property of damaging the cell wall of bacterial pathogens, especially Staphylococcus aureus [13]. However, here, the yeast cell wall appeared intact, which could be explained by the difference in the structural components of the fungal wall in relation to the bacterial wall. Future tests will be carried out to elucidate cell permeability completely.
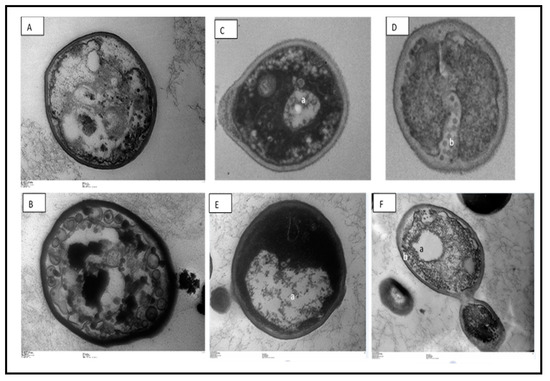
Figure 2.
Cellular alterations in Candida glabrata cells after exposure to Copaifera leaf extracts, observed with transmission electron microscopy. (A)—control (untreated), (B)—treatment with 2 µg/mL amphotericin, (B,C)—0.5 × MIC Copaifera paupera, (D)—0.5 × MIC Copaifera reticulata, (E)—1 × MIC C. paupera, and (F)—1 × MIC C. reticulata leaf extracts. There is intense vacuolization (a) and plasma membrane destruction (b). Magnification (A,B,D): 25,000×; (C,E): 20,000×, (F): 5000×.
The use of invertebrate hosts has recently arisen and facilitated studies on fungal pathogenesis. Among these hosts, the nematode C. elegans stands out and has been successfully used to study the toxicity and effectiveness of substances against some fungi [12,23,45]. Therefore, we evaluated the toxicity to C. elegans of Copaifera reticulata and C. paupera extracts at sub-MIC (0.5 × MIC) concentration, equivalent to 2.9 µg/mL, at MIC of 5.8 µg/mL, and supra-MIC (2 × MIC, 4 × MIC) concentrations, equivalent to 11.6 and 23.4 µg/mL, respectively (Figure 3).
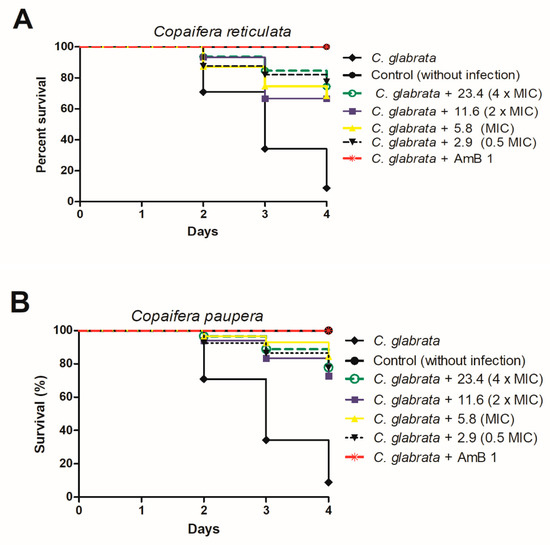
Figure 3.
Survival curve of C. elegans infected with C. glabrata ATCC 2001 and treated with concentrations equivalent to 0.5 MIC, MIC, 2 × MIC, and 4 × MIC of Copaifera reticulata (A) and Copaifera paupera (B). Amphotericin B at 1 µg/mL was used as control. Statistical differences were observed between the groups treated with all the C. reticulata concentrations and the nontreated control group (C. glabrata) (p < 0.0001). In addition, statistical differences were observed between the groups treated with all the C. paupera concentrations and the nontreated control group (C. glabrata) (p < 0.0001). Log-rank test (Mantel–Cox).
All the C. reticulata extract concentrations (A) increased C. elegans survival by between 68% and 75% as compared to infected and untreated larvae (Figure 3). Similarly, treatment with C. paupera (B) provided survival percentages higher than 75% (Figure 3). Previously, we had reported the absence of genotoxic effects on the micronucleus frequencies of Chinese hamster lung fibroblast (V79) cell cultures and even a cytoprotective effect of Copaifera spp leaf extracts, which we associated with the large number of phenolic compounds in the extracts [5,46]. All these data indicated the efficacy and safe use of Copaifera leaf extracts against C. glabrata infection.
