Early Virulence Predictors during the Candida Species–Galleria mellonella Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Galleria mellonella Survival Assays
2.3. Analysis of Hemocyte Levels, Melanin Production, and Phenoloxidase Activity
2.4. Ethics Statement
2.5. Statistical Analysis
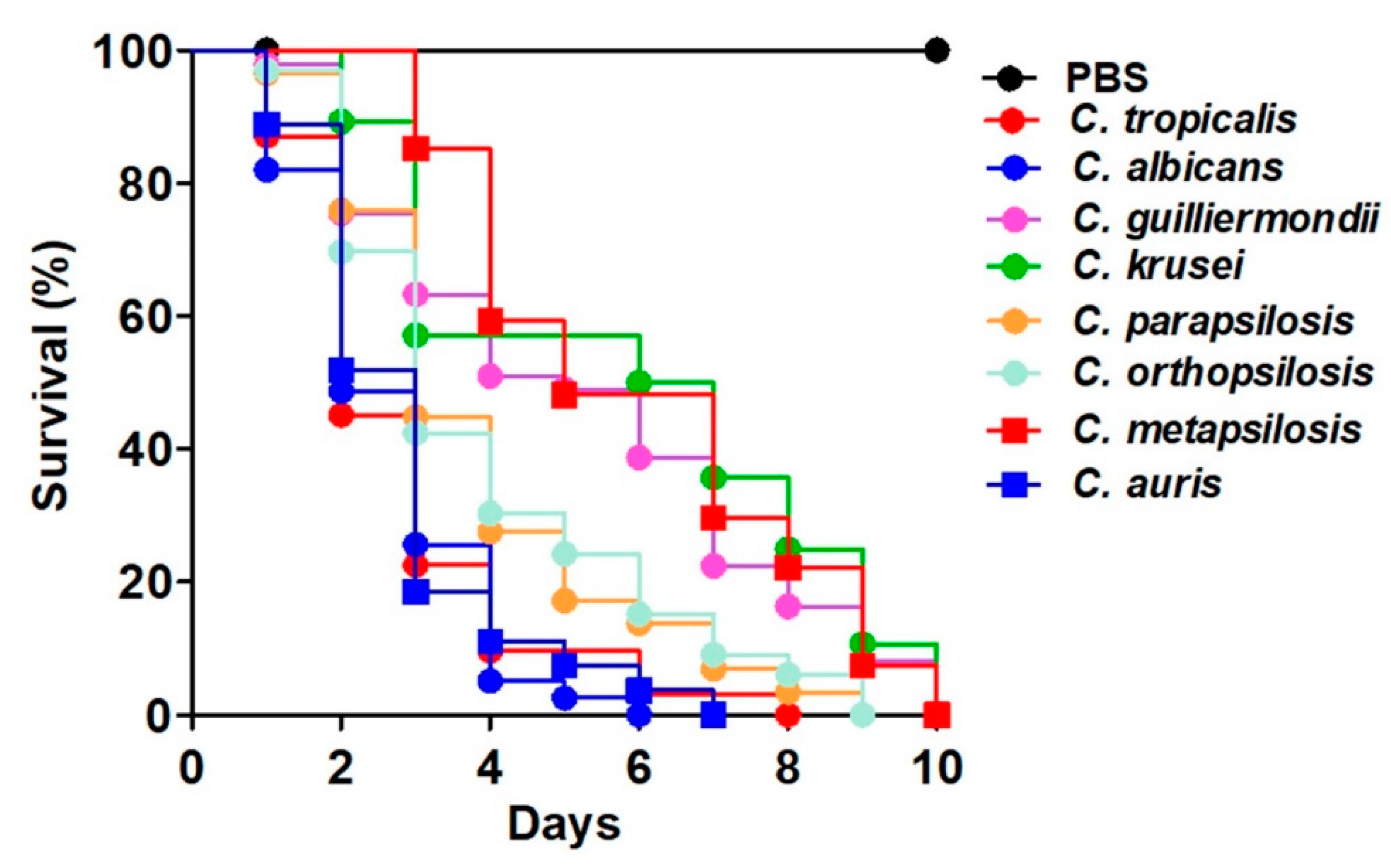
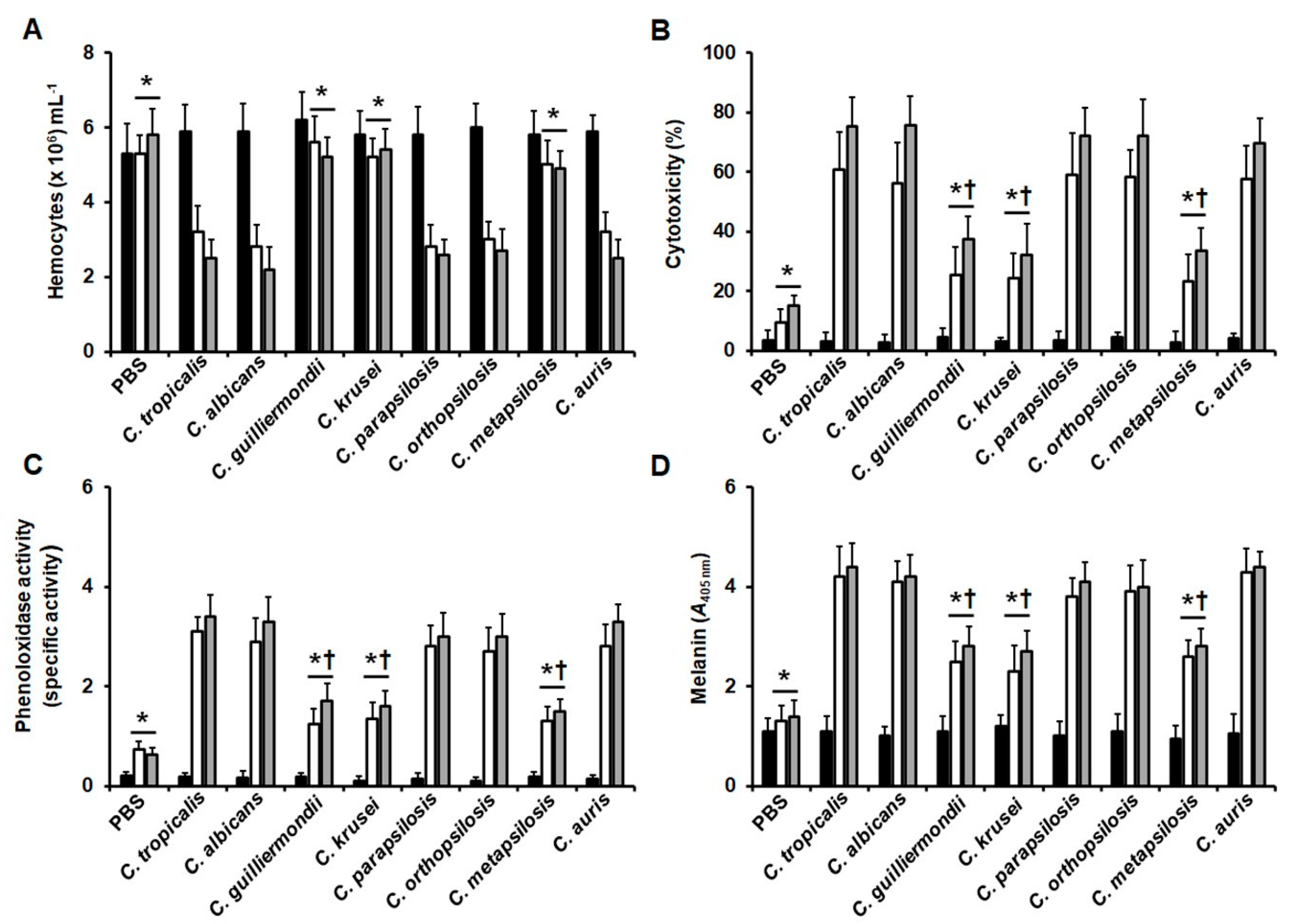
3. Results
3.1. The Virulence Defect of C. tropicalis mnn4Δ, och1Δ, and pmr1Δ Null Mutants in G. mellonella Larvae
3.2. Early Virulence Predictors in Larva Infected with C. tropicalis Clinical Isolates
3.3. Early Virulence Predictors in Larva Infected with Candida Species
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Mata, E.; Navarro-Arias, M.J.; Pérez-García, L.A.; Mellado-Mojica, E.; López, M.G.; Csonka, K.; Gácser, A.; Mora-Montes, H.M. Members of the Candida parapsilosis Complex and Candida albicans are Differentially Recognized by Human Peripheral Blood Mononuclear Cells. Front. Microbiol. 2016, 6, 1527. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Arias, M.J.; Hernández-Chávez, M.J.; Garcia-Carnero, L.C.; Amezcua-Hernández, D.G.; Lozoya-Pérez, E.N.; Estrada-Mata, E.; Martínez-Duncker, I.; Franco, B.; Mora-Montes, H.M. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect. Drug Resist. 2019, 12, 783–794. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Mora-Montes, H.M. Current Aspects in the Biology, Pathogeny, and Treatment of Candida krusei, a Neglected Fungal Pathogen. Infect. Drug Resist. 2020, 13, 1673–1689. [Google Scholar] [CrossRef]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18. [Google Scholar] [CrossRef]
- Friedman, D.Z.P.; Schwartz, I.S. Emerging fungal infections: New patients, new patterns, and new pathogens. J. Fungi 2019, 5, 67. [Google Scholar] [CrossRef]
- Mora-Montes, H.M.; Ponce-Noyola, P.; Villagómez-Castro, J.C.; Gow, N.A.R.; Flores-Carreón, A.; López-Romero, E. Protein glycosylation inCandida. Future Microbiol. 2009, 4, 1167–1183. [Google Scholar] [CrossRef]
- Brown, G.D. Innate antifungal immunity: The key role of phagocytes. Ann. Rev. Immunol. 2011, 29, 1–21. [Google Scholar] [CrossRef]
