An Overview on Conventional and Non-Conventional Therapeutic Approaches for the Treatment of Candidiasis and Underlying Resistance Mechanisms in Clinical Strains
Abstract
1. Relevance of Candidiasis within the Spectrum of Fungal Infections
2. Available Antifungals against Candida spp. and Their Modes of Action
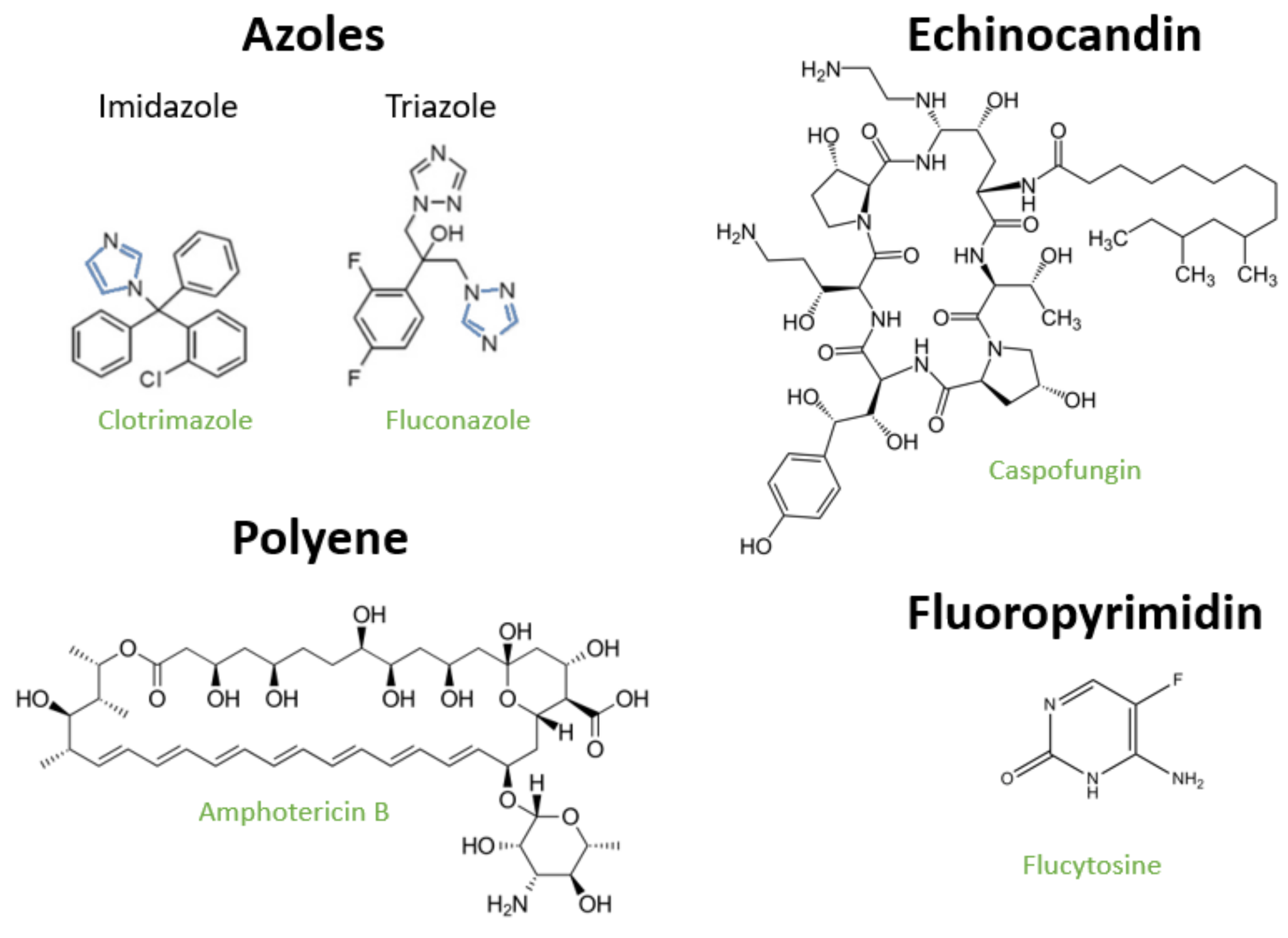
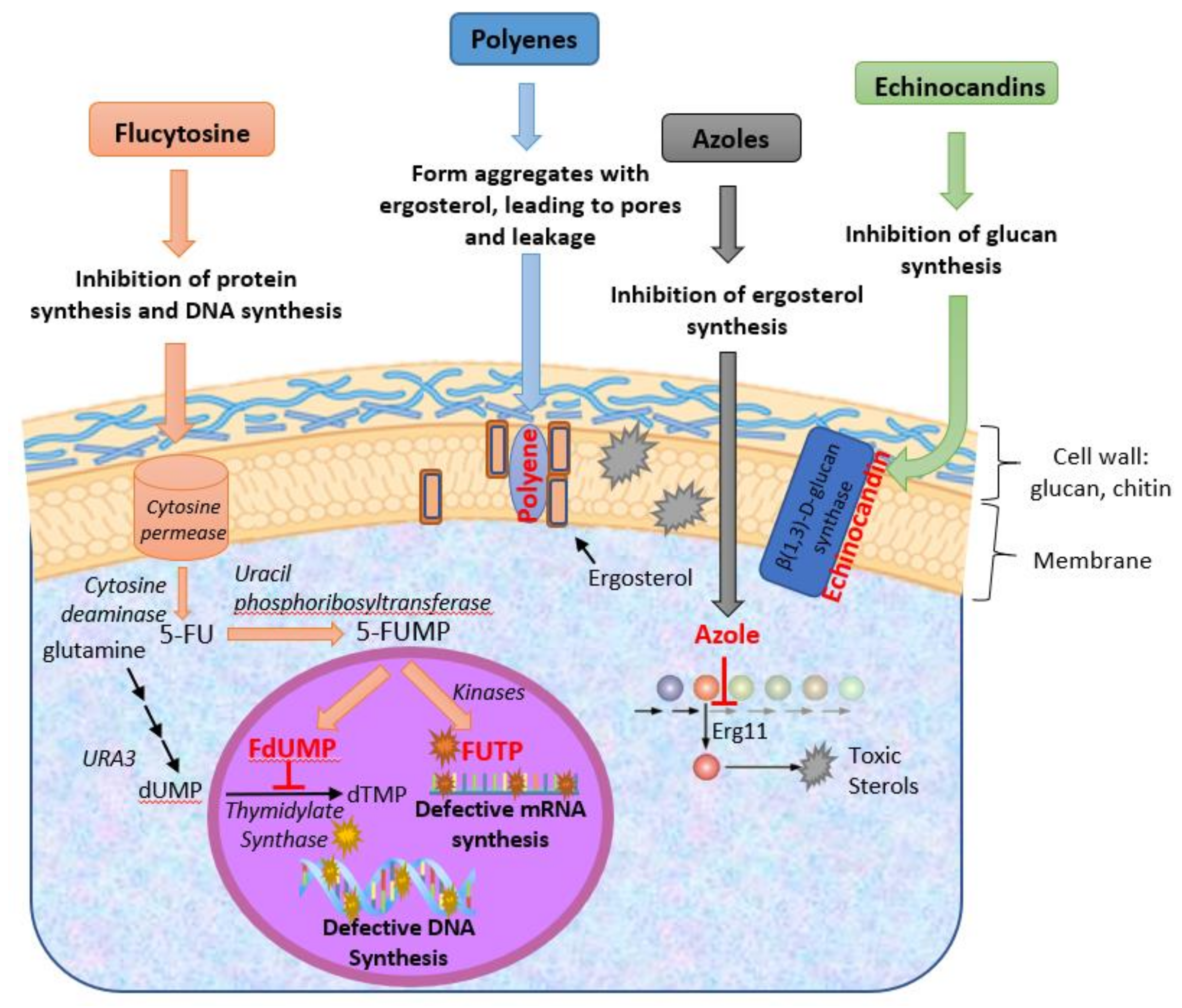
2.1. Polyenes
2.2. Azoles
2.3. Fluoropyrimidines
2.4. Echinocandins
3. Incidence of Antifungal Resistance and Underlying Mechanisms
3.1. Molecular Mechanisms Underlying Resistance to Antifungals in Clinical Strains
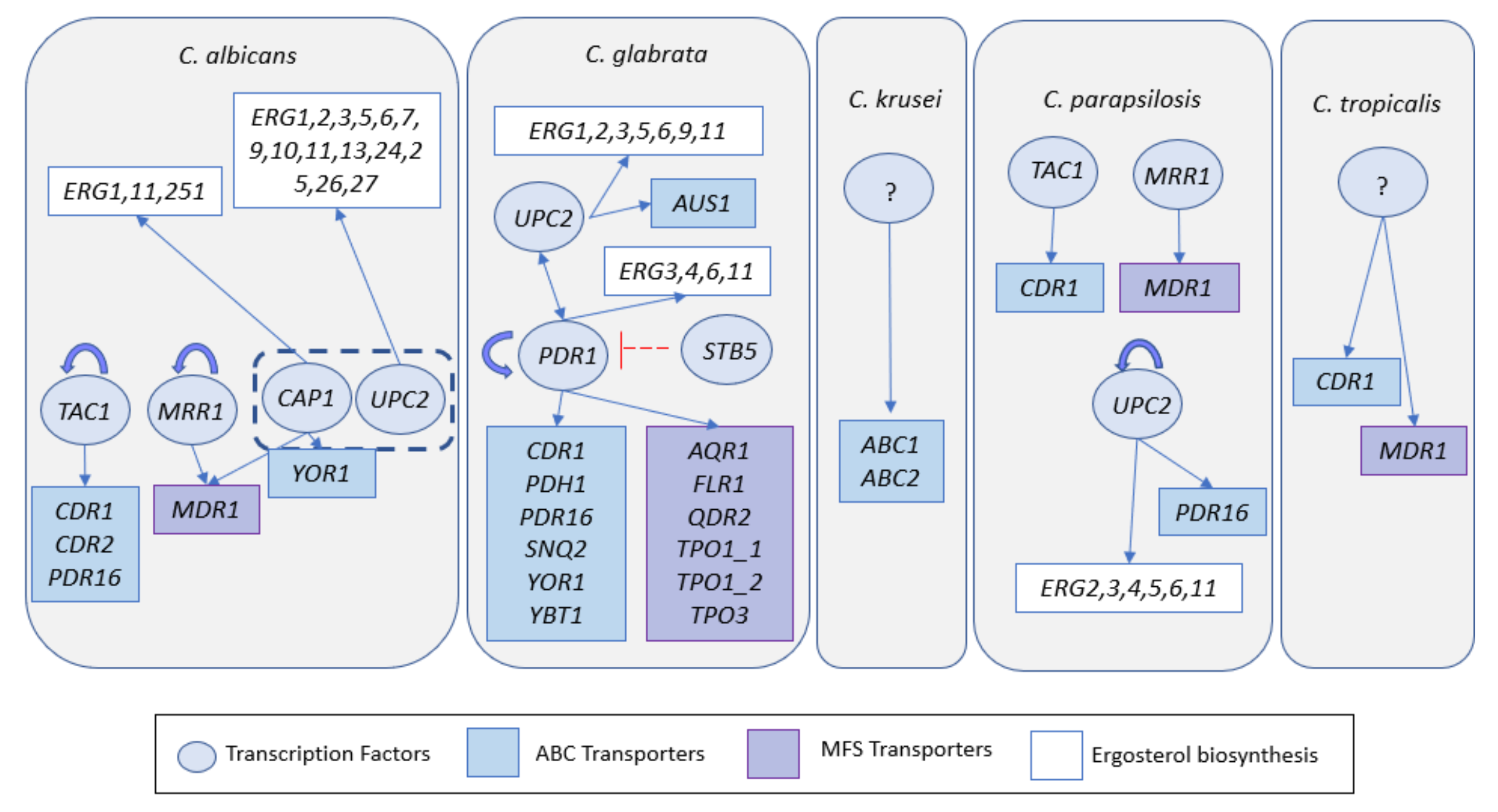
3.1.1. Azoles
3.1.2. Flucytosine, Echinocandines and Polyenes
3.2. Antifungal Resistance Driven by Large-Scale Genomic Alterations
4. Novel Approaches for the Development of Anti-Candida Agents
4.1. Phytotherapeutics
4.2. Redesign of “Old Antifungals”
4.3. New Compounds Obtained by Chemical Synthesis
4.4. Nanoparticles
4.5. Use of Probiotics and Antimicrobial Peptides
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Trans. Med 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Miranda, L.N.; van der Heijden, I.M.; Costa, S.F.; Sousa, A.P.; Sienra, R.A.; Gobara, S.; Santos, C.R.; Lobo, R.D.; Pessoa, V.P., Jr.; Levin, A.S. Candida colonisation as a source for candidaemia. J. Hosp. Infect. 2009, 72, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, M. Epidemiology of Candida albicans infections and role of non-Candida-albicans yeasts. Curr. Drug Targets 2006, 7, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Castanheira, M. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int. J. Antimicrob. Agents 2017, 50, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Criseo, G.; Scordino, F.; Romeo, O. Current methods for identifying clinically important cryptic Candida species. J. Microbiol. Methods 2015, 111, 50–56. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Wilson, D.; Drew, R.; Perfect, J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev. Anti-Infect. Ther. 2015, 13, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, W.J.; Garvey, E.P.; Moore, W.R.; Rafferty, S.W.; Yates, C.M.; Schotzinger, R.J. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 3455–3458. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Dzajic, E.; Jensen, R.H.; Johansen, H.K.; Kjaeldgaard, P.; Knudsen, J.D.; Kristensen, L.; Leitz, C.; Lemming, L.E.; Nielsen, L.; et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: Data from a nationwide fungaemia surveillance programme. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2013, 19, e343–e353. [Google Scholar] [CrossRef]
- Vermes, A.; Guchelaar, H.J.; Dankert, J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef]
- Sofjan, A.K.; Mitchell, A.; Shah, D.N.; Nguyen, T.; Sim, M.; Trojcak, A.; Beyda, N.D.; Garey, K.W. Rezafungin (CD101), a next-generation echinocandin: A systematic literature review and assessment of possible place in therapy. J. Glob. Antimicrob. Resist. 2018, 14, 58–64. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Castanheira, M. Activity of a Long-Acting Echinocandin (CD101) and Seven Comparator Antifungal Agents Tested against a Global Collection of Contemporary Invasive Fungal Isolates in the SENTRY 2014 Antifungal Surveillance Program. Antimicrob. Agents Chemother. 2017, 61, e02045-16. [Google Scholar] [CrossRef]
- Sandison, T.; Ong, V.; Lee, J.; Thye, D. Safety and Pharmacokinetics of CD101 IV, a Novel Echinocandin, in Healthy Adults. Antimicrob. Agents Chemother. 2017, 61, e01627-16. [Google Scholar] [CrossRef]
- Pappas, P.G.; Rex, J.H.; Sobel, J.D.; Filler, S.G.; Dismukes, W.E.; Walsh, T.J.; Edwards, J.E.; Infectious Diseases Society of America. Guidelines for treatment of candidiasis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004, 38, 161–189. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.S.; Galask, R.P.; Messer, S.A.; Hollis, R.J.; Diekema, D.J.; Pfaller, M.A. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J. Clin. Microbiol. 2005, 43, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Andes, D.; Diekema, D.J.; Espinel-Ingroff, A.; Sheehan, D.; Testing, C.S.f.A.S. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: Time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist. Updates 2010, 13, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Boyken, L.; Hollis, R.J.; Kroeger, J.; Messer, S.A.; Tendolkar, S.; Diekema, D.J. Wild-type MIC distributions and epidemiological cutoff values for posaconazole and voriconazole and Candida spp. as determined by 24-hour CLSI broth microdilution. J. Clin. Microbiol. 2011, 49, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Espinel-Ingroff, A.; Canton, E.; Castanheira, M.; Cuenca-Estrella, M.; Diekema, D.J.; Fothergill, A.; Fuller, J.; Ghannoum, M.; Jones, R.N.; et al. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B, flucytosine, and itraconazole and Candida spp. as determined by CLSI broth microdilution. J. Clin. Microbiol. 2012, 50, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Wingard, J.R.; Merz, W.G.; Rinaldi, M.G.; Johnson, T.R.; Karp, J.E.; Saral, R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 1991, 325, 1274–1277. [Google Scholar] [CrossRef]
- Autmizguine, J.; Smith, P.B.; Prather, K.; Bendel, C.; Natarajan, G.; Bidegain, M.; Kaufman, D.A.; Burchfield, D.J.; Ross, A.S.; Pandit, P.; et al. Effect of fluconazole prophylaxis on Candida fluconazole susceptibility in premature infants. J. Antimicrob. Chemother. 2018, 73, 3482–3487. [Google Scholar] [CrossRef]
- Goldman, M.; Cloud, G.A.; Smedema, M.; LeMonte, A.; Connolly, P.; McKinsey, D.S.; Kauffman, C.A.; Moskovitz, B.; Wheat, L.J. Does long-term itraconazole prophylaxis result in in vitro azole resistance in mucosal Candida albicans isolates from persons with advanced human immunodeficiency virus infection? Antimicrob. Agents Chemother. 2000, 44, 1585–1587. [Google Scholar]
- Bennett, J.E.; Izumikawa, K.; Marr, K.A. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob. Agents Chemother. 2004, 48, 1773–1777. [Google Scholar] [CrossRef]
- Verweij, P.E.; Snelders, E.; Kema, G.H.; Mellado, E.; Melchers, W.J. Azole resistance in Aspergillus fumigatus: A side-effect of environmental fungicide use? Lancet. Infect. Dis. 2009, 9, 789–795. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Messer, S.A.; Jones, R.N.; Farrell, D.J.; Pfaller, M.A. Activity of echinocandins and triazoles against a contemporary (2012) worldwide collection of yeast and moulds collected from invasive infections. Int. J. Antimicrob. Agents 2014, 44, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.; Tullio, V.; Rodloff, A.; Fu, W.; Ling, T.A.; Global Antifungal Surveillance Group. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Hajjeh, R.A.; Sofair, A.N.; Harrison, L.H.; Lyon, G.M.; Arthington-Skaggs, B.A.; Mirza, S.A.; Phelan, M.; Morgan, J.; Lee-Yang, W.; Ciblak, M.A.; et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 2004, 42, 1519–1527. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Iqbal, N.; Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Bolden, C.B.; Baughman, W.; Stein, B.; Hollick, R.; Park, B.J.; et al. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J. Clin. Microbiol. 2012, 50, 3435–3442. [Google Scholar] [CrossRef]