4. Conclusions
According to the results, namely, the lack of in vivo toxicity and the potent antifungal effect of Copaifera paupera and C. reticulata leaf extracts, these extracts should be further studied in order to develop new drugs, especially drugs designed for the treatment of Candida glabrata infections.
Author Contributions
Conceptualization, R.H.P. and C.H.G.M.; methodology, G.A. and H.C.S.O.; software, J.K.B.; validation, G.A., H.C.S.O., R.S.P. and L.S.; formal analysis, L.S. and R.H.P.; investigation, G.A.; resources, M.J.S.M.-G.; data curation, F.A., M.T.M.C.; writing—original draft preparation, G.A. and R.H.P.; writing—review and editing, R.H.P. and R.C.S.V.; visualization, J.K.B. and M.J.S.M.-G.; supervision, R.H.P.; project administration, R.H.P. and C.H.G.M.; funding acquisition, S.R.A., R.C.S.V. and H.C.S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially funded by Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES) Finance Code 001. The authors are thankful to the São Paulo Research Foundation (FAPESP) for financial support, grants 2011/13630-7, 2017/03250-9, and 2018/02333-0. S.R.A. and R.C.S.V. thank CNPq (grants 306441/2017–9, 306432/2017-0, respectively) for the research fellowships.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Global Conference on Primary Health Care. World Health Organization. 2018. Available online: https://www.who.int/primary-health/conference-phc (accessed on 2 May 2020).
- Oliveira, U.; Soares-Filho, B.S.; Santos, A.J.; Paglia, A.P.; Brescovit, A.D.; de Carvalho, C.J.B.; Silva, D.P.; Rezende, D.T.; Leite, F.S.F.; Batista, J.A.N.; et al. Modelling highly biodiverse areas in Brazil. Sci. Rep. 2019, 9, 6355. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.J.; Bianchi, T.C.; da Silva, J.J.M.; Oliveira, L.C.; Borges, C.H.G.; Lemes, D.C.; Bastos, J.K.; Veneziani, R.C.S.; Ambrósio, S.R. Development and validation of a rapid and reliable RP-HPLC-PDA method for the quantification of six diterpenes in Copaifera duckei, Copaifera reticulata and Copaifera multijuga oleoresins. J. Braz. Chem. Soc. 2018, 29, 729–737. [Google Scholar] [CrossRef]
- Arruda, C.; Mejia, J.A.A.; Ribeiro, V.P.; Borges, C.H.G.; Martins, C.H.G.; Veneziani, R.C.S.; Ambrósio, S.R.; Bastos, J.K. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus-A review. Biomed. Pharmacother. 2019, 109, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.A.; de Oliveira, P.F.; Senedese, J.M.; Ozelin, S.D.; Ribeiro de Souza, L.D.; Leandro, L.F.; Oliveira, W.L.; Silva, J.J.M.; Oliveira, L.C.; Rogez, H.; et al. Assessment of toxicogenetic activity of oleoresins and leaves extracts of six Copaifera species for prediction of potential human risks. J. Ethnopharmacol. 2018, 221, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Brancalion, A.P.S.; Oliveira, R.B.; Sousa, J.P.B.; Groppo, M.; Berretta, A.A.; Barros, M.E.; Boim, M.; Bastos, J.K. Effect of hydroalcoholic extract from Copaifera langsdorffii leaves on urolithiasis induced in rats. Urol. Res. 2012, 40, 475–481. [Google Scholar] [CrossRef]
- Motta, E.V.; Lemos, M.; Costa, J.C.; Bandero-Filho, V.C.; Sasse, A.; Sheridan, H.; Bastos, J.K. Galloylquinic acid derivatives from Copaifera langsdorffii leaves display gastroprotective activity. Chem. Biol. Interact. 2017, 261, 145–155. [Google Scholar] [CrossRef]
- Ruhnke, M. Epidemiology of Candida albicans infections and role of non-Candida-albicans yeasts. Curr. Drug Targets 2006, 7, 495–504. [Google Scholar] [CrossRef]
- Brandt, M.E.; Lockhart, S.R. Recent taxonomic developments with Candida and other opportunistic yeasts. Curr. Fungal Infect. Rep. 2012, 6, 170–177. [Google Scholar] [CrossRef]