- Hernandez-Chavez, M.J.; Perez-Garcia, L.A.; Nino-Vega, G.A.; Mora-Montes, H.M. Fungal strategies to evade the host immune recognition. J. Fungi 2017, 3, 51. [Google Scholar] [CrossRef]
- Odds, F.C.; Van Nuffel, L.; Gow, N.A.R. Survival in experimental Candida albicans infections depends on inoculum growth conditions as well as animal host. Microbiology 2000, 146, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Szabo, E.K.; MacCallum, D.M. The contribution of mouse models to our understanding of systemic candidiasis. FEMS Microbiol. Lett. 2011, 320, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maccallum, D.M.; Odds, F.C. Safety aspects of working with Candida albicans-infected mice. Med. Mycol. 2004, 42, 305–309. [Google Scholar] [CrossRef]
- Borman, A.M. Of mice and men and larvae: Galleria mellonella to model the early host-pathogen interactions after fungal infection. Virulence 2018, 9, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Desalermos, A.; Fuchs, B.B.; Mylonakis, E. Selecting an invertebrate model host for the study of fungal pathogenesis. PLoS Pathog. 2012, 8, e1002451. [Google Scholar] [CrossRef]
- Clavijo-Giraldo, D.M.; Matínez-Alvarez, J.A.; Lopes-Bezerra, L.M.; Ponce-Noyola, P.; Franco, B.; Almeida, R.S.; Mora-Montes, H.M. Analysis of Sporothrix schenckii sensu stricto and Sporothrix brasiliensis virulence in Galleria mellonella. J. Microbiol. Methods 2016, 122, 73–77. [Google Scholar] [CrossRef]
- Souza, P.; Morey, A.T.; Castanheira, G.M.; Bocate, K.C.P.; Panagio, L.A.; Ito, F.A.; Furlaneto, M.C.; Yamada-Ogatta, S.F.; Da Costa, I.N.; Mora-Montes, H.M.; et al. Tenebrio molitor (Coleoptera: Tenebrionidae) as an alternative host to study fungal infections. J. Microbiol. Methods 2015, 118, 182–186. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Lozoya-Pérez, N.E.; Staniszewska, M.; Franco, B.; Niño-Vega, G.A.; Mora-Montes, H.M. Loss of Kex2 Affects the Candida albicans Cell Wall and Interaction with Innate Immune Cells. J. Fungi 2020, 6, 57. [Google Scholar] [CrossRef]