- Colombo, A.L.; Nucci, M.; Park, B.J.; Nouer, S.A.; Arthington-Skaggs, B.; da Matta, D.A.; Warnock, D.; Morgan, J.; Brazilian Network Candidemia Study. Epidemiology of candidemia in Brazil: A nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 2006, 44, 2816–2823. [Google Scholar] [CrossRef]
- Wang, F.J.; Zhang, D.; Liu, Z.H.; Wu, W.X.; Bai, H.H.; Dong, H.Y. Species Distribution and In Vitro Antifungal Susceptibility of Vulvovaginal Candida Isolates in China. Chin. Med J. 2016, 129, 1161–1165. [Google Scholar] [CrossRef]
- Ng, K.P.; Saw, T.L.; Na, S.L.; Soo-Hoo, T.S. Systemic Candida infection in University hospital 1997-1999: The distribution of Candida biotypes and antifungal susceptibility patterns. Mycopathologia 2001, 149, 141–146. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Bruun, B.; Christensen, J.J.; Fuursted, K.; Johansen, H.K.; Kjaeldgaard, P.; Knudsen, J.D.; Kristensen, L.; Moller, J.; Nielsen, L.; et al. National surveillance of fungemia in Denmark (2004 to 2009). J. Clin. Microbiol. 2011, 49, 325–334. [Google Scholar] [CrossRef]
- Morio, F.; Loge, C.; Besse, B.; Hennequin, C.; Le Pape, P. Screening for amino acid substitutions in the Candida albicans Erg11 protein of azole-susceptible and azole-resistant clinical isolates: New substitutions and a review of the literature. Diagn. Microbiol. Infect. Dis. 2010, 66, 373–384. [Google Scholar] [CrossRef]
- Berkow, E.L.; Manigaba, K.; Parker, J.E.; Barker, K.S.; Kelly, S.L.; Rogers, P.D. Multidrug Transporters and Alterations in Sterol Biosynthesis Contribute to Azole Antifungal Resistance in Candida parapsilosis. Antimicrob. Agents Chemother. 2015, 59, 5942–5950. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Won, E.J.; Shin, J.H.; Kim, S.H.; Lee, W.G.; Kim, M.N.; Lee, K.; Shin, M.G.; Suh, S.P.; Ryang, D.W.; et al. Resistance Mechanisms and Clinical Features of Fluconazole-Nonsusceptible Candida tropicalis Isolates Compared with Fluconazole-Less-Susceptible Isolates. Antimicrob. Agents Chemother. 2016, 60, 3653–3661. [Google Scholar] [CrossRef]
- Vandeputte, P.; Larcher, G.; Berges, T.; Renier, G.; Chabasse, D.; Bouchara, J.P. Mechanisms of azole resistance in a clinical isolate of Candida tropicalis. Antimicrob. Agents Chemother. 2005, 49, 4608–4615. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.L.; Lamb, D.C.; Loeffler, J.; Einsele, H.; Kelly, D.E. The G464S amino acid substitution in Candida albicans sterol 14alpha-demethylase causes fluconazole resistance in the clinic through reduced affinity. Biochem. Biophys. Res. Commun. 1999, 262, 174–179. [Google Scholar] [CrossRef]
- Feng, W.; Yang, J.; Xi, Z.; Qiao, Z.; Lv, Y.; Wang, Y.; Ma, Y.; Wang, Y.; Cen, W. Mutations and/or Overexpressions of ERG4 and ERG11 Genes in Clinical Azoles-Resistant Isolates of Candida albicans. Microb. Drug Resist. 2017, 23, 563–570. [Google Scholar] [CrossRef]
- Neji, S.; Hadrich, I.; Trabelsi, H.; Abbes, S.; Cheikhrouhou, F.; Sellami, H.; Makni, F.; Ayadi, A. Virulence factors, antifungal susceptibility and molecular mechanisms of azole resistance among Candida parapsilosis complex isolates recovered from clinical specimens. J. Biomed. Sci. 2017, 24, 67. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.J.; Schneider, S.; Barker, K.S.; Rogers, P.D.; Morschhauser, J. An A643T mutation in the transcription factor Upc2p causes constitutive ERG11 upregulation and increased fluconazole resistance in Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 353–359. [Google Scholar] [CrossRef]
- Jiang, C.; Ni, Q.; Dong, D.; Zhang, L.; Li, Z.; Tian, Y.; Peng, Y. The Role of UPC2 Gene in Azole-Resistant Candida tropicalis. Mycopathologia 2016, 181, 833–838. [Google Scholar] [CrossRef]
- Silva, A.P.; Miranda, I.M.; Guida, A.; Synnott, J.; Rocha, R.; Silva, R.; Amorim, A.; Pina-Vaz, C.; Butler, G.; Rodrigues, A.G. Transcriptional profiling of azole-resistant Candida parapsilosis strains. Antimicrob. Agents Chemother. 2011, 55, 3546–3556. [Google Scholar] [CrossRef]
- Hull, C.M.; Bader, O.; Parker, J.E.; Weig, M.; Gross, U.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Two clinical isolates of Candida glabrata exhibiting reduced sensitivity to amphotericin B both harbor mutations in ERG2. Antimicrob. Agents Chemother. 2012, 56, 6417–6421. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.F.; Sammons, L.R.; Zhang, X.; Suffis, S.D.; Su, Q.; Myers, T.G.; Marr, K.A.; Bennett, J.E. Microarray and molecular analyses of the azole resistance mechanism in Candida glabrata oropharyngeal isolates. Antimicrob. Agents Chemother. 2010, 54, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Fiori, B.; Ranno, S.; Torelli, R.; Fadda, G. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob. Agents Chemother. 2005, 49, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Berila, N.; Borecka, S.; Dzugasova, V.; Bojnansky, J.; Subik, J. Mutations in the CgPDR1 and CgERG11 genes in azole-resistant Candida glabrata clinical isolates from Slovakia. Int. J. Antimicrob. Agents 2009, 33, 574–578. [Google Scholar] [CrossRef]
- Brun, S.; Berges, T.; Poupard, P.; Vauzelle-Moreau, C.; Renier, G.; Chabasse, D.; Bouchara, J.P. Mechanisms of azole resistance in petite mutants of Candida glabrata. Antimicrob. Agents Chemother. 2004, 48, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Ischer, F.; Monod, M.; Bille, J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: Characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 1997, 143 Pt 2, 405–416. [Google Scholar] [CrossRef]
- Sanglard, D.; Kuchler, K.; Ischer, F.; Pagani, J.L.; Monod, M.; Bille, J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 1995, 39, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Silver, P.M.; Oliver, B.G.; White, T.C. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot. Cell 2004, 3, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Saidane, S.; Weber, S.; De Deken, X.; St-Germain, G.; Raymond, M. PDR16-mediated azole resistance in Candida albicans. Mol. Microbiol. 2006, 60, 1546–1562. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Calabrese, D.; Majcherczyk, P.A.; Bille, J. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 1999, 43, 2753–2765. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Bille, J. Role of ATP-binding-cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 2001, 45, 1174–1183. [Google Scholar] [CrossRef]
