1. Introduction
The causal agent of the maize late wilt disease is the soil- and seed-borne pathogen
Magnaporthiopsis maydis (former names—
Harpophora maydis and
Cephalosporium maydis) [
1]. The pathogen penetrates the plant’s root system at an early stage of the growth period and may cause delayed or reduced seedling sprouting [
2,
3]. The most prominent disease symptoms appear months later [
4,
5]. During this time, the pathogen gradually spreads upwards inside the host plant’s water vascular system without any visible symptoms. At the age of 50–60 days (depending on the maize cultivar susceptibility degree and environmental growth conditions, especially the watering regime) [
6,
7], the plant’s lower parts (mainly the leaves) begin to lose their green color. This begins a relatively rapid dehydration process that peaks 10–20 days later, resulting in the plant’s death [
8]. The disease is considered to be the predominant devastating maize disease in Egypt and Israel, and a serious threat to other areas, including India [
9], Hungary [
10], Romania [
11], Spain and Portugal [
12], and Nepal [
13].
The pathogen can survive in the absence of susceptible maize hosts over extended periods in the soil as sclerotia, spores, or hyphae on plant remains [
14]. The sclerotia consist of a packed fungal mycelium surrounded by a thick tissue enriched with high melanin content that protects it from UV light [
15].
M. maydis can also survive by developing inside an alternative host plant. To date, these include
Lupinus termis L. (lupine) [
16],
Citrullus lanatus (watermelon),
Gossypium hirsutum L. (cotton), and
Setaria viridis (green foxtail) [
17,
18].
A traditional method for determining soil infestation level includes soil plating onto semi-selective media and counting colonies of the fungus following incubation for several weeks. However, this timetable can be problematic when maize growers have to make planting decisions; therefore, methods that could guarantee more quickly and accurate results would be advantageous to economic production. Another approach to provide a faster technique of fungal pathogen detection and quantification in the soil is the use of polymerase chain reaction (PCR) molecular identification [
19]. Customary PCR tests using nested or single amplifications have been suggested for detecting other fungi (summarized, for example, for
Verticillium dahliae by [
20]). A TaqMan probe assay has been developed lately to target
M. maydis [
21] and could be used for the same purpose, as demonstrated for the potato pathogen,
Synchytrium endobioticum [
22].
However, in the case of
M. maydis, the goal of developing an effective soil assay has yet to be achieved. This assay may be challenging to develop due to low quantities of the fungus in the soil and the scattered nature of the disease (that spreads in patches in the field). To make this task even harder, DNA extracted from soil samples may include PCR inhibitors [
23]. With the gradual canceling of soil disinfecting using methyl bromide [
24], lengthier intervals are needed between soil treatments, using alternative fungicides, and sowing. This challenge, together with the increase in production of organic cultivars, necessitates an urgent need for an accurate and rapid method for the determination of
M. maydis soil inoculum level.
On the other hand,
M. maydis can be isolated and identified from symptomatic plant tissues using microscopic morphological characteristics, but this method requires taxonomic expertise and time investment. Additionally, as noted earlier by Saleh et al. (2003) [
25],
M. maydis recovery from plant material is challenging. This difficulty is true even with heavily inoculated plant tissues, due to the pathogen’s slow development and the relatively high prevalence of other, more fast-growing fungi, specifically
Fusarium spp. Indeed, late wilt disease is often accompanied by secondary plant pathogenic fungi contamination, enhancing the stem symptoms. Such pathogenic fungi opportunists are
Fusarium verticillioides, causing stalk rot [
15], and
Macrophomina phaseolina, causing charcoal rot [
17]. We invested considerable efforts in
M. maydis isolation from infested commercial fields with a long history of late wilt harsh damages, but with minor success [
26]. The use of Hygromycin-containing growth-media in moderate concentration (up to 50 µg/mL), which allows for
M. maydis development, partly facilitated this task.
The presence of species-specific PCR primers enables the differentiation of
M. maydis from other species in the
Gaeumannomyces-Harpophora group [
25]. These primers were used as a molecular diagnostic method to track disease progression in the tissues of maize plants grown in an infested field in northern Israel [
15,
27] and to study the interactions of
M. maydis with its host [
28]. The molecular assay, together with symptoms evaluation, was used to test chosen fungicides’ ability to restrain the pathogen [
8,
29]. This approach was applied, in vitro, in a series of experiments, comprised of a culture plate screening, followed by a detached root pathogenicity inoculation, and eventually a seedling assay [
4].
The traditional PCR-based method is only capable of exposing changes in the amount of fungal DNA inside the plant tissues, above certain threshold levels. For example, in field experiments, it can detect
M. maydis DNA only from days 40–50 onwards [
15]. In greenhouse sprouts experiments, the PCR was rarely able to detect the fungal DNA in the host tissues before 22 days after sowing (DAS) [
4,
8]. However, in most experiments, the molecular method failed to detect
M. maydis DNA in host tissues, even 40 DAS, due to this technique’s sensitivity limitations.
The present work focuses on the application of the quantitative Real-Time PCR (qPCR) protocol for evaluating the potential use of the maize cultivar Megaton (Hazera Seeds Ltd., Berurim MP Shikmim, Israel) compared to other cultivars for the detection and estimation of soils infested with M. maydis. To this end, in vitro seeds and in vivo sprouts pathogenicity assays were conducted (in an incubator and growth chamber, respectively). Seed germination and early development, seedlings emergence, root and shoot wet biomass, and phenological stage were evaluated and compared to qPCR molecular tracking results to identify a possible correlation between symptoms development and pathogens DNA quantity.
2. Materials and Methods
2.1. Field Observation for Assessing Maize Cultivars’ Resistance/Susceptibility to Late Wilt Disease
A field observation for the estimation of maize cultivars’ resistance levels to late wilt disease was conducted in the spring and summer of 2017 in Kibbutz Amir maize field southern area (Mehogi 1 corn plot), in the Hula Valley (Upper Galilee, northern Israel). This field has been known to be late wilt infested for many years [
8] and has been used since 2013 as an experimental field to examine new maize cultivars’ resistance/sensitivity to late wilt disease.
The experimental field average air temperature during the growth period (May 25–August 28, 2017) was 27.6°C, with a minimum temperature of 11.3°C and a maximum of 41.3°C. The average soil temperature at the top 5 cm was 35.1 °C, with a minimum temperature of 20.4 °C and a maximum of 50.4 °C. Average humidity was 51.3% (with a minimum of 13.2% and a maximum of 85.5%). During this period, no precipitation was measured (data according to the Israel Northern Research and Development meteorological station). The experiment tested 12 fodder and six sweet maize cultivars (
Table 1).
Plots, each containing one row, were arranged in the field using a randomized complete block design. A standard row spacing of 96.5 cm was used. Each row was 12 m long and included 8 maize plants·m−1 (approximately 96 plants per row). The field was watered with a 16 mm drip line with 50 cm drip spacing (Dripnet PC1613 F, Netafim, Tel Aviv-Yafo, Israel) applied for two rows. The drip flow rate was 2 L/h, and the field was irrigated during the overall growth season, with a total of 450 mm. All of the plants received insecticides and fertilization according to a growth protocol recommended by the Israel Ministry of Agriculture and Rural Development, Consultation Service (Shaham, Beit-Dagan, Israel). Sowing conducted on May 25, 2017, and germination occurred one day later by watering the field with a frontal irrigation system. The fertilization stage occurred on July 20, 2017 (56 DAS), and the fruit ripening stage in most cultivars was set 14 days later on August 13, 2017 (80 DAS).