- Hernández-Chávez, M.J.; Clavijo-Giraldo, D.M.; Novák, Á.; Lozoya-Pérez, N.E.; Martínez-Álvarez, J.A.; Salinas-Marín, R.; Hernández, N.V.; Martínez-Duncker, I.; Gácser, A.; Mora-Montes, H.M. Role of Protein Mannosylation in the Candida tropicalis-Host Interaction. Front. Microbiol. 2019, 10, 2743. [Google Scholar] [CrossRef]
- Hernández-Chávez, M.J.; Franco, B.; Clavijo-Giraldo, D.M.; Hernández, N.V.; Estrada-Mata, E.; Mora-Montes, H.M. Role of protein phosphomannosylation in the Candida tropicalis–macrophage interaction. FEMS Yeast Res. 2018, 18, foy053. [Google Scholar] [CrossRef]
- Navarro-Arias, M.J.; Defosse, T.A.; Dementhon, K.; Csonka, K.; Mellado-Mojica, E.; Valério, A.D.; González-Hernández, R.J.; Courdavault, V.; Clastre, M.; Hernández, N.V.; et al. Disruption of Protein Mannosylation Affects Candida guilliermondii Cell Wall, Immune Sensing, and Virulence. Front. Microbiol. 2016, 7, 1951. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, L.A.; Csonka, K.; Flores-Carreón, A.; Estrada-Mata, E.; Mellado-Mojica, E.; Németh, T.; López-Ramírez, L.A.; Toth, R.; López, M.G.; Vízler, C.; et al. Role of Protein Glycosylation in Candida parapsilosis Cell Wall Integrity and Host Interaction. Front. Microbiol. 2016, 7, 306. [Google Scholar]
- Bergin, D.; Brennan, M.; Kavanagh, K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect. 2003, 5, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Forastiero, A.; Bernal-Martínez, L.; Cuenca-Estrella, M.; Mellado, E.; Zaragoza, O. The non-mammalian hostGalleria mellonellacan be used to study the virulence of the fungal pathogenCandida tropicalisand the efficacy of antifungal drugs during infection by this pathogenic yeast. Med. Mycol. 2013, 51, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.; Sheehan, G. The Use of Galleria mellonella Larvae to Identify Novel Antimicrobial Agents against Fungal Species of Medical Interest. J. Fungi 2018, 4, 113. [Google Scholar] [CrossRef]
- Jemel, S.; Guillot, J.; Kallel, K.; Botterel, F.; Dannaoui, E. Galleria mellonella for the Evaluation of Antifungal Efficacy against Medically Important Fungi, a Narrative Review. Microorganisms 2020, 8, 390. [Google Scholar] [CrossRef]