- Miyazaki, H.; Miyazaki, Y.; Geber, A.; Parkinson, T.; Hitchcock, C.; Falconer, D.J.; Ward, D.J.; Marsden, K.; Bennett, J.E. Fluconazole resistance associated with drug efflux and increased transcription of a drug transporter gene, PDH1, in Candida glabrata. Antimicrob. Agents Chemother. 1998, 42, 1695–1701. [Google Scholar] [CrossRef]
- Costa, C.; Pires, C.; Cabrito, T.R.; Renaudin, A.; Ohno, M.; Chibana, H.; Sa-Correia, I.; Teixeira, M.C. Candida glabrata drug:H+ antiporter CgQdr2 confers imidazole drug resistance, being activated by transcription factor CgPdr1. Antimicrob. Agents Chemother. 2013, 57, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- Torelli, R.; Posteraro, B.; Ferrari, S.; La Sorda, M.; Fadda, G.; Sanglard, D.; Sanguinetti, M. The ATP-binding cassette transporter-encoding gene CgSNQ2 is contributing to the CgPDR1-dependent azole resistance of Candida glabrata. Mol. Microbiol. 2008, 68, 186–201. [Google Scholar] [CrossRef]
- Culakova, H.; Dzugasova, V.; Perzelova, J.; Gbelska, Y.; Subik, J. Mutation of the CgPDR16 gene attenuates azole tolerance and biofilm production in pathogenic Candida glabrata. Yeast 2013, 30, 403–414. [Google Scholar] [CrossRef]
- Costa, C.; Nunes, J.; Henriques, A.; Mira, N.P.; Nakayama, H.; Chibana, H.; Teixeira, M.C. Candida glabrata drug:H+ antiporter CgTpo3 (ORF CAGL0I10384g): Role in azole drug resistance and polyamine homeostasis. J. Antimicrob. Chemother. 2014, 69, 1767–1776. [Google Scholar] [CrossRef]
- Costa, C.; Henriques, A.; Pires, C.; Nunes, J.; Ohno, M.; Chibana, H.; Sa-Correia, I.; Teixeira, M.C. The dual role of candida glabrata drug:H+ antiporter CgAqr1 (ORF CAGL0J09944g) in antifungal drug and acetic acid resistance. Front. Microbiol. 2013, 4, 170. [Google Scholar] [CrossRef]
- Pais, P.; Costa, C.; Pires, C.; Shimizu, K.; Chibana, H.; Teixeira, M.C. Membrane Proteome-Wide Response to the Antifungal Drug Clotrimazole in Candida glabrata: Role of the Transcription Factor CgPdr1 and the Drug:H+ Antiporters CgTpo1_1 and CgTpo1_2. Mol. Cell. Proteom. Mcp 2016, 15, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Vermitsky, J.P.; Earhart, K.D.; Smith, W.L.; Homayouni, R.; Edlind, T.D.; Rogers, P.D. Pdr1 regulates multidrug resistance in Candida glabrata: Gene disruption and genome-wide expression studies. Mol. Microbiol. 2006, 61, 704–722. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Edlind, T.D. Identification and expression of multidrug resistance-related ABC transporter genes in Candida krusei. Med. Mycol. 2001, 39, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Ribeiro, J.; Miranda, I.M.; Silva-Dias, A.; Cavalheiro, M.; Costa-de-Oliveira, S.; Rodrigues, A.G.; Teixeira, M.C. Clotrimazole Drug Resistance in Candida glabrata Clinical Isolates Correlates with Increased Expression of the Drug:H(+) Antiporters CgAqr1, CgTpo1_1, CgTpo3, and CgQdr2. Front. Microbiol. 2016, 7, 526. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, D.; Chen, Z.; Cao, Y.; Gao, P.; Jiang, Y. The putative ABC transporter encoded by the orf19.4531 plays a role in the sensitivity of Candida albicans cells to azole antifungal drugs. FEMS Yeast Res. 2016, 16. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Pais, P.; Galocha, M.; Teixeira, M.C. Host-Pathogen Interactions Mediated by MDR Transporters in Fungi: As Pleiotropic as it Gets! Genes 2018, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Sa-Correia, I.; dos Santos, S.C.; Teixeira, M.C.; Cabrito, T.R.; Mira, N.P. Drug:H+ antiporters in chemical stress response in yeast. Trends Microbiol. 2009, 17, 22–31. [Google Scholar] [CrossRef]
- Rizzo, J.; Stanchev, L.D.; da Silva, V.K.A.; Nimrichter, L.; Pomorski, T.G.; Rodrigues, M.L. Role of lipid transporters in fungal physiology and pathogenicity. Comput. Struct. Biotechnol. J. 2019, 17, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.K.; Chauhan, N.; Sarkar, P.; Esquivel, B.D.; Coccetti, P.; Singh, A.; Coste, A.T.; Gupta, M.; Sanglard, D.; White, T.C.; et al. Azole resistance in a Candida albicans mutant lacking the ABC transporter CDR6/ROA1 depends on TOR signaling. J. Biol. Chem. 2018, 293, 412–432. [Google Scholar] [CrossRef] [PubMed]
- Vermitsky, J.P.; Edlind, T.D. Azole resistance in Candida glabrata: Coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob. Agents Chemother. 2004, 48, 3773–3781. [Google Scholar] [CrossRef]
- Coste, A.T.; Karababa, M.; Ischer, F.; Bille, J.; Sanglard, D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 2004, 3, 1639–1652. [Google Scholar] [CrossRef]
- Morschhauser, J.; Barker, K.S.; Liu, T.T.; Bla, B.W.J.; Homayouni, R.; Rogers, P.D. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 2007, 3, e164. [Google Scholar] [CrossRef]
- Alarco, A.M.; Raymond, M. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J. Bacteriol. 1999, 181, 700–708. [Google Scholar] [CrossRef]
- Noble, J.A.; Tsai, H.F.; Suffis, S.D.; Su, Q.; Myers, T.G.; Bennett, J.E. STB5 is a negative regulator of azole resistance in Candida glabrata. Antimicrob. Agents Chemother. 2013, 57, 959–967. [Google Scholar] [CrossRef]
- Whaley, S.G.; Caudle, K.E.; Vermitsky, J.P.; Chadwick, S.G.; Toner, G.; Barker, K.S.; Gygax, S.E.; Rogers, P.D. UPC2A is required for high-level azole antifungal resistance in Candida glabrata. Antimicrob. Agents Chemother. 2014, 58, 4543–4554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, M.; Watts, M.R.; Wang, H.; Fan, X.; Kong, F.; Xu, Y.C. Development of fluconazole resistance in a series of Candida parapsilosis isolates from a persistent candidemia patient with prolonged antifungal therapy. BMC Infect. Dis. 2015, 15, 340. [Google Scholar] [CrossRef]
- Pais, P.; Costa, C.; Cavalheiro, M.; Romao, D.; Teixeira, M.C. Transcriptional Control of Drug Resistance, Virulence and Immune System Evasion in Pathogenic Fungi: A Cross-Species Comparison. Front. Cell. Infect. Microbiol. 2016, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.T.; Pais, P.; Costa, C.; Manna, S.; Sa-Correia, I.; Teixeira, M.C. The PathoYeastract database: An information system for the analysis of gene and genomic transcription regulation in pathogenic yeasts. Nucleic Acids Res. 2017, 45, D597–D603. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Turner, V.; Ischer, F.; Morschhauser, J.; Forche, A.; Selmecki, A.; Berman, J.; Bille, J.; Sanglard, D. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 2006, 172, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yang, J.; Yang, L.; Li, Q.; Zhu, X.; Xi, Z.; Qiao, Z.; Cen, W. Research of Mrr1, Cap1 and MDR1 in Candida albicans resistant to azole medications. Exp. Ther. Med. 2018, 15, 1217–1224. [Google Scholar] [CrossRef]
- Morio, F.; Pagniez, F.; Besse, M.; Gay-andrieu, F.; Miegeville, M.; Le Pape, P. Deciphering azole resistance mechanisms with a focus on transcription factor-encoding genes TAC1, MRR1 and UPC2 in a set of fluconazole-resistant clinical isolates of Candida albicans. Int. J. Antimicrob. Agents 2013, 42, 410–415. [Google Scholar] [CrossRef]
