Diagnosis of Progressive Disseminated Histoplasmosis in Advanced HIV: A Meta-Analysis of Assay Analytical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection Criteria
2.3. Statistical Analysis and Data Synthesis
2.4. Calculation of Assays Analytical Performance
2.5. Meta-Analysis Forest Plot
2.6. Plot of Fitted Model
2.7. Hierarchical Summary Receiver Operating Characteristic (HSROC) Curves
3. Results
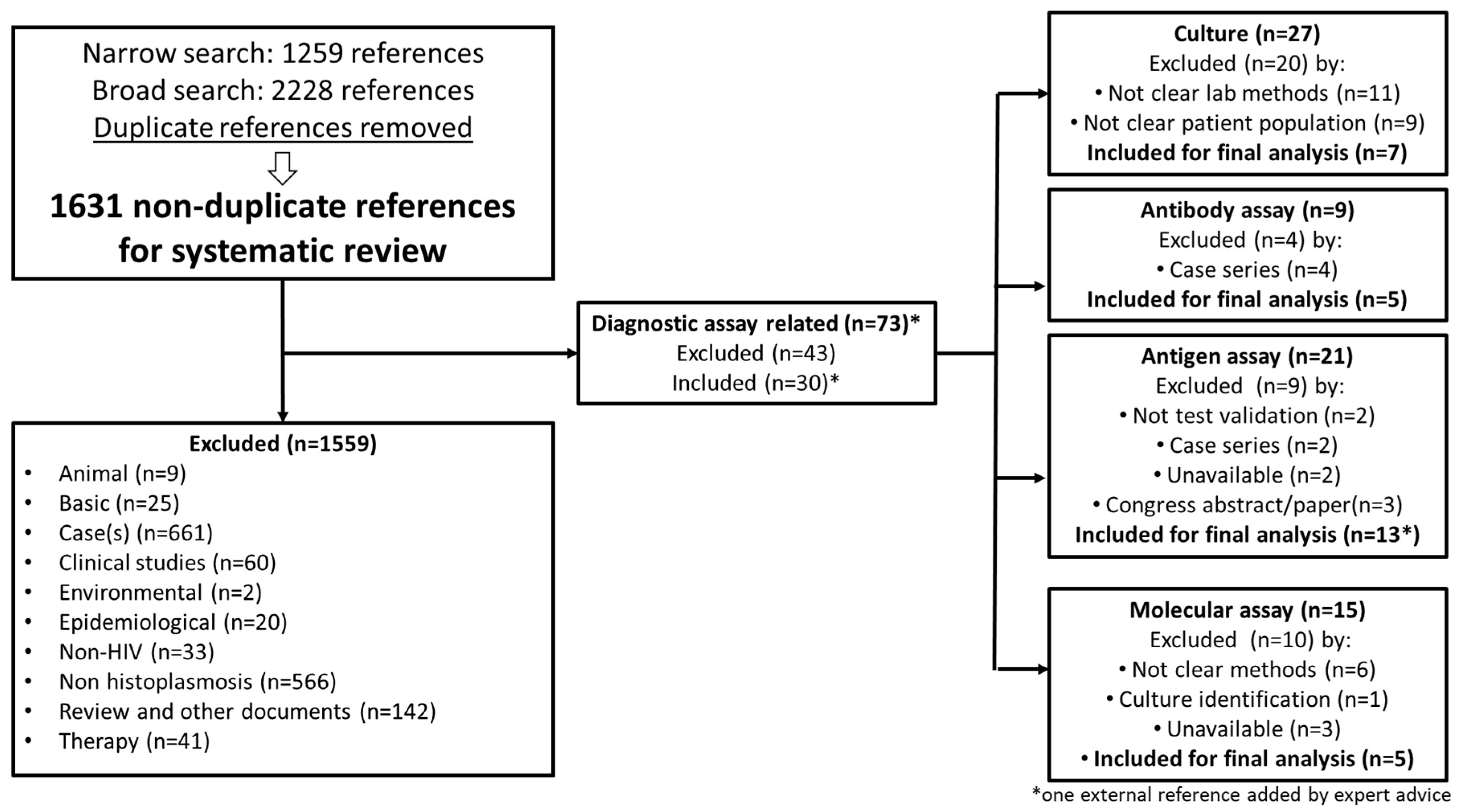
3.1. Literature Search
3.2. Meta-Analysis
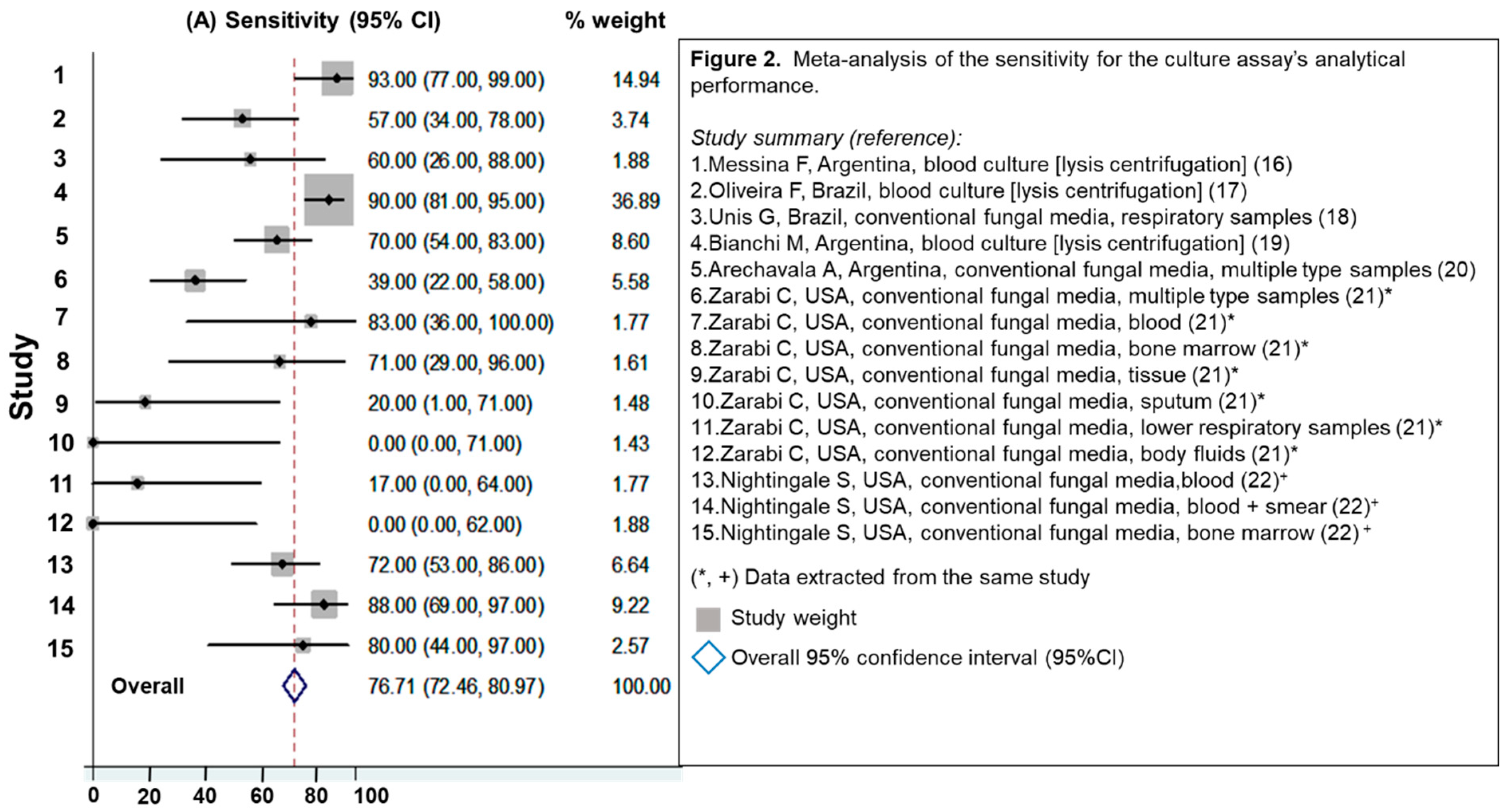
3.2.1. Evaluation of Culture Assays
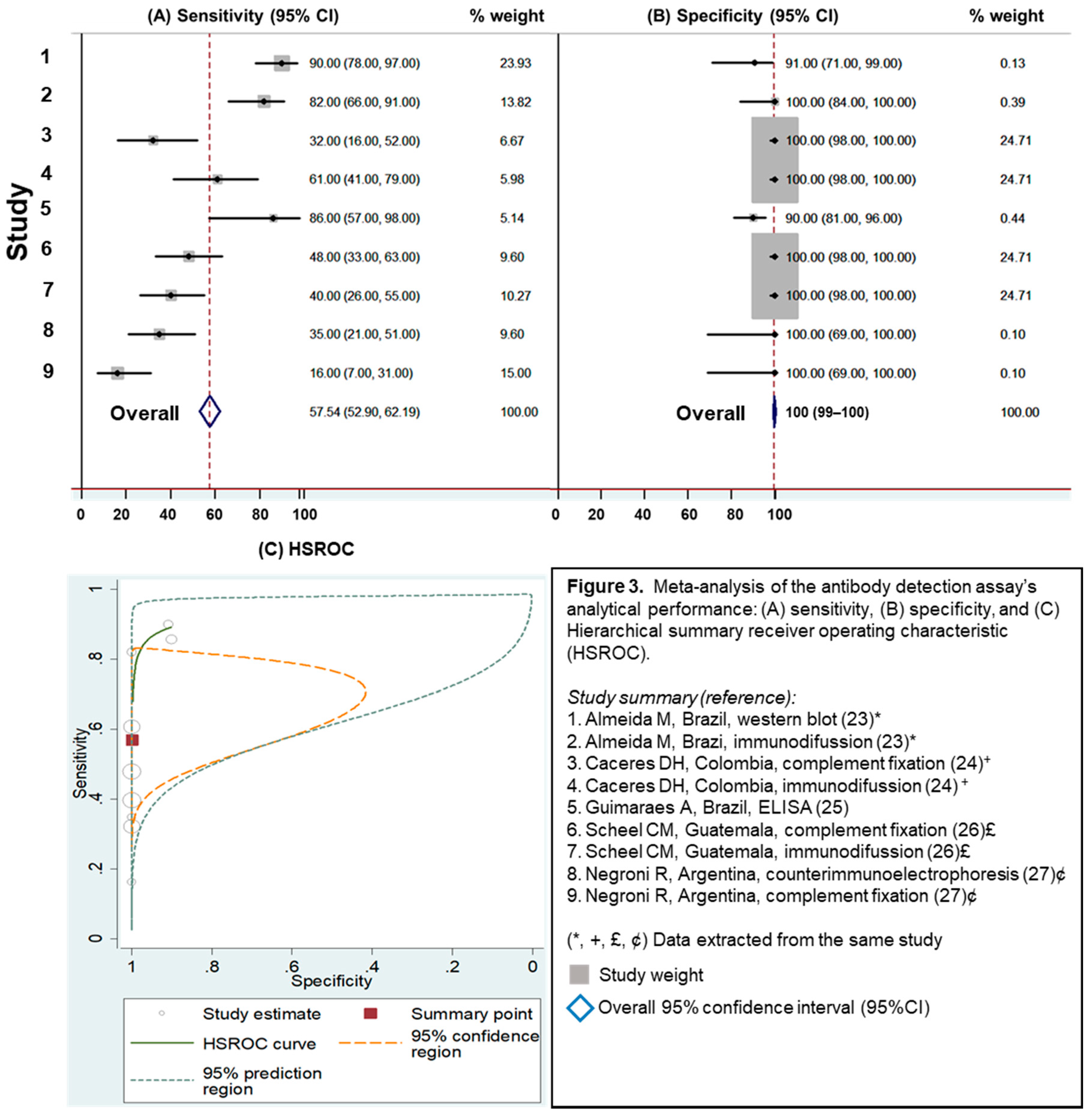
3.2.2. Evaluation of Antibody Detection Assays