- Scorzoni, L.; De Lucas, M.P.; Mesa-Arango, A.C.; Fusco-Almeida, A.M.; Lozano, E.; Cuenca-Estrella, M.; Mendes-Giannini, M.J.S.; Zaragoza, O. Antifungal Efficacy during Candida krusei Infection in Non-Conventional Models Correlates with the Yeast In Vitro Susceptibility Profile. PLoS ONE 2013, 8, e60047. [Google Scholar] [CrossRef]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, Q.; Zhang, J.; Yang, B.; Wu, K.; Xie, W.; Luan, Y.-X.; Ling, E. Insect prophenoloxidase: The view beyond immunity. Front. Physiol. 2014, 5, 252. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095505. [Google Scholar] [CrossRef]
- Chan, F.K.; Moriwaki, K.; De Rosa, M.J. Detection of Necrosis by Release of Lactate Dehydrogenase Activity. In Methods in Molecular Biology; Clifton, N.J., Ed.; Humana Press: Totowa, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Bergin, D.; Murphy, L.; Keenan, J.; Clynes, M.; Kavanagh, K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006, 8, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.C.; De Barros, P.P.; Fugisaki, L.R.D.O.; Rossoni, R.D.; Ribeiro, F.; De Menezes, R.T.; Junqueira, J.C.; Scorzoni, L. Recent Advances in the Use of Galleria mellonella Model to Study Immune Responses against Human Pathogens. J. Fungi 2018, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, A.; Davidson, A.D.; Johnson, E.M.; Maiden, M.C.J.; Shaw, D.J.; Gow, N.A.R.; Odds, F.C. Multilocus Sequence Typing for Differentiation of Strains of Candida tropicalis. J. Clin. Microbiol. 2005, 43, 5593–5600. [Google Scholar] [CrossRef] [PubMed]
- Gillum, A.M.; Tsay, E.Y.H.; Kirsch, D.R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Genet. Genom. 1984, 198, 179–182. [Google Scholar] [CrossRef]
- Millerioux, Y.; Clastre, M.; Simkin, A.J.; Marais, E.; Sibirny, A.; Noël, T.; Crèche, J.; Giglioli-Guivarc’H, N.; Papon, N. Development of a URA5 integrative cassette for gene disruption in the Candida guilliermondii ATCC 6260 strain. J. Microbiol. Methods 2011, 84, 355–358. [Google Scholar] [CrossRef]