- Ferrari, S.; Ischer, F.; Calabrese, D.; Posteraro, B.; Sanguinetti, M.; Fadda, G.; Rohde, B.; Bauser, C.; Bader, O.; Sanglard, D. Gain of function mutations in CgPDR1 of Candida glabrata not only mediate antifungal resistance but also enhance virulence. PLoS Pathog. 2009, 5, e1000268. [Google Scholar] [CrossRef]
- Salazar, S.B.; Wang, C.; Munsterkotter, M.; Okamoto, M.; Takahashi-Nakaguchi, A.; Chibana, H.; Lopes, M.M.; Guldener, U.; Butler, G.; Mira, N.P. Comparative genomic and transcriptomic analyses unveil novel features of azole resistance and adaptation to the human host in Candida glabrata. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef]
- Popp, C.; Hampe, I.A.I.; Hertlein, T.; Ohlsen, K.; Rogers, P.D.; Morschhauser, J. Competitive Fitness of Fluconazole-Resistant Clinical Candida albicans Strains. Antimicrob. Agents Chemother. 2017, 61, e00584-17. [Google Scholar] [CrossRef]
- Ferrari, S.; Sanguinetti, M.; Torelli, R.; Posteraro, B.; Sanglard, D. Contribution of CgPDR1-regulated genes in enhanced virulence of azole-resistant Candida glabrata. PLoS ONE 2011, 6, e17589. [Google Scholar] [CrossRef] [PubMed]
- Nolte, F.S.; Parkinson, T.; Falconer, D.J.; Dix, S.; Williams, J.; Gilmore, C.; Geller, R.; Wingard, J.R. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 1997, 41, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Facultative sterol uptake in an ergosterol-deficient clinical isolate of Candida glabrata harboring a missense mutation in ERG11 and exhibiting cross-resistance to azoles and amphotericin B. Antimicrob. Agents Chemother. 2012, 56, 4223–4232. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Tronchin, G.; Berges, T.; Hennequin, C.; Chabasse, D.; Bouchara, J.P. Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrob. Agents Chemother. 2007, 51, 982–990. [Google Scholar] [CrossRef]
- Woods, R.A.; Bard, M.; Jackson, I.E.; Drutz, D.J. Resistance to polyene antibiotics and correlated sterol changes in two isolates of Candida tropicalis from a patient with an amphotericin B-resistant funguria. J. Infect. Dis. 1974, 129, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Forastiero, A.; Mesa-Arango, A.C.; Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Bernal-Martinez, L.; Pelaez, T.; Lopez, J.F.; Grimalt, J.O.; Gomez-Lopez, A.; Cuesta, I.; et al. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob. Agents Chemother. 2013, 57, 4769–4781. [Google Scholar] [CrossRef] [PubMed]
- Lotfali, E.; Kordbacheh, P.; Mirhendi, H.; Zaini, F.; Ghajari, A.; Mohammadi, R.; Noorbakhsh, F.; Moazeni, M.; Fallahi, A.; Rezaie, S. Antifungal Susceptibility Analysis of Clinical Isolates of Candida parapsilosis in Iran. Iran J. Public Health 2016, 45, 322–328. [Google Scholar]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Nagy, E.; Dobiasova, S.; Rinaldi, M.; Barton, R.; Veselov, A.; Global Antifungal Surveillance Group. Candida krusei, a multidrug-resistant opportunistic fungal pathogen: Geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J. Clin. Microbiol. 2008, 46, 515–521. [Google Scholar] [CrossRef]
- Park, S.; Kelly, R.; Kahn, J.N.; Robles, J.; Hsu, M.J.; Register, E.; Li, W.; Vyas, V.; Fan, H.; Abruzzo, G.; et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 2005, 49, 3264–3273. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Lee, S.; Park, S.; Cleary, J.D.; Perlin, D.S. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-D-glucan synthase: Implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 2009, 53, 3690–3699. [Google Scholar] [CrossRef]
- Dannaoui, E.; Desnos-Ollivier, M.; Garcia-Hermoso, D.; Grenouillet, F.; Cassaing, S.; Baixench, M.T.; Bretagne, S.; Dromer, F.; Lortholary, O.; French Mycoses Study, G. Candida spp. with acquired echinocandin resistance, France, 2004–2010. Emerg. Infect. Dis. 2012, 1890, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Pfaller, M.; Edlind, T. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 2006, 50, 2892–2894. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Effron, G.; Chua, D.J.; Tomada, J.R.; DiPersio, J.; Perlin, D.S.; Ghannoum, M.; Bonilla, H. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob. Agents Chemother. 2010, 54, 2225–2227. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Effron, G.; Katiyar, S.K.; Park, S.; Edlind, T.D.; Perlin, D.S. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 2008, 52, 2305–2312. [Google Scholar] [CrossRef]
- Desnos-Ollivier, M.; Bretagne, S.; Bernede, C.; Robert, V.; Raoux, D.; Chachaty, E.; Forget, E.; Lacroix, C.; Dromer, F.; Yeasts, G. Clonal population of flucytosine-resistant Candida tropicalis from blood cultures, Paris, France. Emerg. Infect. Dis. 2008, 14, 557–565. [Google Scholar] [CrossRef]
- Hoeprich, P.D.; Ingraham, J.L.; Kleker, E.; Winship, M.J. Development of resistance to 5-fluorocytosine in Candida parapsilosis during therapy. J. Infect. Dis. 1974, 130, 112–118. [Google Scholar] [CrossRef]
- Chapeland-Leclerc, F.; Hennequin, C.; Papon, N.; Noel, T.; Girard, A.; Socie, G.; Ribaud, P.; Lacroix, C. Acquisition of flucytosine, azole, and caspofungin resistance in Candida glabrata bloodstream isolates serially obtained from a hematopoietic stem cell transplant recipient. Antimicrob. Agents Chemother. 2010, 54, 1360–1362. [Google Scholar] [CrossRef]
- Dodgson, A.R.; Dodgson, K.J.; Pujol, C.; Pfaller, M.A.; Soll, D.R. Clade-specific flucytosine resistance is due to a single nucleotide change in the FUR1 gene of Candida albicans. Antimicrob. Agents Chemother. 2004, 48, 2223–2227. [Google Scholar] [CrossRef]
- Hope, W.W.; Tabernero, L.; Denning, D.W.; Anderson, M.J. Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrob. Agents Chemother. 2004, 48, 4377–4386. [Google Scholar] [CrossRef]
- Costa, C.; Ponte, A.; Pais, P.; Santos, R.; Cavalheiro, M.; Yaguchi, T.; Chibana, H.; Teixeira, M.C. New Mechanisms of Flucytosine Resistance in C. glabrata Unveiled by a Chemogenomics Analysis in S. cerevisiae. PLoS ONE 2015, 10, e0135110. [Google Scholar] [CrossRef]
- Xiang, M.J.; Liu, J.Y.; Ni, P.H.; Wang, S.; Shi, C.; Wei, B.; Ni, Y.X.; Ge, H.L. Erg11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res. 2013, 13, 386–393. [Google Scholar] [CrossRef]
- Flowers, S.A.; Colon, B.; Whaley, S.G.; Schuler, M.A.; Rogers, P.D. Contribution of clinically derived mutations in ERG11 to azole resistance in Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 450–460. [Google Scholar] [CrossRef]
- Feng, L.J.; Wan, Z.; Wang, X.H.; Li, R.Y.; Liu, W. Relationship between antifungal resistance of fluconazole resistant Candida albicans and mutations in ERG11 gene. Chin. Med. J. 2010, 123, 544–548. [Google Scholar]