- Herkert, P.F.; Gomes, R.R.; Muro, M.D.; Pinheiro, R.L.; Fornari, G.; Vicente, V.A.; Queiroz-Telles, F. In vitro susceptibility and molecular characterization of Candida spp. from candidemic patients. Rev. Iberoam. Micol. 2015, 32, 221–228. [Google Scholar] [CrossRef]
- Sanguinetti, M.; Posteraro, B.; Lass-Florl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58 Suppl. 2, 2–13. [Google Scholar] [CrossRef]
- Breger, J.; Fuchs, B.B.; Aperis, G.; Moy, T.I.; Ausubel, F.M.; Mylonakis, E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007, 3, e18. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga Junior, V.F.; Pinto, A.C.; Nakamura, C.V. Antimicrobial activity of Brazilian copaiba oils obtained from different species of the Copaifera genus. Mem. Inst. Oswaldo Cruz 2008, 103, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement (Document M27-S4); CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute. M60: Performance Standards for Antifungal Susceptibility Testing of Yeasts; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Far, F.E.; Al-Obaidi, M.M.J.; Desa, M.N.M. Efficacy of modified Leeming-Notman media in a resazurin microtiter assay in the evaluation of in-vitro activity of fluconazole against Malassezia furfur ATCC 14521. J. Mycol. Med. 2018, 28, 486–491. [Google Scholar] [CrossRef]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Dias, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz. 2002, 97, 1027–1031. [Google Scholar] [CrossRef]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-derived products as antibacterial and antifungal agents in human health care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; MacDougall, C.; Ostrosky-Zeichner, L.; Perfect, J.R.; Rex, J.H. Combination antifungal therapy. Antimicrob. Agents Chemother. 2004, 48, 693–715. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E. Combination chemotherapy for invasive fungal infections: What laboratory and clinical studies tell us so far. Drug Resist. Updat. 2003, 6, 257–269. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Aneja, B.; Irfan, M.; Kapil, C.; Jairajpuri, M.A.; Maguire, R.; Kavanagh, K.M.; Rizvi, M.A.; Manzoor, N.; Azam, A.; Abid, M. Effect of novel triazole-amino acid hybrids on growth and virulence of Candida species: In vitro and in vivo studies. Org. Biomol. Chem. 2016, 14, 10599–10619. [Google Scholar] [CrossRef]
- Mylonakis, E.; Ausubel, F.M.; Perfect, J.R.; Heitman, J.; Calderwood, S.B. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 5675–5680. [Google Scholar] [CrossRef]
- Scorzoni, L.; Lucas, M.P.; Singulani, J.L.; Oliveira, H.C.; Assato, P.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Evaluation of Caenorhabditis elegans as a host model for Paracoccidioides brasiliensis and Paracoccidioides lutzii. Path. Dis. 2018, 76, fty004. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, A.L.; Muniz, F.; Oballe, H.J.R.; Rosing, C.K. Antimicrobial activity of copaiba oil (Copaifera ssp.) on oral pathogens: Systematic review. Phytother. Res. 2018, 32, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Zimmermam-Franco, D.C.; Bolutari, E.B.; Polonini, H.C.; do Carmo, A.M.; Chaves, M.; Raposo, N.R. Antifungal activity of Copaifera langsdorffii Desf oleoresin against dermatophytes. Molecules 2013, 18, 12561–12570. [Google Scholar] [CrossRef] [PubMed]
- Tobouti, P.L.; de Andrade Martins, T.C.; Pereira, T.J.; Mussi, M.C.M. Antimicrobial activity of copaiba oil: A review and a call for further research. Biomed. Pharmacother. 2017, 94, 93–99. [Google Scholar] [CrossRef]