- Szenzenstein, J.; Gácser, A.; Grózer, Z.; Farkas, Z.; Nagy, K.; Vágvölgyi, C.; Márki-Zay, J.; Pfeiffer, I. Differential Sensitivity of the Species of Candida parapsilosis Sensu Lato Complex Against Statins. Mycopathologia 2013, 176, 211–217. [Google Scholar] [CrossRef]
- Sharma, C.; Kumar, N.; Meis, J.F.; Pandey, R.; Chowdhary, A. Draft Genome Sequence of a Fluconazole-Resistant Candida auris Strain from a Candidemia Patient in India. Genome Announc. 2015, 3, e00722-15. [Google Scholar] [CrossRef]
- Martínez-Álvarez, J.A.; García-Carnero, L.C.; Kubitschek-Barreira, P.H.; Lozoya-Pérez, E.N.; Belmonte-Vázquez, J.L.; De Almeida, J.R.; Gómez-Infante, A.D.J.; Curty, N.; Villagómez-Castro, J.C.; Peña-Cabrera, E.; et al. Analysis of some immunogenic properties of the recombinant Sporothrix schenckii Gp70 expressed in Escherichia coli. Future Microbiol. 2019, 14, 397–410. [Google Scholar] [CrossRef]
- Wu, G.-Q.; Yi, Y.; Lv, Y.; Li, M.; Wang, J.; Qiu, L. The lipopolysaccharide (LPS) of Photorhabdus luminescens TT01 can elicit dose- and time-dependent immune priming in Galleria mellonella larvae. J. Invertebr. Pathol. 2015, 127, 63–72. [Google Scholar] [CrossRef]
- Bidla, G.; Hauling, T.; Dushay, M.S.; Theopold, U. Activation of Insect Phenoloxidase after Injury: Endogenous versus Foreign Elicitors. J. Innate Immun. 2009, 1, 301–308. [Google Scholar] [CrossRef]
- Ciesielczuk, H.; Betts, J.W.; Phee, L.; Doumith, M.; Hope, R.; Woodford, N.; Wareham, D.W. Comparative virulence of urinary and bloodstream isolates of extra-intestinal pathogenic Escherichia coli in a Galleria mellonella model. Virulence 2015, 6, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Frenkel, M. Experimental In Vivo Models of Candidiasis. J. Fungi 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Maccallum, D.M.; Castillo, L.; Brown, A.J.P.; Gow, N.A.R.; Odds, F.C. Early-Expressed Chemokines Predict Kidney Immunopathology in Experimental Disseminated Candida albicans Infections. PLoS ONE 2009, 4, e6420. [Google Scholar] [CrossRef] [PubMed]