- Zhang, L.; Yang, H.F.; Liu, Y.Y.; Xu, X.H.; Ye, Y.; Li, J.B. Reduced susceptibility of Candida albicans clinical isolates to azoles and detection of mutations in the ERG11 gene. Diagn. Microbiol. Infect. Dis. 2013, 77, 327–329. [Google Scholar] [CrossRef]
- Vale-Silva, L.A.; Coste, A.T.; Ischer, F.; Parker, J.E.; Kelly, S.L.; Pinto, E.; Sanglard, D. Azole resistance by loss of function of the sterol Delta(5),(6)-desaturase gene (ERG3) in Candida albicans does not necessarily decrease virulence. Antimicrob. Agents Chemother. 2012, 56, 1960–1968. [Google Scholar] [CrossRef]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.; Rolley, N.; Kelly, D.E.; Kelly, S.L. Identification and characterization of four azole-resistant erg3 mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 4527–4533. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. A clinical isolate of Candida albicans with mutations in ERG11 (encoding sterol 14alpha-demethylase) and ERG5 (encoding C22 desaturase) is cross resistant to azoles and amphotericin B. Antimicrob. Agents Chemother. 2010, 54, 3578–3583. [Google Scholar] [CrossRef] [PubMed]
- Kalkandelen, K.T.; Doluca Dereli, M. Investigation of mutations in transcription factors of efflux pump genes in fluconazole-resistant Candida albicans strains overexpressing the efflux pumps. Mikrobiyol. Bul. 2015, 49, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Lohberger, A.; Coste, A.T.; Sanglard, D. Distinct roles of Candida albicans drug resistance transcription factors TAC1, MRR1, and UPC2 in virulence. Eukaryot. Cell 2014, 13, 127–142. [Google Scholar] [CrossRef]
- Dunkel, N.; Blass, J.; Rogers, P.D.; Morschhauser, J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 2008, 69, 827–840. [Google Scholar] [CrossRef]
- Flowers, S.A.; Barker, K.S.; Berkow, E.L.; Toner, G.; Chadwick, S.G.; Gygax, S.E.; Morschhauser, J.; Rogers, P.D. Gain-of-function mutations in UPC2 are a frequent cause of ERG11 upregulation in azole-resistant clinical isolates of Candida albicans. Eukaryot. Cell 2012, 11, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Hoot, S.J.; Smith, A.R.; Brown, R.P.; White, T.C. An A643V amino acid substitution in Upc2p contributes to azole resistance in well-characterized clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 2011, 55, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Caudle, K.E.; Simonicova, L.; Zhang, Q.; Moye-Rowley, W.S.; Rogers, P.D. Jjj1 Is a Negative Regulator of Pdr1-Mediated Fluconazole Resistance in Candida glabrata. mSphere 2018, 3, e00466-17. [Google Scholar] [CrossRef] [PubMed]
- Marichal, P.; Vanden Bossche, H.; Odds, F.C.; Nobels, G.; Warnock, D.W.; Timmerman, V.; Van Broeckhoven, C.; Fay, S.; Mose-Larsen, P. Molecular biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob. Agents Chemother. 1997, 41, 2229–2237. [Google Scholar] [CrossRef]
- Abbes, S.; Mary, C.; Sellami, H.; Michel-Nguyen, A.; Ayadi, A.; Ranque, S. Interactions between copy number and expression level of genes involved in fluconazole resistance in Candida glabrata. Front. Cell. Infect. Microbiol. 2013, 3, 74. [Google Scholar] [CrossRef]
- Lamping, E.; Ranchod, A.; Nakamura, K.; Tyndall, J.D.; Niimi, K.; Holmes, A.R.; Niimi, M.; Cannon, R.D. Abc1p is a multidrug efflux transporter that tips the balance in favor of innate azole resistance in Candida krusei. Antimicrob. Agents Chemother. 2009, 53, 354–369. [Google Scholar] [CrossRef]
- Grossman, N.T.; Pham, C.D.; Cleveland, A.A.; Lockhart, S.R. Molecular mechanisms of fluconazole resistance in Candida parapsilosis isolates from a U.S. surveillance system. Antimicrob. Agents Chemother. 2015, 59, 1030–1037. [Google Scholar] [CrossRef]
- Feng, W.; Yang, J.; Wang, Y.; Chen, J.; Xi, Z.; Qiao, Z. ERG11 mutations and upregulation in clinical itraconazole-resistant isolates of Candida krusei. Can. J. Microbiol. 2016, 62, 938–943. [Google Scholar] [CrossRef]
- Healey, K.R.; Zhao, Y.; Perez, W.B.; Lockhart, S.R.; Sobel, J.D.; Farmakiotis, D.; Kontoyiannis, D.P.; Sanglard, D.; Taj-Aldeen, S.J.; Alexander, B.D.; et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat. Commun. 2016, 7, 11128. [Google Scholar] [CrossRef]
- Delliere, S.; Healey, K.; Gits-Muselli, M.; Carrara, B.; Barbaro, A.; Guigue, N.; Lecefel, C.; Touratier, S.; Desnos-Ollivier, M.; Perlin, D.S.; et al. Fluconazole and Echinocandin Resistance of Candida glabrata Correlates Better with Antifungal Drug Exposure Rather than with MSH2 Mutator Genotype in a French Cohort of Patients Harboring Low Rates of Resistance. Front. Microbiol. 2016, 7, 2038. [Google Scholar] [CrossRef]
- Byun, S.A.; Won, E.J.; Kim, M.N.; Lee, W.G.; Lee, K.; Lee, H.S.; Uh, Y.; Healey, K.R.; Perlin, D.S.; Choi, M.J.; et al. Multilocus Sequence Typing (MLST) Genotypes of Candida glabrata Bloodstream Isolates in Korea: Association With Antifungal Resistance, Mutations in Mismatch Repair Gene (Msh2), and Clinical Outcomes. Front. Microbiol. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Hou, X.; Xiao, M.; Wang, H.; Yu, S.Y.; Zhang, G.; Zhao, Y.; Xu, Y.C. Profiling of PDR1 and MSH2 in Candida glabrata Bloodstream Isolates from a Multicenter Study in China. Antimicrob. Agents Chemother. 2018, 62, e00153-18. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Chan, C.L.; Jauert, P.A.; Kirkpatrick, D.T. Role of DNA mismatch repair and double-strand break repair in genome stability and antifungal drug resistance in Candida albicans. Eukaryot. Cell 2007, 6, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Polakova, S.; Blume, C.; Zarate, J.A.; Mentel, M.; Jorck-Ramberg, D.; Stenderup, J.; Piskur, J. Formation of new chromosomes as a virulence mechanism in yeast Candida glabrata. Proc. Natl. Acad. Sci. USA 2009, 106, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Selmecki, A.; Forche, A.; Diogo, D.; Bougnoux, M.E.; d’Enfert, C.; Berman, J.; Sanglard, D. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot. Cell 2007, 6, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Selmecki, A.; Forche, A.; Berman, J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 2006, 313, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Weil, T.; Santamaria, R.; Lee, W.; Rung, J.; Tocci, N.; Abbey, D.; Bezerra, A.R.; Carreto, L.; Moura, G.R.; Bayes, M.; et al. Adaptive Mistranslation Accelerates the Evolution of Fluconazole Resistance and Induces Major Genomic and Gene Expression Alterations in Candida albicans. mSphere 2017, 2, e00167-17. [Google Scholar] [CrossRef]
- Bezerra, A.R.; Simoes, J.; Lee, W.; Rung, J.; Weil, T.; Gut, I.G.; Gut, M.; Bayes, M.; Rizzetto, L.; Cavalieri, D.; et al. Reversion of a fungal genetic code alteration links proteome instability with genomic and phenotypic diversification. Proc. Natl. Acad. Sci. USA 2013, 110, 11079–11084. [Google Scholar] [CrossRef]
- Chen, Y.N.; Lo, H.J.; Wu, C.C.; Ko, H.C.; Chang, T.P.; Yang, Y.L. Loss of heterozygosity of FCY2 leading to the development of flucytosine resistance in Candida tropicalis. Antimicrob. Agents Chemother. 2011, 55, 2506–2514. [Google Scholar] [CrossRef]