The dehydration degree was evaluated 66, 80, 84, and 95 DAS. Calculating the percentage of plants with typical maize late wilt dehydration symptoms was performed according to the upper leaves’ color change to light-silver and then to light-brown. A plant was considered dried when it shows harsh drought symptoms—over 50% of its parts are dehydrated. Classification of the maize cultivars according to their degree of late wilt disease sensitivity was performed using four criteria (previously determined by Dr. Tsafrir Weinberg), as described in
Table 2. Later, on the harvest day of sweet maize cultivars (78 DAS), the levels of
M. maydis DNA were measured using qPCR analysis for all experiment cultivars, as will be detailed below. For each cultivar, the qPCR analysis was conducted on three representative replications (plants). The fodder maize cultivars were harvested 95 days post-sowing.
2.2. Fungal Isolates and Growth Conditions
One selected isolate of
M. maydis,
Hm2 (CBS 133,165), was used for this work. This isolate is a pure monosporic, pathogenic strain validated by its morphological, microscopic, and molecular characteristics [
15,
25]. The
Hm2 strain is kept at the CBS-KNAW Fungal Biodiversity Center, Utrecht, The Netherlands. All fungal colonies were grown routinely on potato dextrose agar (PDA) (Difco, Detroit, MI, USA) in complete darkness at 28 ± 1 °C.
2.3. In Vitro Seed Infection
To examine the pathogens’ ability to infect maize seeds in vitro, seeds were inoculated with the fungus and then tested for the fungus’ DNA presence in the inner tissues in a previously developed method [
8]. The sweet maize hybrids, Megaton cv., Prelude cv. (from SRS Snowy-River seeds, Australia, supplied by Green 2000 Ltd., Bitan Aharon, Israel), or Royalty cv. (from Pop Vriend Seeds B.V., Andijk, The Netherlands, provided by Eden Seeds, Reut, Israel) were chosen for the seed pathogenicity trial. The Megaton cv. is a highly susceptible maize hybrid studied here in the context of late wilt disease for the first time. The other two cultivars, Prelude (late wilt disease high susceptible cv.) and Royalty (moderately resistant cv.), are representative maize hybrids well studied in the lab [
25] and the field [
4,
8,
15,
29].
Ten seeds of each selected maize genotype were dipped in 1% (v/v) sodium hypochlorite for 3 min, thoroughly washed in autoclaved double-distilled water (DDW), and then maintained with 20 mL sterile DDW in a 250 mL Erlenmeyer flask. Three 6-mm-diameter M. maydis colony agar disks were added to each Erlenmeyer flask. These agar disks were cut previously from M. maydis colonies margins. These colonies grew on PDA in the dark at 28 °C for about five days. The Erlenmeyer flasks were maintained at 28 °C in the dark in a rotary shaker at 150 revolutions per minute (RPM) for one week. At the end of the experiment, determination of seeds germination percentage, wet biomass of all the seeds in each treatment, and wet biomass of the germinated seeds was conducted using analytical scales. A germinating seed was defined as a seed in which the radicle scored the seed coat. Each treatment and the non-inoculated control (seeds soaked only in water) comprised six independent replications. The whole experiment was repeated twice, and similar results were obtained. The molecular detection at the experiment end was conducted on samples of three seeds from each repeat for every treatment. The samples were disinfected with an ethanol suspension (70%) and then washed with sterile DDW to ensure no fungus hyphae are attached to the seeds’ surface. The seeds were ground to a powder in liquid nitrogen, and DNA was extracted and used as a template for the qPCR reactions, as will be detailed below.
2.4. Growth Chamber Seedlings Assay
Maize sprout susceptibility assays were performed under controlled conditions and aimed at achieving two goals. The first goal was to identify variations in the severity of the symptoms of plants cultivated on naturally infested field soils taken from two different locations. Soil samples were taken from commercial fields, having a long history of
M. maydis contamination: the Kibbutz Amir field soil [
8,
29] and the Kibbutz Neot Mordechai field soil [
15,
27]. The direct examination of those soils impact on late wilt disease symptoms progression was carried out without the enrichment with the pathogen (added in the other growth chamber experiment). Additionally, the symptoms evaluation was performed by studying the differences among selected maize cultivars and between those cultivars and
S. viridis (green foxtail), a recently discovered new host of this fungus [
18]. This is important to identify the most appropriate test plant for the soil assay. Our second aim was to use the preferable cultivar and determine the optimal growth period duration that will be used to inspect susceptibility to late wilt infested soils.
Each experimental group comprised of six biological replicates (pots). Each pot was sown with five seeds. Maize hybrids Megaton cv. and Royalty cv., and
S. viridis were inoculated with
M. maydis as previously described [
25]. Briefly, seeding was performed in a two-liter pot about 4 cm beneath the ground surface. The soil was naturally infested peat soil from the Kibbutz Amir maize field (Hula Valley, Upper Galilee, northern Israel), which has been
M. maydis infested for many years, mixed with 30% Perlite No. 4 (for aerating the soil(. The negative control (uninfected soil) in the pot experiments was taken from a commercial field alongside soil that had no history of late wilt disease. If
M. maydis infestation did present in the negative control soil, it was estimated to be very low. Watering was performed by adding 100 ± 10 mL tap water every 72 h. All the plants were raised in a controlled condition in a growth chamber at a temperature of 25 ± 2 °C, a relative humidity of 30% and a 16-h photoperiod illuminated by cool-white fluorescent tubes (Philips, Eindhoven, The Netherlands).
To ensure as high and uniform a disease pressure as possible, the inoculum method comprised two steps. First, 20 g sterilized infected wheat seeds were added to each pot with the seeding. These seeds had been incubated previously for three weeks in the dark at 28 °C with 10
M. maydis colony agar disks (per 100 g seeds). These sterilized infected seeds were used to scatter the pathogen in the soil, as previously described [
15,
30]. Second, according to [
4], two culture agar disks (6-mm-diameter) from five-day-old
M. maydis colonies (grown in the dark at 28 °C) were added to each plant’s upper roots (4 cm beneath the surface) four days after seeding (with the emergence of the plants above the ground surface).
At three intervals at the age of 10, 20, and 30 days post-sowing, the seedlings were examined for phenological development and plant height. Also, roots and above-ground fresh-weight parts were measured. At the above time points, the seedlings were removed gently from the ground, rinsed thoroughly under running tap water and then dried softly with paper towels. The root and above-ground parts of each seedling were segregated by a scalpel, and the wet biomass was measured separately using analytical scales. The above-ground parts height (from the first node to the shoot tip) of each sprout was measured individually. The whole experiment was repeated twice, and similar results were obtained.
For DNA extraction, plants were washed twice in DDW for 30 s. Tissue testing was achieved by cutting a cross-section of about 2 cm in length from the root’s uppermost part and the near-surface hypocotyl of each plant. Five plants from each pot were sampled, the samples were combined, and the total fresh weight was adjusted to 0.7 g and regarded to be one replicate. DNA was extracted and used as a template for the qPCR reactions, as will be detailed below.