- Souza, A.B.; Martins, C.H.G.; Souza, M.G.M.; Furtado, N.A.J.C.; Heleno, V.C.G.; Sousa, J.P.B.; Rocha, E.M.P.; Bastos, J.K.; Cunha, W.R.; Veneziani, R.C.S.; et al. Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf. against cariogenic bacteria. Phytother. Res. 2011, 25, 215–220. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2014, 5, 019752. [Google Scholar] [CrossRef]
- De Leo, M.; Braca, A.; De Tommasi, N.; Norscia, I.; Morelli, L.; Battinelli, L.; Mazzanti, G. Phenolic compounds from Baseonema acuminatum leaves: Isolation and antimicrobial activity. Planta Med. 2004, 70, 841–846. [Google Scholar] [CrossRef]
- Li, X.C.; Jacob, M.R.; Pasco, D.S.; ElSohly, H.N.; Nimrod, A.C.; Walker, L.A.; Clark, A.M. Phenolic compounds from Miconia myriantha inhibiting Candida aspartic proteases. J. Nat. Prod. 2001, 64, 1282–1285. [Google Scholar] [CrossRef]
- Turchetti, B.; Pinelli, P.; Buzzini, P.; Romani, A.; Heimler, D.; Franconi, F.; Martini, A. In vitro antimycotic activity of some plant extracts towards yeast and yeast-like strains. Phytother. Res. 2005, 19, 44–49. [Google Scholar] [CrossRef]
- Latte, K.P.; Kolodziej, H. Antifungal effects of hydrolysable tannins and related compounds on dermatophytes, mould fungi and yeasts. Z. für Naturforsch. C J. Biosci. 2000, 55, 467–472. [Google Scholar] [CrossRef]
- Kolodzeij, H.; Kayser, O.; Latte, K.P.; Ferreira, D. Evaluation of the antimicrobial potency of tannins and related compounds using the microdilution broth method. Planta Med. 1999, 65, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, B.E.; Zhang, S.W.; Yang, S.M.; Wang, H.; Ren, A.M.; Yie, E.-T. Isolation of antifungal compound from Paeonia suffruticosa and its antifungal mechanism. Chin. J. Integr. Med. 2015, 21, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.; Mavrianos, J.; Chauhan, N. Candida glabrata Pwp7p and Aed1p are required for adherence to human endothelial cells. FEMS Yeast Res. 2011, 11, 595–601. [Google Scholar] [CrossRef]
- Tam, P.; Gee, K.; Piechocinski, M.; Macreadie, I. Candida glabrata, friend and foe. J. Fungi 2015, 1, 277–292. [Google Scholar] [CrossRef]
- Selim, S.A.; Adam, M.E.; Hassan, S.M.; Albalawi, A.R. Chemical composition, antimicrobial and antibiofilm activity of the essential oil and methanol extract of the Mediterranean cypress (Cupressus sempervirens L.). BMC Complement. Altern. Med. 2014, 14, 179. [Google Scholar] [CrossRef]
- Evensen, N.A.; Braun, P.C. The effects of tea polyphenols on Candida albicans: Inhibition of biofilm formation and proteasome inactivation. Can. J. Microbiol. 2009, 55, 1033–1039. [Google Scholar] [CrossRef]
- Groot, P.W.J.; Kraneveld, E.A.; Yin, Q.Y.; Dekker, H.L.; Gross, U.; Crielaard, W.; Koster, C.G.; Bader, O.; Klis, F.M.; Weig, M. The cell wall of the human pathogen Candida glabrata: Differential incorporation of novel adhesin-like wall proteins. Eukaryot. Cell 2008, 7, 1951–1964. [Google Scholar] [CrossRef]
- Takahashi, S.; Kudoh, A.; Okawa, Y.; Shibata, N. Significant differences in the cell-wall mannans from three Candida glabrata strains correlate with antifungal drug sensitivity. FEBS J. 2012, 279, 1844–1856. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Aldana, J.A.; De Grandis, R.A.; Nicolella, H.; Guissoni, A.P.P.; Squarisi, I.; Arruda, C.; Ribeiro, V.P.; Tavares, D.C.; Barcelos, G.R.M.; Antunes, L.M.G.; et al. Evaluation of cytoprotective effects of compounds isolated from Copaifera langsdorffii Desf. against induced cytotoxicity by exposure to methylmercury and lead. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).