- Thamburan, S.; Klaasen, J.; Mabusela, W.T.; Cannon, J.F.; Folk, W.; Johnson, Q. Tulbaghia alliacea phytotherapy: A potential anti-infective remedy for candidiasis. Phytother. Res. 2006, 20, 844–850. [Google Scholar] [CrossRef]
- Ogbolu, D.O.; Oni, A.A.; Daini, O.A.; Oloko, A.P. In vitro antimicrobial properties of coconut oil on Candida species in Ibadan, Nigeria. J. Med. Food 2007, 10, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Pianalto, K.M.; Alspaugh, J.A. New Horizons in Antifungal Therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Emami, S.; Vaezi, A.; Hashemi, S.M.; Faeli, L.; Diba, K.; Dannaoui, E.; Badali, H. In Vitro Activities of Novel Azole Compounds ATTAF-1 and ATTAF-2 against Fluconazole-Susceptible and -Resistant Isolates of Candida Species. Antimicrob. Agents Chemother. 2017, 61, e01106-16. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.K.; Garzan, A.; Garneau-Tsodikova, S. Novel alkylated azoles as potent antifungals. Eur. J. Med. Chem. 2017, 133, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.M.S.; Guerreiro, S.I.; Lourenco, A.; Alves, M.M.; Montemor, M.F.; Mira, N.P.; Leitao, J.H.; Carvalho, M. Ag(I) camphorimine complexes with antimicrobial activity towards clinically important bacteria and species of the Candida genus. PLoS ONE 2017, 12, e0177355. [Google Scholar] [CrossRef] [PubMed]
- Dileepan, A.G.B.; Kumar, A.G.; Mathumidha, R.; Rajaram, R.; Rajam, S. Dinuclear rectangular-shaped assemblies of bis-benzimidazolydine salt coordinated to Ag(I) and Cu(I) N-heterocyclic carbene complexes and their biological applications. Chem. Pap. 2018, 72, 3017–3031. [Google Scholar] [CrossRef]
- Yasmeen, S.; Sumrra, S.H.; Akram, M.S.; Chohan, Z.H. Antimicrobial metal-based thiophene derived compounds. J. Enzym. Inhib. Med. Chem. 2017, 32, 106–112. [Google Scholar] [CrossRef]
- Andrejević, T.P.; Nikolić, A.M.; Glišić, B.D.; Wadepohl, H.; Vojnovic, S.; Zlatović, M.; Petković, M.; Nikodinovic-Runic, J.; Opsenica, I.M.; Djuran, M.I. Synthesis, structural characterization and antimicrobial activity of silver(I) complexes with 1-benzyl-1H-tetrazoles. Polyhedron 2018, 154, 325–333. [Google Scholar] [CrossRef]
- Menezes, D.C.; Vieira, F.T.; de Lima, G.M.; Wardell, J.L.; Cortés, M.E.; Ferreira, M.P.; Soares, M.A.; Boas, A.V. The in vitro antifungal activity of some dithiocarbamate organotin(IV) compounds on Candida albicans—A model for biological interaction of organotin complexes. Appl. Organomet. Chem 2008, 22, 221–226. [Google Scholar] [CrossRef]
- El-Sonbati, A.Z.; El-Bindary, A.A.; Mohamed, G.G.; Morgan, S.M.; Hassan, W.M.I.; Elkholy, A.K. Geometrical structures, thermal properties and antimicrobial activity studies of azodye complexes. J. Mol. Liq. 2016, 218, 16–34. [Google Scholar] [CrossRef]
- El-Sonbati, A.Z.; Diab, M.A.; El-Bindary, A.A.; Abou-Dobara, M.I.; Seyam, H.A. Molecular docking, DNA binding, thermal studies and antimicrobial activities of Schiff base complexes. J. Mol. Liq. 2016, 218, 434–456. [Google Scholar] [CrossRef]
- El-Ghamry, H.A.; Fathalla, S.K.; Gaber, M. Synthesis, structural characterization and molecular modelling of bidentate azo dye metal complexes: DNA interaction to antimicrobial and anticancer activities. Appl. Organomet. Chem 2017, 32. [Google Scholar] [CrossRef]
- Fathima, S.S.A.; Paulpandiyan, R.; Nagarajan, E.R. Expatiating biological excellence of aminoantipyrine derived novel metal complexes: Combined DNA interaction, antimicrobial, free radical scavenging studies and molecular docking simulations. J. Mol. Struct. 2019, 1178, 179–191. [Google Scholar] [CrossRef]
- Arun, T.; Subramanian, R.; Raman, N. Novel bio-essential metal based complexes linked by heterocyclic ligand: Synthesis, structural elucidation, biological investigation and docking analysis. J. Photochem. Photobiol. Biol. 2016, 154, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Philip, J.E.; Antony, S.A.; Eeettinilkunnathil, S.J.; Kurup, M.R.P.; Velayudhan, M.P. Design, synthesis, antimicrobial and antioxidant activity of 3-formyl chromone hydrazone and their metal (II) complexes. Inorg. Chim. Acta 2018, 469, 87–97. [Google Scholar] [CrossRef]
- Singh, U.; Bukhari, M.; Anayutullah, S.; Alam, H.; Manzoor, N.; Hashmi, A. Synthesis, Characterization and Biological Evaluation of Metal Complexes with Water-Soluble Macromolecular Dendritic Ligand. Pharm. Chem. J. 2016, 49, 868–877. [Google Scholar] [CrossRef]
- Gull, P.; Malik, M.A.; Dar, O.A.; Hashmi, A.A. Design, synthesis and spectroscopic characterization of metal (II) complexes derived from a tetradentate macrocyclic ligand: Study on antimicrobial and antioxidant capacity of complexes. Microb. Pathog. 2017, 104, 212–216. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sheng, J.; Yin, D.W.; Xin, H.; Yang, X.M.; Qiao, Q.Y.; Yang, Z.J. Ferrocenyl chalcone-based Schiff bases and their metal complexes: Highly efficient, solvent-free synthesis, characterization, biological research. J. Organometal. Chem. 2018, 856, 27–33. [Google Scholar] [CrossRef]
- Maia, P.J.S.; de Aguiar, I.; Velloso, M.S.; Zhang, D.; Santos, E.R.; Oliveira, J.R.; Junqueira, J.C.; Selke, M.; Carlos, R.M. Singlet oxygen production by a polypyridine ruthenium (II) complex with a perylene monoimide derivative: A strategy for photodynamic inactivation of Candida albicans. J. Photochem. Photobiol. A Chem. 2018, 353, 536–545. [Google Scholar] [CrossRef]
- Panacek, A.; Kvitek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizurova, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Silva, S.; Negri, M.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver colloidal nanoparticles: Antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef]
- Panacek, A.; Kolar, M.; Vecerova, R.; Prucek, R.; Soukupova, J.; Krystof, V.; Hamal, P.; Zboril, R.; Kvitek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Sung, W.S.; Suh, B.K.; Moon, S.K.; Choi, J.S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Matsubara, V.H.; Bandara, H.M.; Mayer, M.P.; Samaranayake, L.P. Probiotics as Antifungals in Mucosal Candidiasis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, 1143–1153. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Zhou, X.; Pierson, J.D.; Ravel, J.; Forney, L.J. Understanding vaginal microbiome complexity from an ecological perspective. Transl. Res. 2012, 160, 267–282. [Google Scholar] [CrossRef]
- Martin, D.H. The microbiota of the vagina and its influence on women’s health and disease. Am. J. Med. Sci. 2012, 343, 2–9. [Google Scholar] [CrossRef]