2.5. DNA Isolation and Extraction
Tissue samples were moved to universal extraction bags (Bioreba, Reinach, Switzerland) and 4 mL CTAB buffer (0.7 M NaCl, 1% cetyltriammonium bromide, 50 mM Tris- hydrochloric acid pH 8.8, 10 mM EDTA and 1% 2-mercaptoethanol) were added to each bag. The samples were ground with a hand tissue homogenizer (Bioreba, Reinach, Switzerland) for 5 min until the samples were fully homogenous. The homogenized tissues underwent DNA extraction, according to [
31], with minor modifications [
25]. After grinding the tissue samples, 1.2 mL from this mixture was kept for 20 min at 65 °C. The samples were centrifuged for 5 min at 25 °C at 14,000 rpm. The upper lysate part (usually 700 µL) was extracted with an equal volume of chloroform/isoamyl alcohol (24:1). Following mixing by vortex, the mixture was repeated twice. The supernatant (approximately 300 µL) was then moved to a new Eppendorf tube and mixed with cold isopropanol (2:3). The DNA suspension was mixed gently by inverting the tube several times, maintained for 20–60 min at 20 °C, and centrifuged (14,000 rpm for 20 min at 4 °C). The precipitated DNA isolated was resuspended in 0.5 mL 70% ethanol. After additional centrifugation (14,000 rpm at 4 °C for 10 min), the DNA pellet was dried in a sterile hood overnight. The DNA was suspended by the addition of 100 µL of HPLC-grade water and maintained at −20 °C until use in the qPCR reactions.
2.6. Molecular Diagnosis
The molecular method used was recently described in detail [
17]. Briefly, three representative plants from each treatment were selected arbitrarily for qPCR analysis. Each biological replicate was tested in triplicate using qPCR to confirm the reliability of the results. The qPCR reactions were executed using the ABI PRISM
® 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) for 384-well plates. A reaction volume of 5 µL (in total) contained 2 µL of the DNA extract sample, 2.5 µL of the Universal SYBR
® Green Supermix iTaq™ (Bio-Rad Laboratories Ltd., Rishon Le Zion, Israel), 0.25 µL of each of the forward and reverse primers (at a concentration of 10 µM of each primer per well). The qPCR plan was: 1 min at 95 °C precycle activation stage, 40 cycles of denaturation (15 s at 95 °C), annealing and extension (30 s at 60 °C), and eventually a melting curve analysis. The target A200a,
M. maydis-specific DNA, was evaluated against a reference “housekeeping” gene—the mitochondria-cytochrome c oxidase, COXI gene (sequences in [
32]). This reference gene encoding the eukaryotic mitochondria respiratory electron transport chain’s last enzyme was used to normalize the amount of DNA. Calculating the relative DNA abundance was according to the ΔCt model. The same efficacy was assumed for all samples. All amplifications were performed in triplicate.
2.7. Statistical Analyses
In all experiments, a completely randomized statistical design was used. Data analysis and statistics were fulfilled using the JMP program, 7th edition, SAS Institute Inc., Cary, NC, USA. The Student’s t-test was used for the analysis of
M. maydis infection results (with a significance threshold of
p = 0.05) and for comparisons of treatment means to the control. In the field trials’ qPCR-based-molecular DNA tracking, there is an objective difficulty to achieve uniform repeats, due to changes in environmental conditions, and the non-uniform spreading nature of the late wilt disease pathogen [
32]. Consequently, this resulted in relatively high standard error values, and in most of those tests, no statistically significant difference could be measured in comparison to the control. This is also true for indoor growth experiments, whereas achieving a uniform infection with this particular pathogen Israeli strains is challenging [
8].
3. Results
Annual field assessments of new maize varieties for the determination of their degree of resistance to late wilt disease have been conducted by the Consultation Service (Shaham, Beit-Dagan), Israel Ministry of Agriculture and Rural Development, and by the Israel Northern R&D for over a decade. In this procedure inspection, each cultivar was tested over three growth seasons (years) in order to provide a trustworthy recommendation to farmers as to whether to grow a specific maize cultivar on
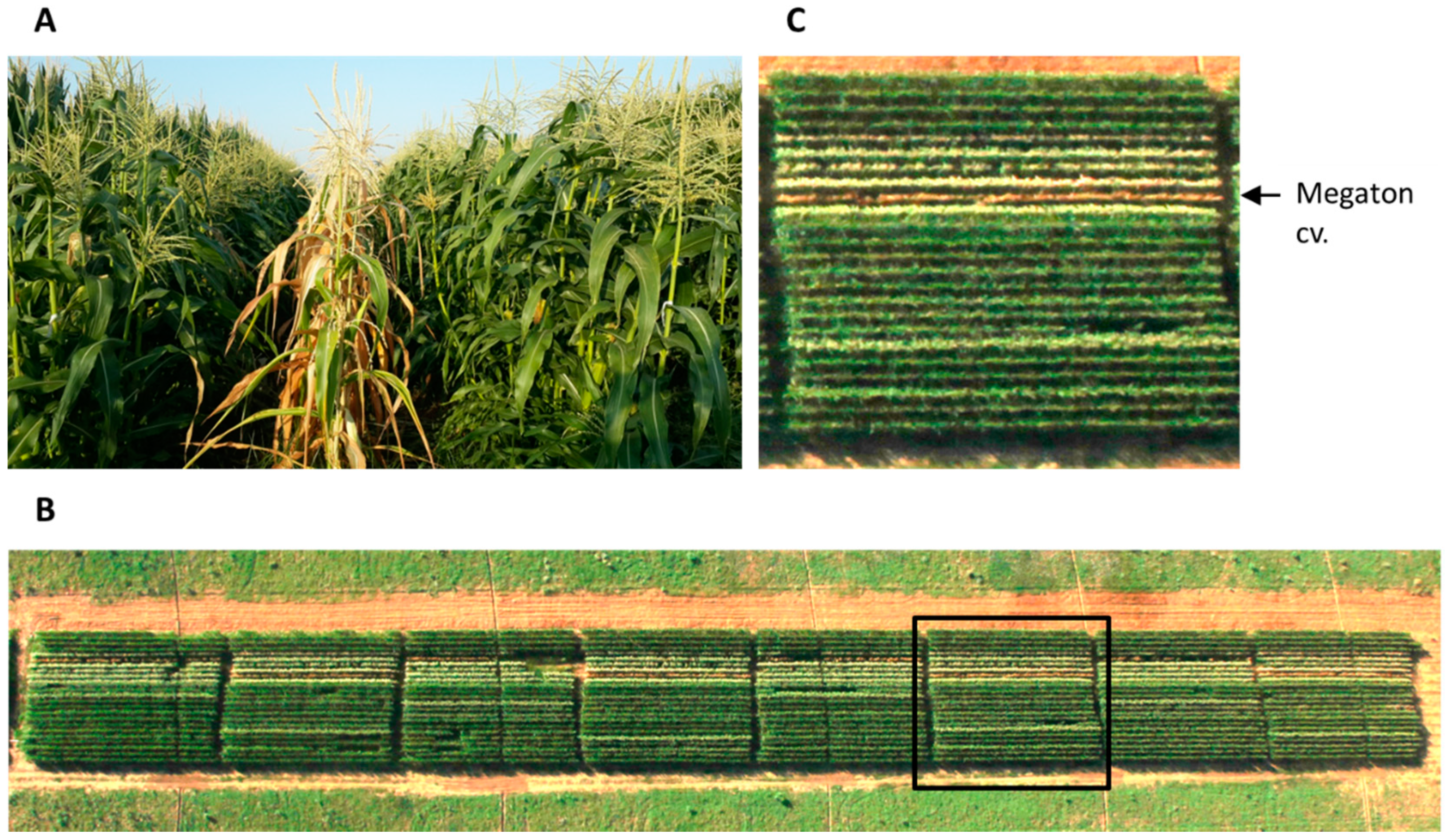
M. maydis-infested soil. During this routine evaluation in 2017, a newly developed hybrid, Megaton cv., was discovered to have late wilt disease hyper-susceptibility (
Figure 1).