- Maccallum, D.M.; Odds, F.C. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 2005, 48, 151–161. [Google Scholar] [CrossRef]
- Gago, S.; García-Rodas, R.; Cuesta, I.; Mellado, E.; Alastruey-Izquierdo, A.; Gago, S. Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosisvirulence in the non-conventional host Galleria mellonella. Virulence 2014, 5, 278–285. [Google Scholar] [CrossRef]
- Matha, V.; Acek, Z. Changes in Haemocyte Counts in Galleria Mellonella (L.) (Lepidoptera: Galleriidae) Larvae Infected With Steinernema Sp. (Nematoda: Steinernematidae). Nematologica 1984, 30, 86–89. [Google Scholar] [CrossRef]
- McKenzie, C.G.J.; Koser, U.; Lewis, L.E.; Bain, J.M.; Mora-Montes, H.M.; Barker, R.N.; Gow, N.A.R.; Erwig, L.-P. Contribution of Candida albicans Cell Wall Components to Recognition by and Escape from Murine Macrophages. Infect. Immun. 2010, 78, 1650–1658. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Zaragoza, O. Immune response of Galleria mellonella against human fungal pathogens. J. Fungi 2018, 5, 3. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; Rowley, A.F.; Fitzgerald, S.W.; Rhodes, C.P. Invertebrate immunity: Basic concepts and recent advances. In International Review of Cytology; Bourne, G.H., Ed.; Academic Press: Cambridge, MA, USA, 1985; Volume 97, pp. 183–350. [Google Scholar]
- Marcos-Zambrano, L.J.; Bordallo-Cardona, M.Á.; Borghi, E.; Falleni, M.; Tosi, D.; Muñoz, P.; Escribano, P.; Guinea, J. Candida isolates causing candidemia show different degrees of virulence in Galleria mellonella. Med. Mycol. 2020, 58, 83–92. [Google Scholar] [CrossRef]
- Ames, L.; Duxbury, S.; Pawlowska, B.; Ho, H.-L.; Haynes, K.; Bates, S. Galleria mellonella as a host model to study Candida glabrata virulence and antifungal efficacy. Virulence 2017, 8, 1909–1917. [Google Scholar] [CrossRef]