- Hu, H.; Merenstein, D.J.; Wang, C.; Hamilton, P.R.; Blackmon, M.L.; Chen, H.; Calderone, R.A.; Li, D. Impact of eating probiotic yogurt on colonization by Candida species of the oral and vaginal mucosa in HIV-infected and HIV-uninfected women. Mycopathologia 2013, 176, 175–181. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Parolin, C.; Nahui Palomino, R.A.; Vitali, B.; Lanciotti, R. Determination of Antibacterial and Technological Properties of Vaginal Lactobacilli for Their Potential Application in Dairy Products. Front. Microbiol. 2017, 8, 166. [Google Scholar] [CrossRef]
- Ehrstrom, S.; Daroczy, K.; Rylander, E.; Samuelsson, C.; Johannesson, U.; Anzen, B.; Pahlson, C. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microb. Infect. 2010, 12, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Nahui Palomino, R.A.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of Vaginal Lactobacilli and Characterization of Anti-Candida Activity. PLoS ONE 2015, 10, e0131220. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Pedro, N.; Salazar, S.B.; Mira, N.P. Effect of acetic acid and lactic acid at low pH in growth and azole resistance of Candida albicans and Candida glabrata. Front. Microbiol. 2019, 9, 3265. [Google Scholar]
- Kasper, L.; Miramon, P.; Jablonowski, N.; Wisgott, S.; Wilson, D.; Brunke, S.; Hube, B. Antifungal activity of clotrimazole against Candida albicans depends on carbon sources, growth phase and morphology. J. Med. Microbiol. 2015, 64, 714–723. [Google Scholar] [CrossRef]
- Hofs, S.; Mogavero, S.; Hube, B. Interaction of Candida albicans with host cells: Virulence factors, host defense, escape strategies, and the microbiota. J. Microbiol. 2016, 54, 149–169. [Google Scholar] [CrossRef]
- Thornton, L.; Dixit, V.; Assad, L.O.; Ribeiro, T.P.; Queiroz, D.D.; Kellett, A.; Casey, A.; Colleran, J.; Pereira, M.D.; Rochford, G.; et al. Water-soluble and photo-stable silver(I) dicarboxylate complexes containing 1,10-phenanthroline ligands: Antimicrobial and anticancer chemotherapeutic potential, DNA interactions and antioxidant activity. J. Inorg. Biochem. 2016, 159, 120–132. [Google Scholar] [CrossRef]
- Giulidori, C.; Mosconi, N.; Toplikar, T.; Vega, M.; Williams, P.; Svetaz, L.; Raimondi, M.; Rizzotto, M. Heteroleptic complexes of antifungal drugs with the silver ion. J. Phys. Org. Chem. 2016, 29, 656–664. [Google Scholar] [CrossRef]
- Savic, N.D.; Vojnovic, S.; Glisic, B.D.; Crochet, A.; Pavic, A.; Janjic, G.V.; Pekmezovic, M.; Opsenica, I.M.; Fromm, K.M.; Nikodinovic-Runic, J.; et al. Mononuclear silver(I) complexes with 1,7-phenanthroline as potent inhibitors of Candida growth. Eur. J. Med. Chem. 2018, 156, 760–773. [Google Scholar] [CrossRef]
- Lv, J.; Liu, T.; Cai, S.; Wang, X.; Liu, L.; Wang, Y. Synthesis, structure and biological activity of cobalt(II) and copper(II) complexes of valine-derived schiff bases. J. Inorg. Biochem. 2006, 100, 1888–1896. [Google Scholar] [CrossRef]
- Turecka, K.; Chylewska, A.; Kawiak, A.; Waleron, K.F. Antifungal Activity and Mechanism of Action of the Co(III) Coordination Complexes With Diamine Chelate Ligands Against Reference and Clinical Strains of Candida spp. Front. Microbiol. 2018, 9, 1594. [Google Scholar] [CrossRef]
- Saad, F.A. Nano-synthesis and spectral, thermal, modeling, quantitative structure–activity relationship and docking studies of novel bioactive homo-binuclear metal complexes derived from thiazole drug for therapeutic applications. Appl. Organomet. Chem. 2018, 32, e4352. [Google Scholar] [CrossRef]
- Munoz, J.E.; Rossi, D.C.P.; Ishida, K.; Spadari, C.C.; Melhem, M.S.C.; Garcia, D.M.; Caires, A.C.F.; Taborda, C.P.; Rodrigues, E.G. Antifungal Activity of the Biphosphinic Cyclopalladate C7a against Candida albicans Yeast Forms In Vitro and In Vivo. Front. Microbiol. 2017, 8, 771. [Google Scholar] [CrossRef] [PubMed]
| Species | Imidazoles | Triazoles | Flucytosine | Ampho. B | Echinocandins |
|---|---|---|---|---|---|
| C. albicans | S to R | S | S | S | S |
| C. tropicalis | S | S | S | S | S |
| C. parapsilosis | S | S | S | S | S to I |
| C. glabrata | S-DD to R | S-DD to R | S | S to I | S |
| C. krusei | S to R | S-DD to R | I to R | S to I | S |
| Species | Imidazoles | Triazoles | Echinocandins | Flucytosine | Ampho. B |
|---|---|---|---|---|---|
| C. albicans | 0–54 | 0–16.6 | 0 | 0.3–4.3 | 0 |
| C. glabrata | 0–50.5 | 6.9–15.7 | 1.1–1.5 | 0–0.6 | 0–1.6 |
| C. tropicalis | 4–14 | 4.1–6.1 | 0 | 1–12.5 | 0–1 |
| C. parapsilosis | 0–2 | 1.8–14 | 0 | 0–1.4 | 0 |
| C. krusei | 0–73.1 ** | 2.8–100 ** | 0–2.8 | 1–16 | 0–12 |
| C. albicans | C. glabrata | C. krusei | C. parapsilosis | C. tropicalis | |
|---|---|---|---|---|---|
| Modification of drug target (protein or pathway) | SNPs reducing the inactivation of CaERG11 by azoles Overexpression of CaERG11 | Not found | SNPs identified in CkERG11 in resistant isolates Mild overexpression of CkERG11 | Overexpression of CpERG11 | SNPs reducing the inactivation of CtERG11 by azoles Overexpression of CtERG11 |
| SNPs inactivating CaERG3 or CaERG5/CaERG11 to bypass the inactivation of ergosterol biosynthesis by azoles | SNPs inactivating CaERG3 or CaERG5/CaERG11 to bypass the inactivation of ergosterol biosynthesis by azoles Decreased expression of an acylCoA:sterol acyltransferase resulting in low sterol esterification | - | SNPs that inactivate CpERG11 or CpERG2 to bypass the inactivation of ergosterol biosynthesis by azoles | - | |
| Increased activity of drug-efflux pumps | Overexpression of CaCDR1, CaCDR2, CaMDR1, CaPDR16 Increased activity of CaTac1, CaMrr1, CaCap1, CaUpc2 | Overexpression of CgAQR1, CgCDR1, CgFLR2, CgPDH1, CgQDR2, CgSNQ2, CgTPO1_1, CgTPO1_2, CgTPO3 Increased activity of CaTac1, CaMrr1, CaCap1, CaUpc2 | Overexpression of CkABC1 and CkABC2 | Overexpression of CpMDR1 and CpCDR1 Increased activity of CpUpc2 | Overexpression of CtCDR1 and CtMDR1 |
| C. albicans | C. glabrata | C. krusei | C. parapsilosis | C. tropicalis | ||
|---|---|---|---|---|---|---|
| Modification of drug target/pathway | Echinocandins | SNPs reducing the inactivation of CaGSC1 by echinocandins | SNPs reducing the inactivation of CgFKS1 and/or CgFKS2 by echinocandins | SNPs reducing the inactivation of CgFKS1 by echinocandins | SNPs reducing the inactivation of CgFKS1 by echinocandins | SNPs reducing the inactivation of CgFKS1 by echinocandins |
| Polyenes | SNPs inactivating ERG3 resulting in reduced ergosterol in the membrane | SNPs inactivating ERG2, ERG6 or ERG11 hypothesized to result in reduced ergosterol in the membrane | - | - | SNPs inactivating CtERG11 hypothesized to result in reduced ergosterol in the membrane | |
| 5-Flucytosine | SNPs reducing the inactivation of CaFUR1 or CaFCA1 by 5-FC Potential inactivation of CaFCY21 or CaFCY22 | SNPs reducing the inactivation of CgFUR1 by 5-FC | - | SNPs reducing the inactivation of CpFUR1 by 5-FC | Possible hyper activation of CtUra3 to increase formation of UMP |
| Metallic Center | Ligand | MIC/Diameter of Inhibition of the Complex (or Ligand) against C. albicans* | Ref |
|---|---|---|---|