At 66 days post-seeding (10 days after fertilization, DAF), the new Megaton cv. was dried entirely (
Figure 1), with total yield loss (
Table 3). At the same time, most of the other cultivars tested showed some degree of resistance and had a healthy appearance with or without minor dehydration symptoms.
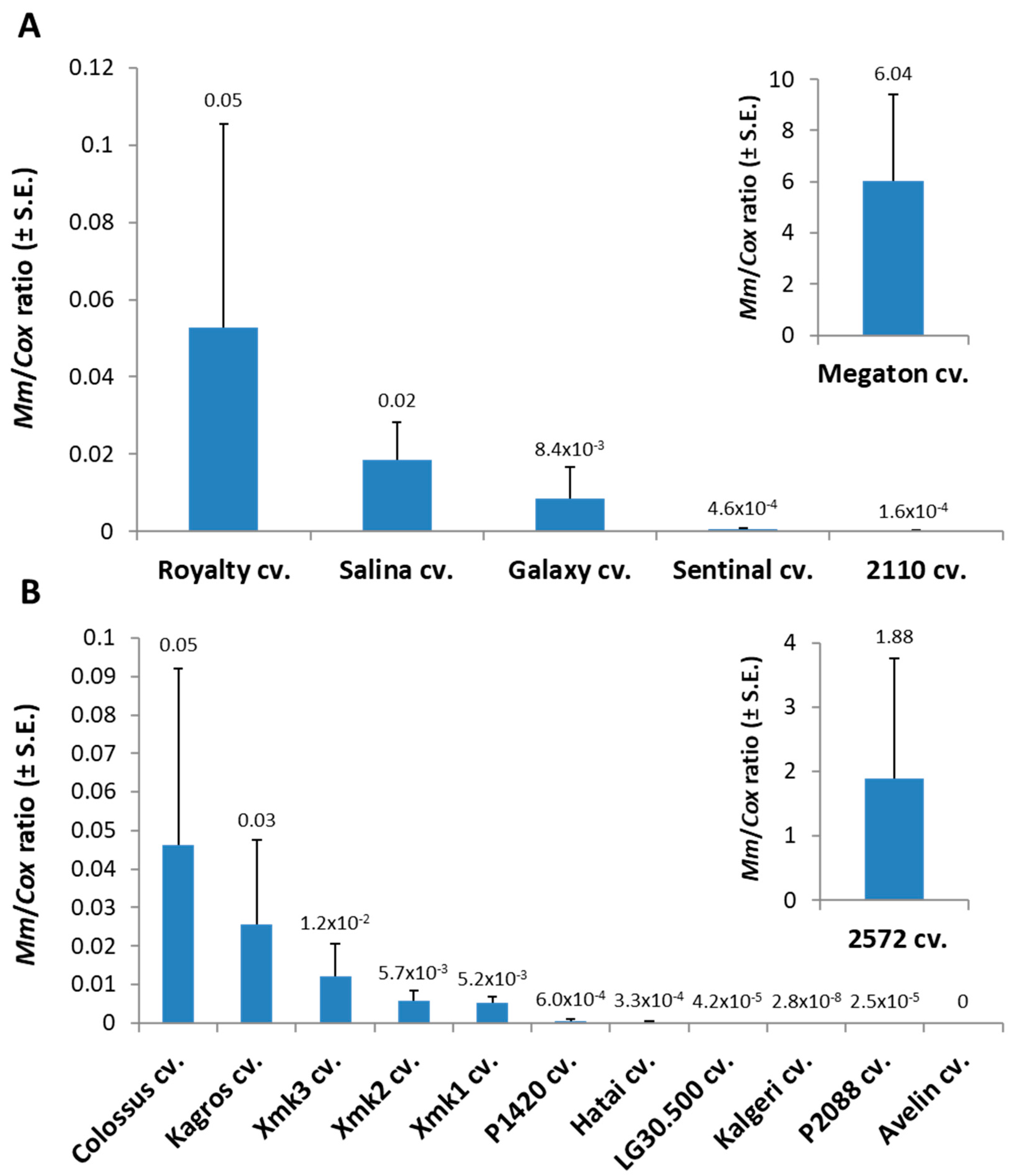
Tracking the
M. maydis fungal DNA within the Megaton cv. root tissues (at 66 DAS) supported the severe above-ground symptoms with over 120-fold higher relative
M. maydis DNA levels compared to the nearest most sensitive sweet maize cultivar (Royalty cv.,
Figure 2). The pathogen spreading within the Megaton cultivar’s root system was prominently higher also in comparison to the most late-wilt-sensitive fodder maize cultivar tested this year, the 2572 cv. Overall,
M. maydis DNA fluctuation among the different maize varieties (
Figure 2) well reflects the degree of their sensitivity or resistance to the disease expressed in their dehydration symptoms (
Table 3).
A dedicated set of experiments aimed at adjusting the seedling assay under controlled conditions will allow us to make a relatively rapid determination of the degree of soil infestation with the late wilt causal agent. To this end, we used the newly discovered Megaton cv. as a potential check genotype and measured its disease severity in response to the soils’ prevalence of the pathogen (
Figure 3). Indeed, two soil samples from commercial fields having a long history of
M. maydis contamination, the Kibbutz Amir field soil [
8,
29], and the Kibbutz Neot Mordechai field soil [
15,
27] evoked a noticeable response. Planting the highly susceptible Megaton cv. in those field soils caused an inhibitory effect on the plants’ growth parameters after 30 days of growth in a controlled environment growth chamber (
Figure 3).