- Vertyporokh, L.; Wojda, I. Immune response of Galleria mellonella after injection with non-lethal and lethal dosages of Candida albicans. J. Invertebr. Pathol. 2020, 170, 107327. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Vautier, S.; Potrykus, J.; Walker, L.A.; Shepardson, K.M.; Hopke, A.; Mora-Montes, H.M.; Kerrigan, A.; Netea, M.G.; Murray, G.I.; et al. Differential Adaptation of Candida albicans In Vivo Modulates Immune Recognition by Dectin-1. PLoS Pathog. 2013, 9, e1003315. [Google Scholar] [CrossRef]
- Wand, M.; McCowen, J.W.I.; Nugent, P.G.; Sutton, J. Complex interactions of Klebsiella pneumoniae with the host immune system in a Galleria mellonella infection model. J. Med. Microbiol. 2013, 62, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Okawa, Y.; Miyauchi, M.; Kobayashi, H. Comparison of Pathogenicity of Various Candida tropicalis Strains. Boil. Pharm. Bull. 2008, 31, 1507–1510. [Google Scholar] [CrossRef] [PubMed]
- Zuza-Alves, D.L.; De Medeiros, S.S.T.Q.; De Souza, L.B.F.C.; Silva-Rocha, W.P.; Francisco, E.C.; De Araújo, M.C.B.; Lima-Neto, R.G.; Neves, R.P.; Melo, A.S.D.A.; Chaves, G.M. Evaluation of Virulence Factors In Vitro, Resistance to Osmotic Stress and Antifungal Susceptibility of Candida tropicalis Isolated from the Coastal Environment of Northeast Brazil. Front. Microbiol. 2016, 7, 1783. [Google Scholar] [CrossRef]
- Yu, S.; Li, W.; Liu, X.; Che, J.; Wu, Y.; Lu, J. Distinct Expression Levels of ALS, LIP, and SAP Genes in Candida tropicalis with Diverse Virulent Activities. Front. Microbiol. 2016, 7, 1175. [Google Scholar] [CrossRef]
- Borman, A.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef]
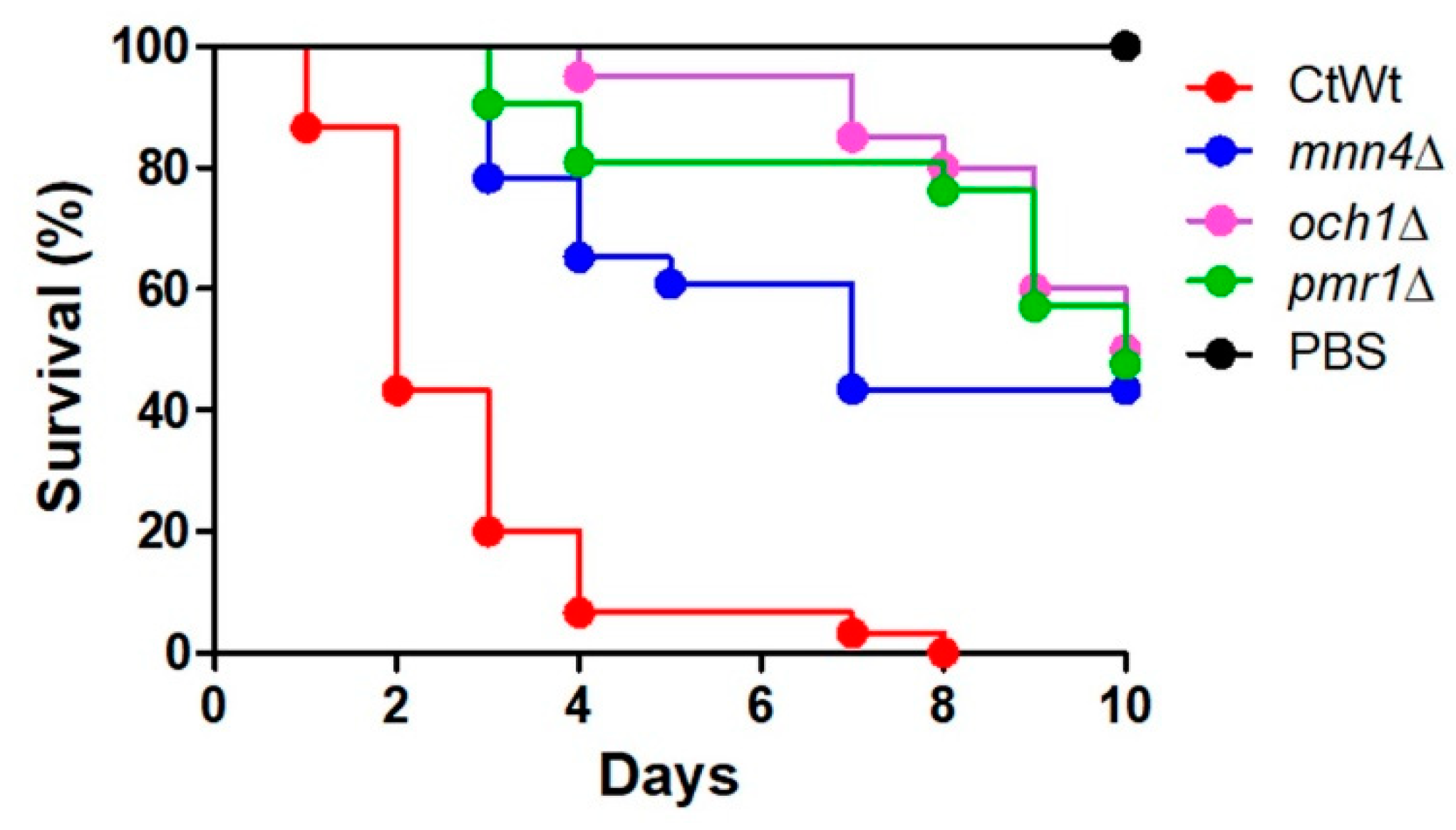
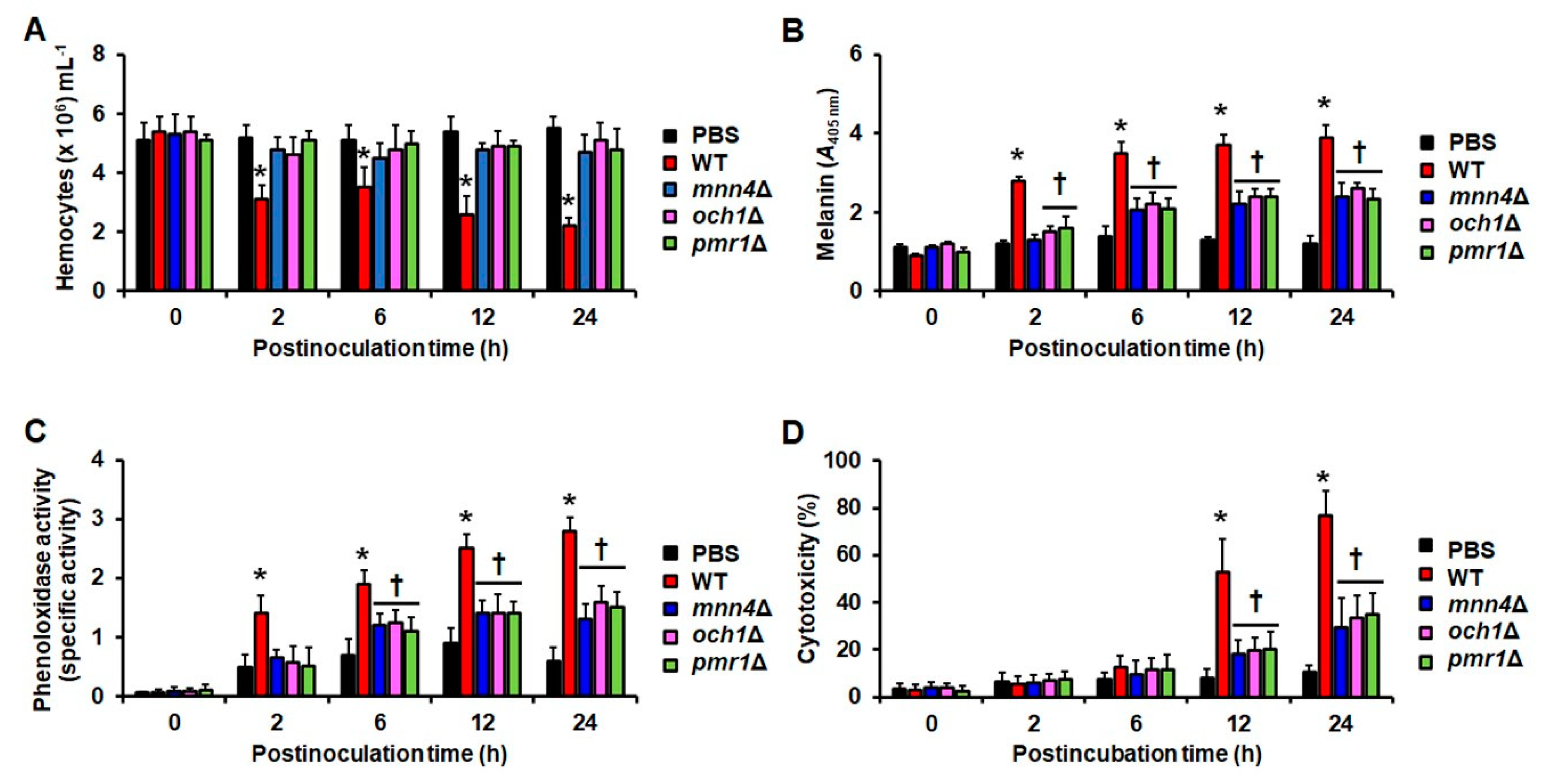
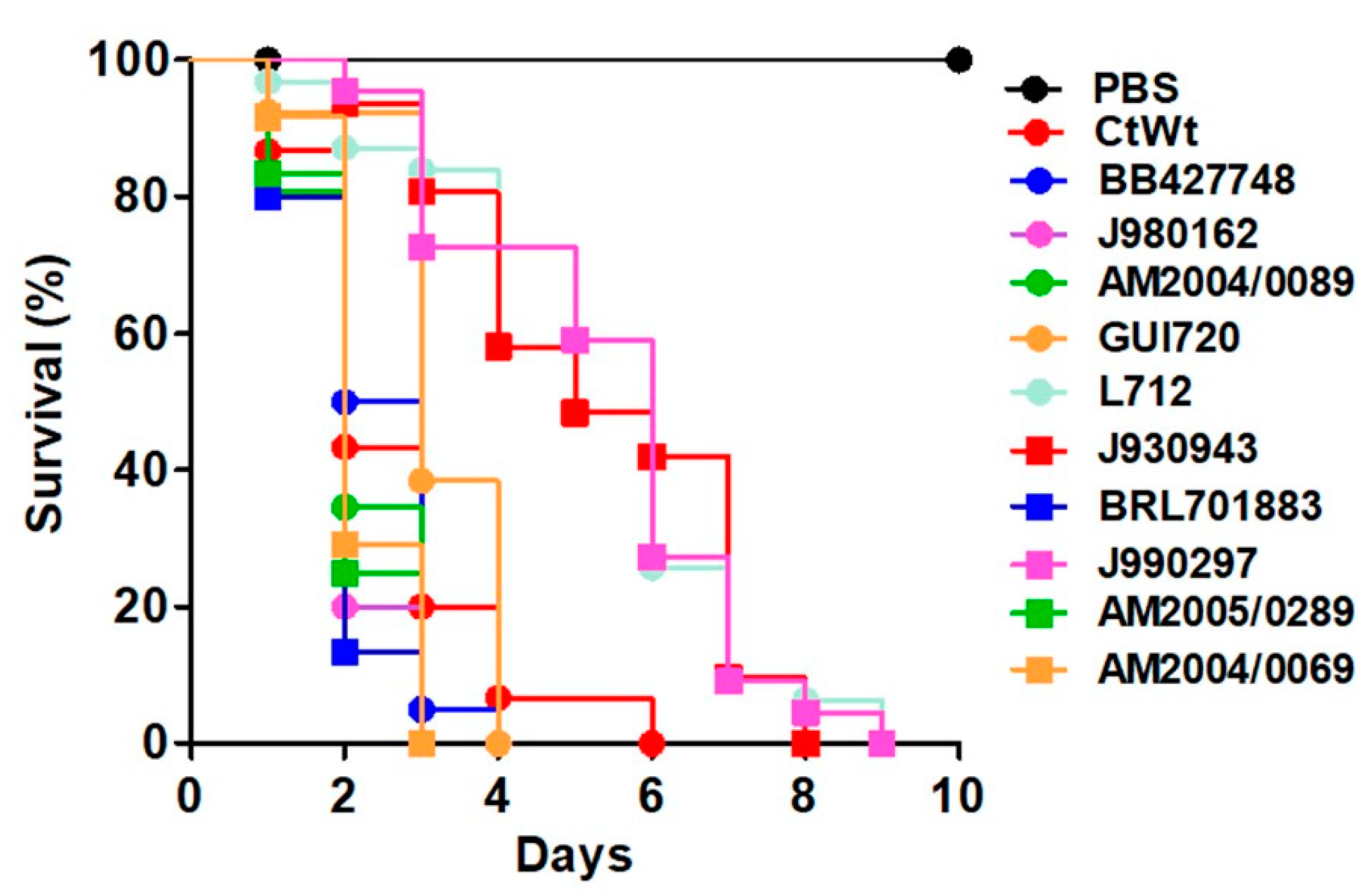
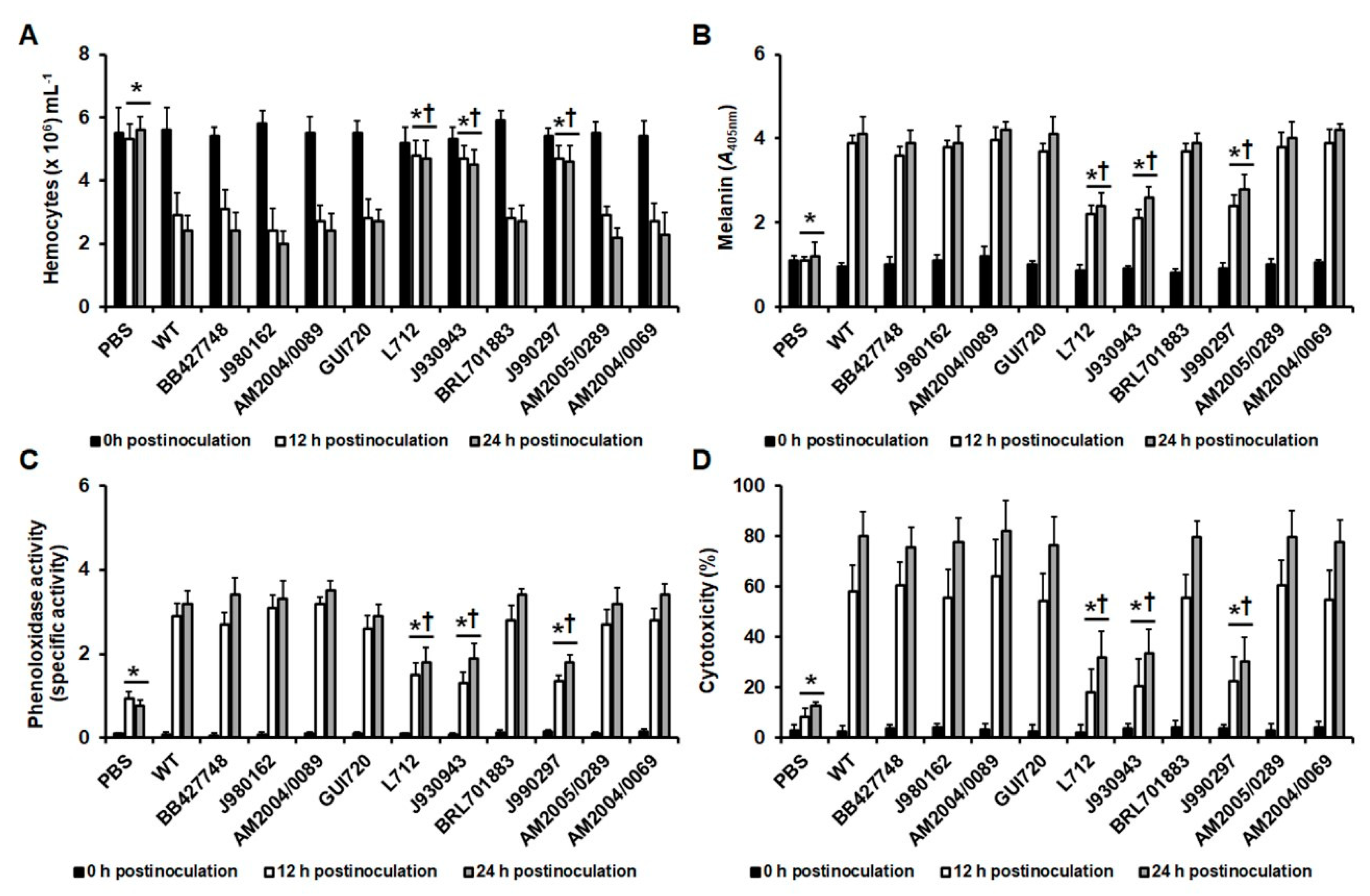
| Strain | Organism | Genotype | Reference |
|---|---|---|---|
| ATCC MYA-3404 | Candida tropicalis | Wild-type | ATCC |
| HMY175 | Candida tropicalis | As ATCC MYA-3404, but mnn4Δ::sat1/mnn4Δ::sat1 | [20] |
| HMY181 | Candida tropicalis | As ATCC MYA-3404, but och1Δ::sat1/och1Δ::sat1 | [19] |
| HMY207 | Candida tropicalis | As ATCC MYA-3404, but pmr1Δ::sat1/pmr1Δ::sat1 | [19] |
| BB427748 | Candida tropicalis | Wild-type | [34] |
| J980162 | Candida tropicalis | Wild-type | [34] |