| Ag | Dicarboxylic acid Phenanthroline | 1–490 mM (ligand has antifungal activity at >1000 mM) 0.9–1.7 mM (ligand has antifungal activity at 149.4 mM) | [176] |
| Phenanthroline | 7.8 µg/mL (ligand has antifungal activity at 31.25 µg/mL) | [177] | |
| Tetrazole nitrogen | 0.62–1.25 µg/mL (information regarding the activity of the ligand was not provided) | [148] | |
| Phenanthroline | 12–113 mM (ligand has antifungal activity at 5000 mM) | [178] | |
| Benzimidazolydine | 18 mm (ligand has no antifungal activity) | [146] | |
| Cu | Schiff base | 4 µg/mL (information regarding the activity of the ligand alone is not provided) | [179] |
| Benzimidazolydine | 12 mm (ligand has no antifungal activity) | [146] | |
| Azo dye | 11 mm diameter (10 mm attributable to the ligand) | [152] | |
| Schiff base type | 115 mM (ligand has antifungal activity at 245 mM) | [153] | |
| Schiff base + 2,2′-bipyridine ancillary | 57 mM (ligand has antifungal activity at 188 mM) | [154] | |
| Chromone hydrazines | 24.8 and 30.7 mm diameter (20.8 and 21.2 mm attributable to ligands) | [155] | |
| Dendrimer | 1 mg/mL (ligand has antifungal activity at 12.9 mg/mL) | [156] | |
| Ferrocenyl chalcone derivatives | 17 and 21 mm diameter (12 and 19 mm attributable to ligand) | [158] | |
| Tetradentate macrocyclic | 22 mm diameter (16 mm attributable to ligand) | [157] | |
| Co | Schiff base type ligand | 32 µg/mL (information regarding the activity of the ligand alone is not provided) | [179] |
| azo dye ligand | 11 mm diameter (10 mm attributable to ligand) | [152] | |
| Schiff base type ligand | 57–75% inhibition (40–60% attributable to ligand) | [147] | |
| Schiff base type ligand | 125 mM (ligand has antifungal activity with 245 mM) | [153] | |
| Schiff base type ligand | 82 mM (ligand has antifungal activity at 188 mM) | [154] | |
| Dendrimer ligand | 0.6 mg/mL (ligand has antifungal activity at 12.9 mg/mL) | [156] | |
| Tetradentate macrocyclic ligand | 22 mm diameter (15 mm attributable to ligand) | [157] | |
| Ethylenediamine derivatives | 62.5 µg/mL (information regarding the activity of the ligand alone is not provided) | [180] | |
| Ni | Bidentate azodye ligand | 15.7 mm diameter (ligand has no antifungal activity) | [150] |
| Schiff base type ligand | 129 mM (ligand has antifungal activity with 245 mM) | [153] | |
| Schiff base type ligand + 2,2′-bipyridine ancillary ligand | 87 mM (ligand has antifungal activity at 188 mM) | [154] | |
| Chromone hydrazone | 22.5 and 25.6mm diameter (20.8 and 21.2 mm attributable to ligand) | [155] | |
| Dendrimer ligand | 0.6 mg/mL (ligand has antifungal activity at 12.9 mg/mL) | [156] | |
| Tetradentate macrocyclic ligand | 19 mm diameter (15 mm attributable to ligand) | [157] | |
| Cd | Bidentate azodye ligand | 17.1 mm diameter (ligand has no antifungal activity) | [150] |
| Ferrocenyl chalcone derivatives | 20 mm diameter (12 mm attributable to ligand) | [158] | |
| Sn | Dithiocarbamate derivatives | 2.5–250 µg/mL (information regarding the activity of the ligand alone is not provided) | [149] |
| Schiff base type ligand | 135 mM (ligand has antifungal activity with 245 mM) | [153] | |
| Schiff base type ligand + 2,2′-bipyridine ancillary ligand | 102 mM (ligand has antifungal activity at 188 mM) | [154] | |
| Chromone hydrazine ligand | 24.8 and 26.3 mm diameter (20.8 and 21.2 mm attributable to ligand) | [155] | |
| Fe | Thiazole derivatives ligand | 18.9 mm diameter (11.9 mm attributable to ligand) | [181] |
| Ferrocenyl chalcone derivatives | 17 mm diameter of inhibition zone (12 mm attributable to ligand) | [158] | |
| Bidentate azodye ligand | 19.6 mm diameter (ligand has no antifungal activity) | [150] | |
| Ferrocenyl chalcone derivatives | 15 mm diameter (12 mm attributable to ligand) | [158] | |
| Ru | Perylene ligand | 125 mM (information regarding the activity of the ligand alone is not provided) | [159] |
| Pb | Ferrocenyl chalcone derivatives | 17 and 21 mm diameter (12 and 19 mm attributable to ligand) | [158] |
| Ba | Ferrocenyl chalcone derivatives | 13 mm diameter (12 mm attributable to ligand) | [158] |
| Pd | Phenylphosphine ligand | 0.5 µg/mL (information regarding the activity of the ligand alone is not provided) | [182] |
| Probiotic | Candida spp. |
|---|---|
| Lactobacillus rhamnosus GG (ATCC 53103), L. rhamnosus LC705, Propionibacterium freudenreichii subsp. shermanii JS | C. albicans, C. glabrata, C. krusei and C. tropicalis |
| Lactobacillus casei and Bifidobacterium breve | C. albicans, C. tropicalis, C. guillermondii, C. glabrata, C. krusei, C. kefyr and C. parapsilosis |
| L. rhamnosus HS111, L. acidophillus HS101, and Bifidobacterium bifidum | C. albicans, C. guillermondii, C. tropicalis, C. glabrata, C. dubliniensis, C. famata and C. parapsilosis |
| L. acidophilus, L. rhamnosus, L. delbrueckii subsp. bulgaricus and S. thermophiles | Candida spp. |
| L. rhamnosus GR-1 and L. reuteri RC-14 | C. albicans and non-C. albicans |
| Lactobacillus fermentum LF10 and L. acidophilus LA02 | C. albicans, C. glabrata, C. parapsilosis and C. krusei |
| Bifidobacterium and Lactobacillus (DanActive or yoPlus yogurt) | C. albicans and non-C. albicans |
| L. casei subsp. rhamnosus | C. albicans and non-C. albicans |
| L. reuterii ATCC 55730 and L. rhamnosus (ATCC 53103) | Candida spp. |
| L. acidophillus, L. rhamnosus, B. longum, B. bifidum, S. boulardii, and Saccharomyces thermophiles | Candida spp. |
| L. acidophilus, Bifidobacterium lactis, B. longum, and B. bifidum | C. albicans and C. glabrata |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, S.B.; Simões, R.S.; Pedro, N.A.; Pinheiro, M.J.; Carvalho, M.F.N.N.; Mira, N.P. An Overview on Conventional and Non-Conventional Therapeutic Approaches for the Treatment of Candidiasis and Underlying Resistance Mechanisms in Clinical Strains. J. Fungi 2020, 6, 23. https://doi.org/10.3390/jof6010023
Salazar SB, Simões RS, Pedro NA, Pinheiro MJ, Carvalho MFNN, Mira NP. An Overview on Conventional and Non-Conventional Therapeutic Approaches for the Treatment of Candidiasis and Underlying Resistance Mechanisms in Clinical Strains. Journal of Fungi. 2020; 6(1):23. https://doi.org/10.3390/jof6010023
Chicago/Turabian StyleSalazar, Sara B., Rita S. Simões, Nuno A. Pedro, Maria Joana Pinheiro, Maria Fernanda N. N. Carvalho, and Nuno P. Mira. 2020. "An Overview on Conventional and Non-Conventional Therapeutic Approaches for the Treatment of Candidiasis and Underlying Resistance Mechanisms in Clinical Strains" Journal of Fungi 6, no. 1: 23. https://doi.org/10.3390/jof6010023
APA StyleSalazar, S. B., Simões, R. S., Pedro, N. A., Pinheiro, M. J., Carvalho, M. F. N. N., & Mira, N. P. (2020). An Overview on Conventional and Non-Conventional Therapeutic Approaches for the Treatment of Candidiasis and Underlying Resistance Mechanisms in Clinical Strains. Journal of Fungi, 6(1), 23. https://doi.org/10.3390/jof6010023