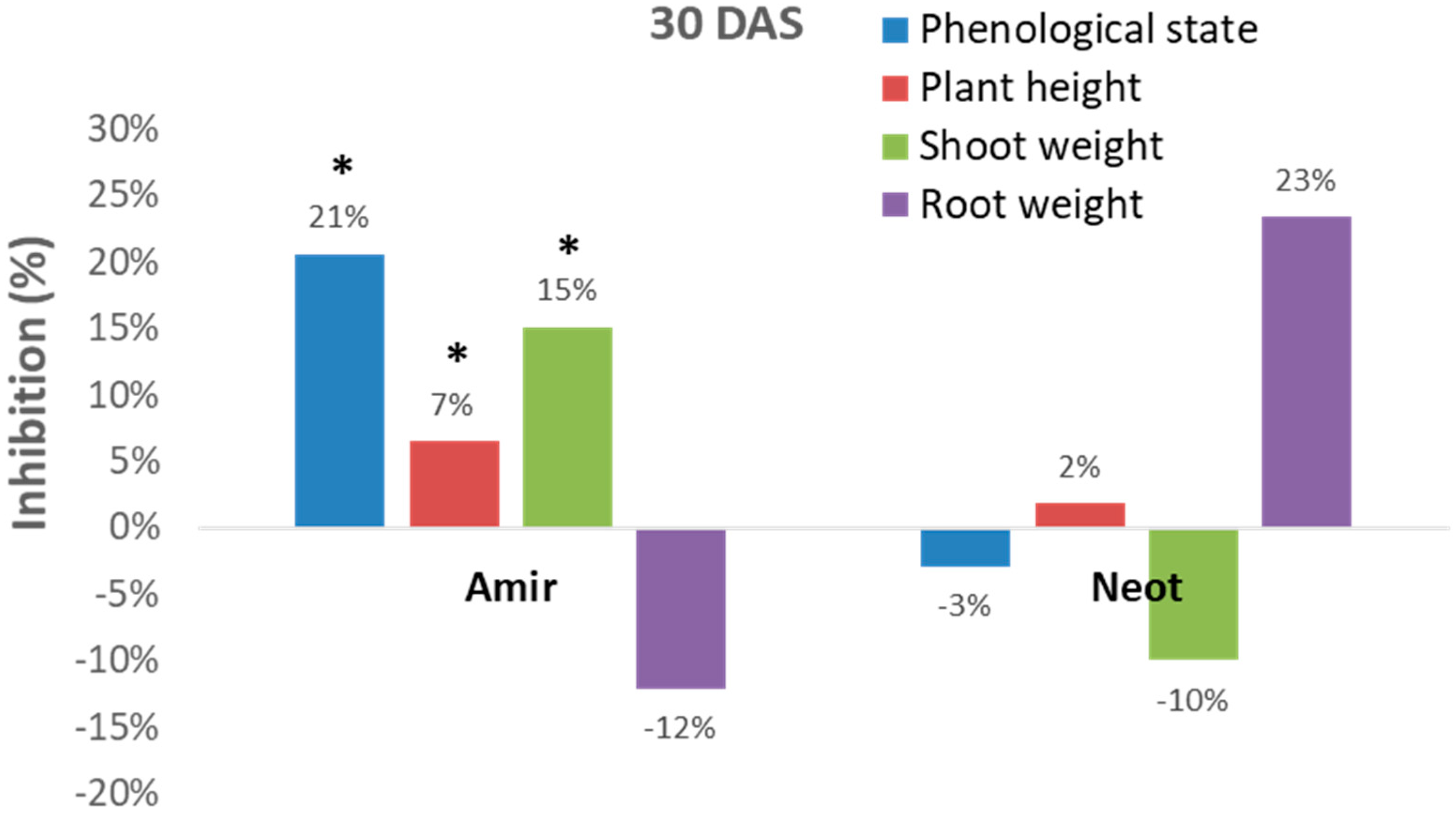
However, these measurements can sometimes be inconsistent. For example, the Kibbutz Amir soil led to significant (p < 0.05) inhibition of the development of the plant’s above-ground parts (phenological state, plant height and shoot fresh weight), while an opposite picture was revealed in the Kibbutz Neot soil (in most measures, except for plant height). Interestingly, the roots of the inspected Megaton cv. reacted differently from the shoot. Plants in the Kibbutz Amir infested soil had a more extensive root system expressed in wet weight (compared to the control) in contrast to those grown in the Kibbutz Neot soil, which had a reduced root wet weight. Thus, relying only on growth parameters is not sufficient for soil contamination evaluation.
To overcome this limitation and to achieve consistent and accurate evaluation of the degree of soil infestation, the sensitive qPCR molecular tool provides an excellent alternative. The qPCR assay consistently detected M. maydis DNA from all sample types except for the negative controls: uninfected maize tissue. The use of this technique for the soil bio-assay will be demonstrated in the following experiments.
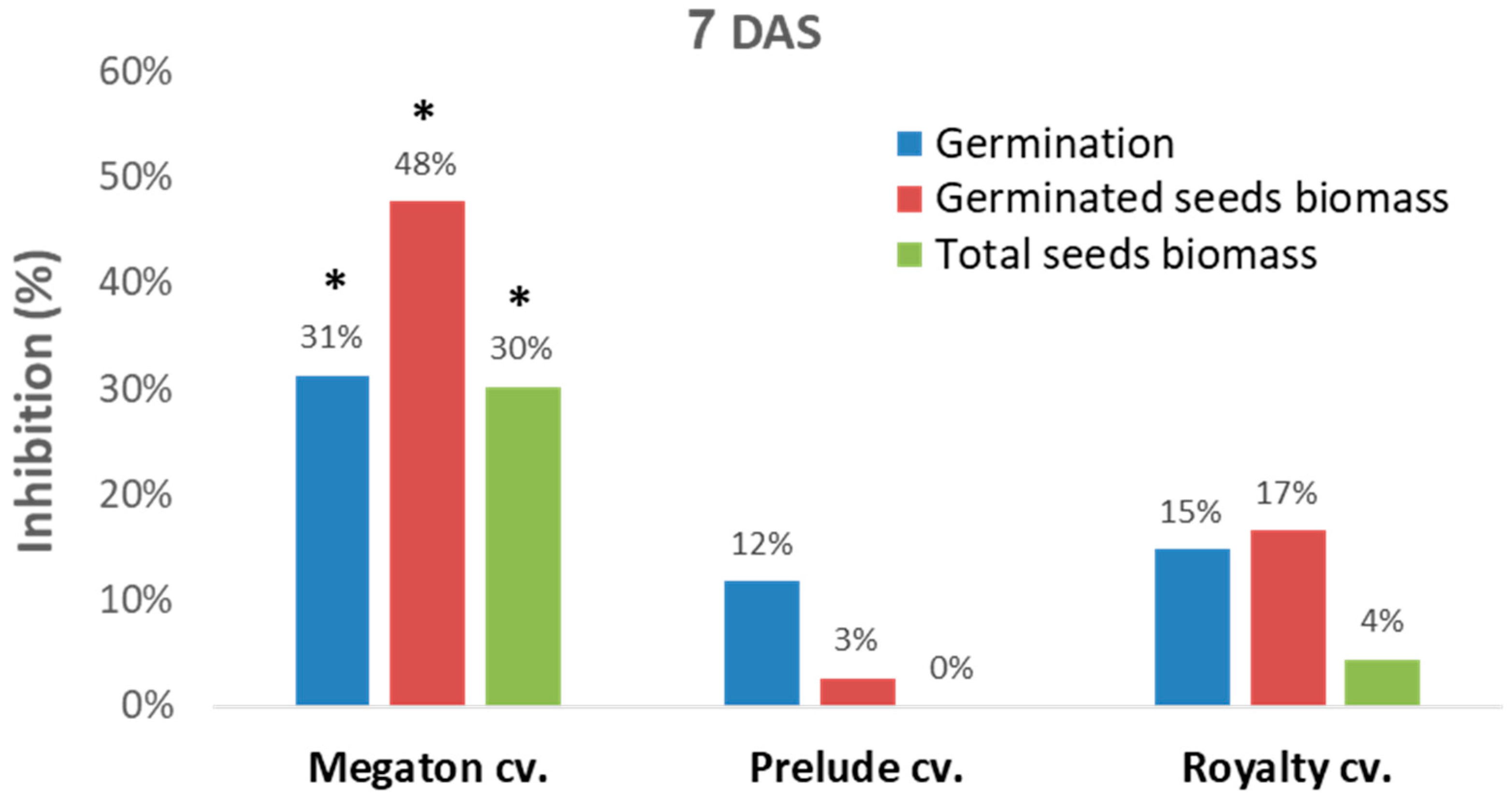
A sequential experiment was designed to evaluate Megaton cv. late wilt sensitivity in comparison to two other well-studied [
25] representative sweet maize cultivars, Prelude cv. (late-wilt-sensitive hybrid) and Royalty cv. (moderately resistant hybrid,
Figure 4). In this setting, in a seed assay in Erlenmeyer flasks, the Megaton cv. was severely affected by the
M. maydis after one week. While the Prelude cv. and Royalty cv. seeds had a moderate response to the pathogen, as reflected in 12–15% germination inhibition and 3–17% germinate seeds biomass reduction, in the Megaton cv., these parameters were significantly higher (
p < 0.05) and exceeded 30% (
Figure 4).