| AM2004/0089 | Candida tropicalis | Wild-type | [34] |
| GUI720 | Candida tropicalis | Wild-type | [34] |
| L712 | Candida tropicalis | Wild-type | [34] |
| J930943 | Candida tropicalis | Wild-type | [34] |
| BRL701883 | Candida tropicalis | Wild-type | [34] |
| J990297 | Candida tropicalis | Wild-type | [34] |
| AM2005/0289 | Candida tropicalis | Wild-type | [34] |
| AM2004/0069 | Candida tropicalis | Wild-type | [34] |
| SC5314 | Candida albicans | Wild-type | [35] |
| ATCC 6260 | Candida guilliermondii | Wild-type | [36] |
| ATCC 6258 | Candida krusei | Wild-type | ATCC |
| SZMC 8110 | Candida parapsilosis | Wild-type | [37] |
| SZMC 1545 | Candida orthopsilosis | Wild-type | [37] |
| SZMC 1548 | Candida metapsilosis | Wild-type | [37] |
| VPCI 479/P/13 | Candida auris | Wild-type | [38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Carnero, L.C.; Clavijo-Giraldo, D.M.; Gómez-Gaviria, M.; Lozoya-Pérez, N.E.; Tamez-Castrellón, A.K.; López-Ramírez, L.A.; Mora-Montes, H.M. Early Virulence Predictors during the Candida Species–Galleria mellonella Interaction. J. Fungi 2020, 6, 152. https://doi.org/10.3390/jof6030152
García-Carnero LC, Clavijo-Giraldo DM, Gómez-Gaviria M, Lozoya-Pérez NE, Tamez-Castrellón AK, López-Ramírez LA, Mora-Montes HM. Early Virulence Predictors during the Candida Species–Galleria mellonella Interaction. Journal of Fungi. 2020; 6(3):152. https://doi.org/10.3390/jof6030152
Chicago/Turabian StyleGarcía-Carnero, Laura C., Diana M. Clavijo-Giraldo, Manuela Gómez-Gaviria, Nancy E. Lozoya-Pérez, Alma K. Tamez-Castrellón, Luz A. López-Ramírez, and Héctor M. Mora-Montes. 2020. "Early Virulence Predictors during the Candida Species–Galleria mellonella Interaction" Journal of Fungi 6, no. 3: 152. https://doi.org/10.3390/jof6030152
APA StyleGarcía-Carnero, L. C., Clavijo-Giraldo, D. M., Gómez-Gaviria, M., Lozoya-Pérez, N. E., Tamez-Castrellón, A. K., López-Ramírez, L. A., & Mora-Montes, H. M. (2020). Early Virulence Predictors during the Candida Species–Galleria mellonella Interaction. Journal of Fungi, 6(3), 152. https://doi.org/10.3390/jof6030152