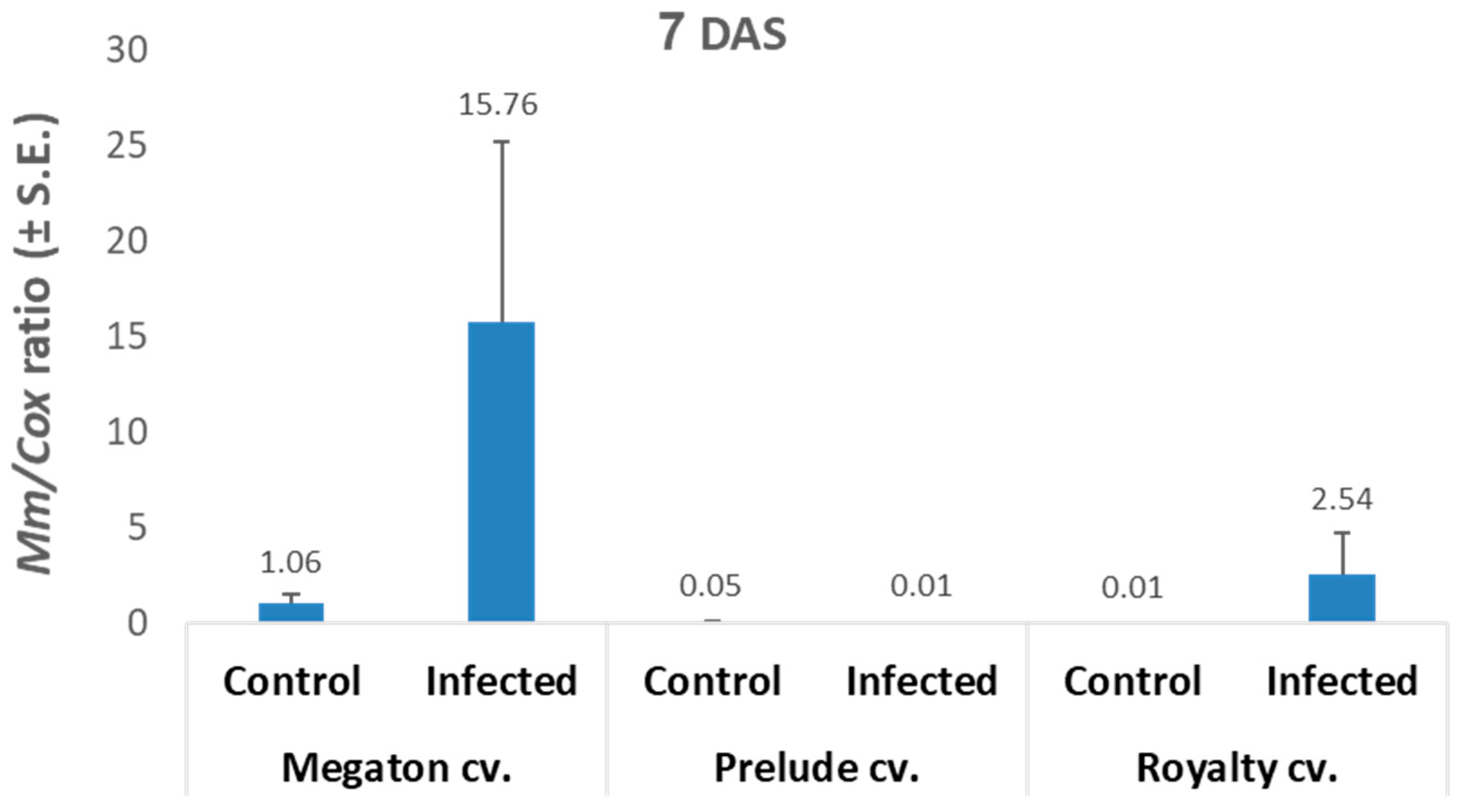
The qPCR analysis results (
Figure 5) were in line with the seeds’ germination results. The infected Megaton cv. seeds had 15.8 relative
M. maydis DNA levels, 6.2 times higher than those of the Royalty cv., and 1,576 times higher than those of the Prelude cv.
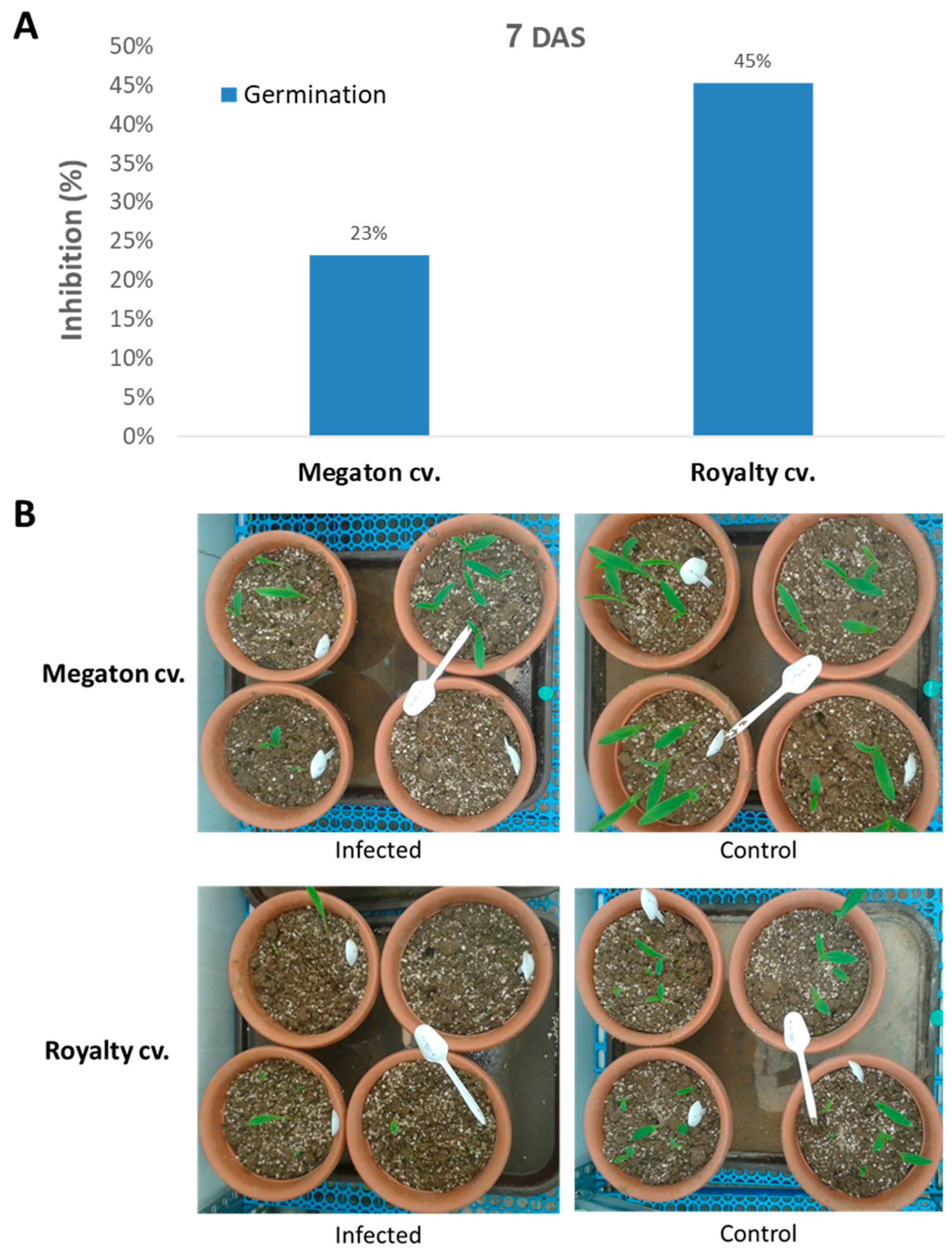
The following step was to inspect Megaton cv. susceptibility in potted sprouts up to the age of 40 days. Here, the Megaton cv. was compared to the Royalty cv., and plant development measures were documented. At 7 DAS, the emergence of seedlings above the ground surface revealed the late wilt response (
Figure 6). Both cultivars suffered from growth inhibition as a result of the pathogen presence. The above-ground emergence inhibition reached 23% of the seeds in the Megaton cv. and 45% in the Royalty cv. (
Figure 6A). Moreover, not only the number of emerging sprouts was affected, but also the number of leaves and their size, as can be seen in the experiment’s photo (
Figure 6B).
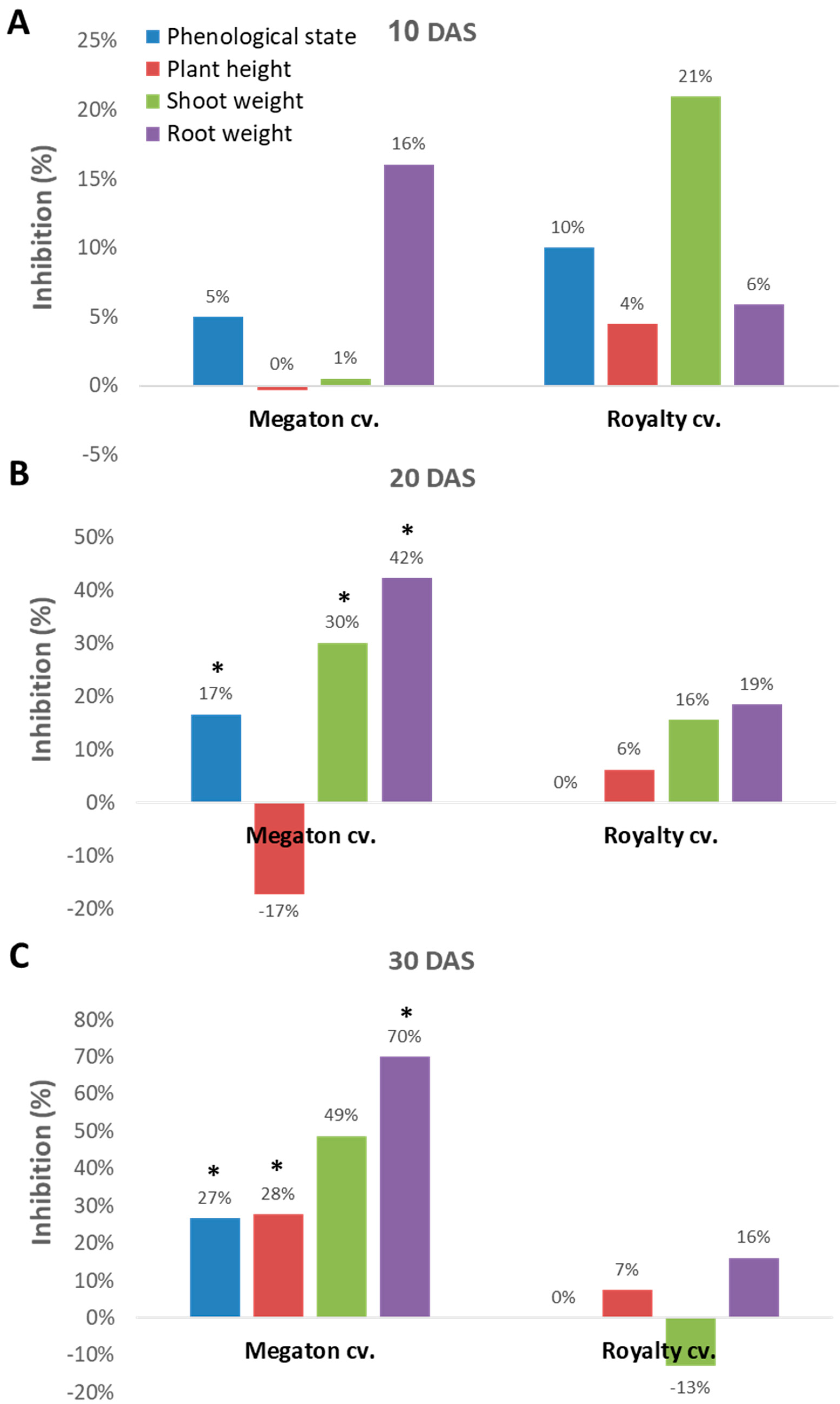
Indeed, close inspection of the growth parameters at 10, 20, and 30 DAS revealed that the phenological state, the above-ground parts fresh weight and height, and the weight of the roots were all inhibited by the
M. maydis presence (
Figure 7). At 10 DAS, the Royalty cv. symptoms were more severe than the Megaton cv. symptoms. However, at 20 DAS, and even more at 30 DAS, the Megaton cv. exterior symptoms were significantly (
p < 0.05) higher, with about a 30% delay in phenological development and plan height growth, and 40% and 70% reduction in shoot and root fresh-weight, respectively. Interestingly, the Royalty cv., which is considered to be a moderately resistant cultivar [
25], showed prominent symptoms at 10 DAS. Still, those symptoms gradually disappeared during the growth period, and eventually, only a minor plant height and root weight reduction were measured (
Figure 7 and
Figure 8A).
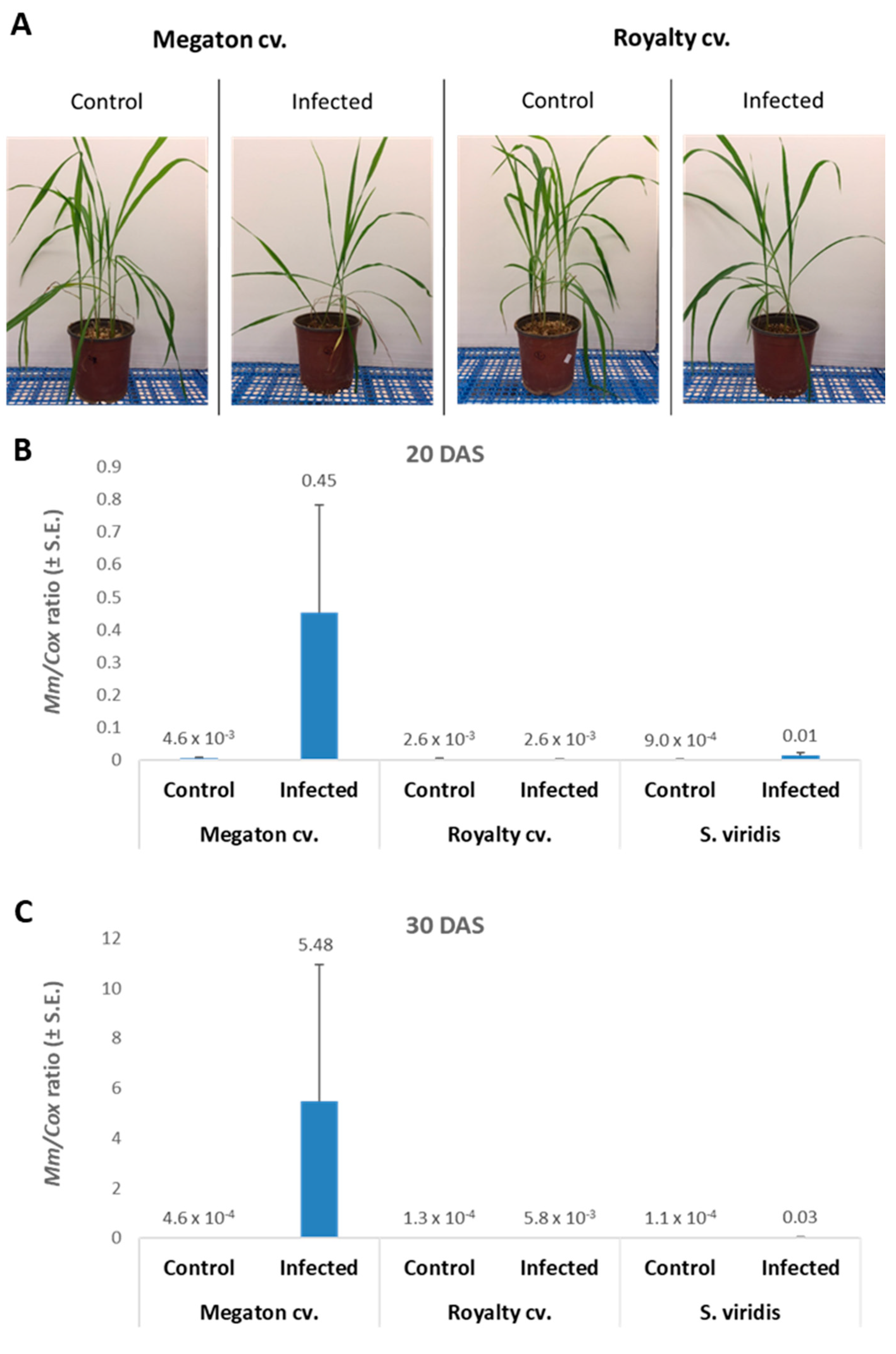
The molecular tracking of
M. maydis DNA in the roots (
Figure 8B,C) of the experiment described above showed an expected elevation in the Megaton cv. plants. The relative DNA levels in those plants increased from 0.45 at 20 DAS to 5.48 at 30 DAS. The DNA levels were 173 and 945 times higher than the pathogen DNA levels in the Royalty cv.’s roots, respectively. We compared the maize plants results to the pathogen DNA variations within the roots of
S. viridis (green foxtail), a recently discovered new host of this fungus [
18]. In the
S. viridis plants that were grown under the same conditions during the same periods,
M. maydis DNA levels were similar to those in the maize Royalty cv. (
Figure 8B,C).
4. Discussion
There is an urgent need to develop a more rapid, accurate, and specific assay to determine the degree of
M. maydis infestation in suspicious soils. Today, no such method exists, and relying on traditional media plant protocols to isolate the pathogen from the ground has several disadvantages that make this solution impractical. A limitation of this technique includes the difficulty in isolating the pathogen from the soil due to its scattered nature and low prevalence. Additionally, the pathogen has a relatively slow growth rate of about 1 cm per day on PDA media plates and optimal temperature [
15], and it is considered a poor competitor in a mixture of microorganisms. Thus, from the soil mycoflora existing in the sample, rapid-growth fungi such as
Fusarium spp. will most probably take over and cover the plate [
33]. This limitation is true even if antibiotics are added to the medium [
25]. Hence, the media plate technique to isolate the pathogen directly from the soil is time-consuming and produces inconsistent results.
The seedling pathogenicity inspection is a preferable method for soil testing. It provides more reliable and relatively rapid results while minimizing the risk of missing M. maydis identification (receiving false-negative results). To this end, we developed and validated a qPCR-based soil bioassay that facilitates detecting and tracking the pathogen in a testing maize plant, the Megaton cv. This Megaton cv. has a high susceptibility to late wilt disease and was discovered during the routine annual inspection of new cultivars conducted in an M. maydis-infested commercial field. Similarly, other highly sensitive maize hybrids could be used for the same purpose. The finding that Megaton is so susceptible in the field provides encouragement to screen diverse inbreeds by the field screening method, which may lead to an identified more ideal trap plants that would allow pathogen detection reproducibly within days of planting.
Interestingly, in the results presented here, the moderate resistance Royalty cv. was relatively more susceptible to fungal infection then the Megaton cv., at the earliest stages of infection, both 7 DAS and 10 DAS (
Figure 6 and
Figure 7, respectively). One possibility for these results is that Royalty cv. has some sort of adult plant resistance that starts acquiring and manifesting after two weeks of growth. It would have been helpful to have conducted a DNA analysis at these earlier stages, but the ability of the qPCR-based DNA analysis to detect the pathogen DNA in potted sprouts before 20 DAS is limited [
25].
The advantage of the proposed bioassay using a susceptible cultivar and a qPCR-based method is its ability to detect the fungus even if it scattered in the soil. This assay enables us to isolate and enrich the maize late wilt pathogen from the soil using a trap plant. The use of this bioassay is essential for study M. maydis distribution and to provide an estimation of its infestation degree in commercial fields. Such data are necessary for decision-making among growers to reduce disease damage and provide risk assessment. The soil bioassay data can provide insurance companies with vital information that will help them to support the farmers. Identifying the fungus will allow the farmer to make decisions about sowing time, choosing the appropriate maize cultivar for planting, the application of disease management strategies, the implementation of a prevention plan, and more. All these means may also help quarantine the infected areas and prevent the disease from spreading to new fields. Together with these advantages, it should be noted that the sensitivity of the bioassay method presented in this work was not specified. It would be beneficial to determine the minimum concentration of the pathogen in the soil that would allow the detection of the pathogen in the plant. This should be the focus of a subsequent study. Additionally, the objective difficulty of achieving a uniform infection with this particular pathogen Israeli strains may be reduced if the number of plants used in each assay is increased.
The proposed assay may also be used to evaluate the virulence of different
M. maydis isolates. Indeed,
M. maydis strains differ in morphology and mode of infection [
34]. For example, four different lines of
M. maydis isolated in Egypt showed different abilities to establish and infect corn plants [
30,
33,
35]. In southern Portugal and Spain, an analysis of 14
M. maydis isolates was performed using 32 different maize varieties [
36]. One of the isolates was extremely virulent and caused intense symptoms that included significant decreases in the fresh weight of both above-ground parts and roots.
Such extremely virulent isolates are an alarming threat to resistant maize cultivars as well. Indeed,
M. maydis may also spread in the tissue of resistant corn genotypes without causing any visible symptoms [
15], and the seeds of the same resistant cultivar may spread the disease. This hints at the possibility that the pathogen acts as an endophyte in resistant maize cultivars, and it may become an opportunist, causing disease under certain circumstances, or it may undergo pathogenic variations and become aggressive.
A TaqMan probe assay was developed recently to target
M. maydis [
21]. This newly developed methodology was demonstrated in a field experiment through the screening of potentially infected maize roots, revealing a high specificity and proving to be a suitable tool to ascertain
M. maydis infection in maize. Its high sensitivity makes it very efficient for the early diagnosis of the diseases and also for certification purposes. It will be most interesting to study the combination of the Megaton cv. pot bioassay together with the TaqMan probe assay in order to achieve an improved soil assay with maximum efficiency and sensitivity.