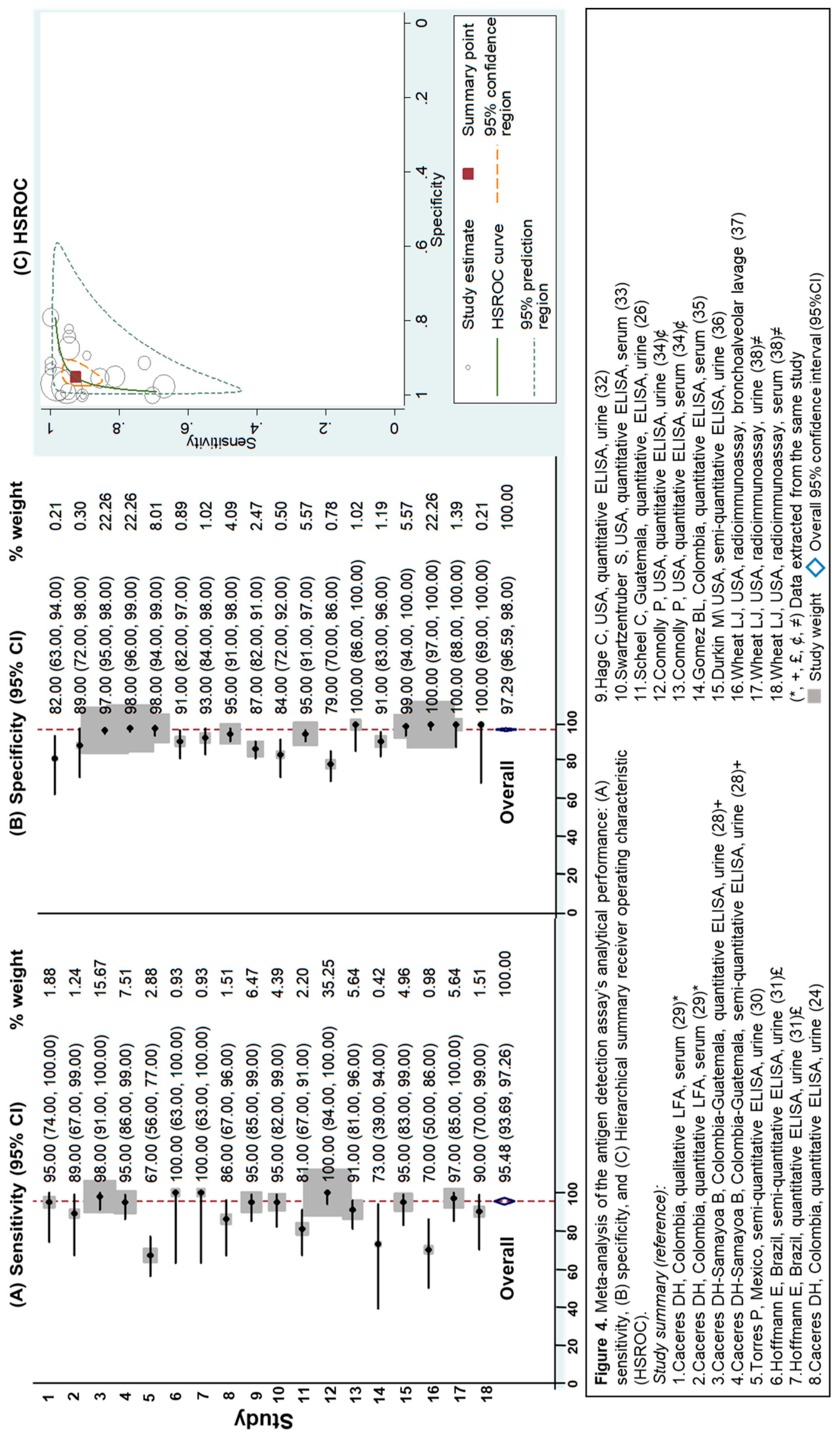
3.2.3. Evaluation of Antigen Detection Assays
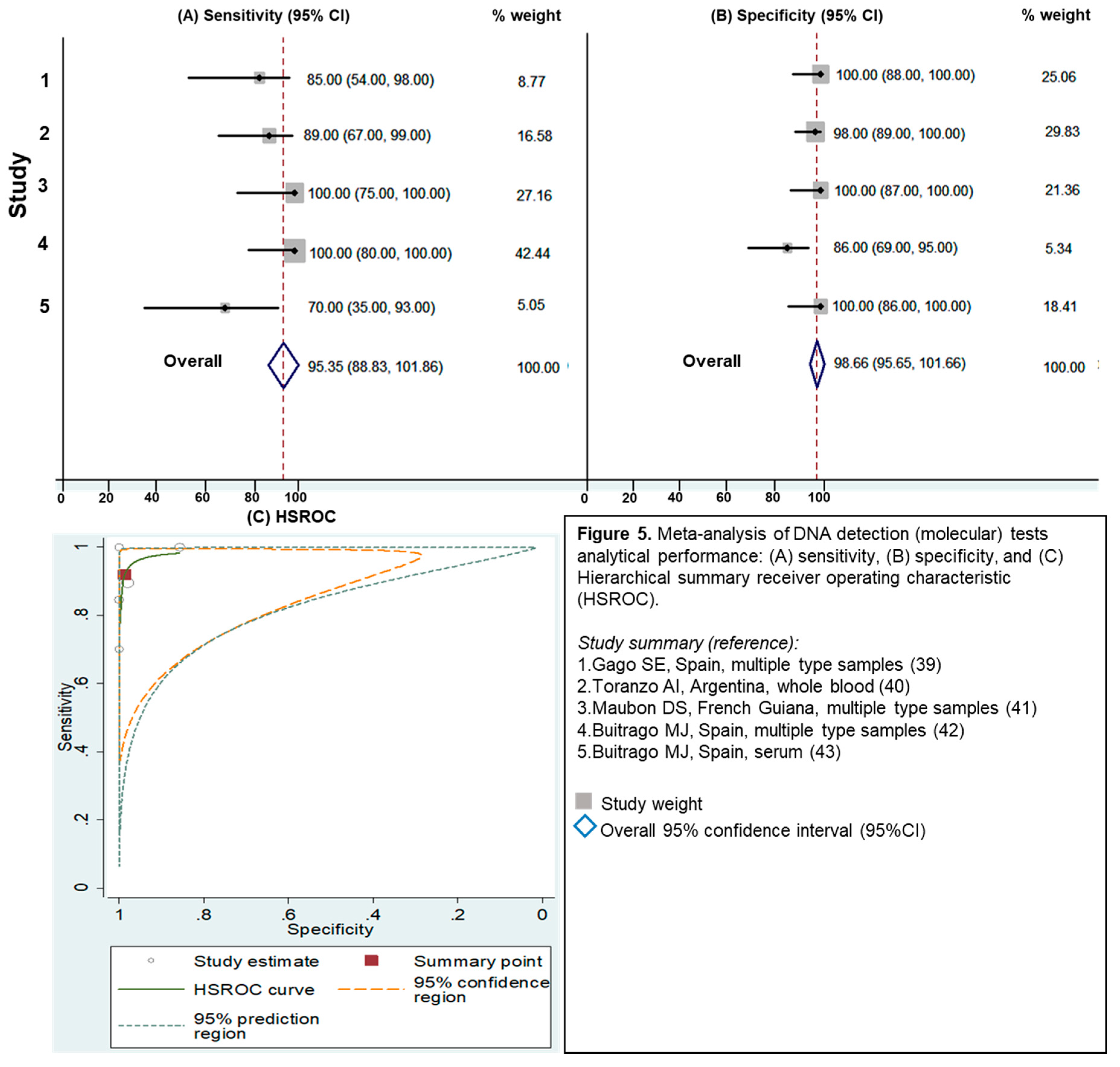
3.2.4. Evaluation of Molecular Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bahr, N.C.; Antinori, S.; Wheat, L.J.; Sarosi, G.A. Histoplasmosis infections worldwide: Thinking outside of the Ohio River valley. Curr. Trop. Med. Rep. 2015, 2, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Deepe, G.S. Histoplasma capsulatum (histoplasmosis). In Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 2949–2962. [Google Scholar]
- Kauffman, C.A. Diagnosis of histoplasmosis in immunosuppressed patients. Curr. Opin. Infect. Dis. 2008, 21, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, D.H.; Gómez, B.L.; Restrepo, Á.; Tobón, Á.M. Histoplasmosis y sida: Factores de riesgo clínicos y de laboratorio asociados al pronóstico de la enfermedad. Infectio 2012, 16, 44–50. [Google Scholar] [CrossRef]
- Adenis, A.A.; Valdes, A.; Cropet, C.; McCotter, O.Z.; Derado, G.; Couppie, P.; Chiller, T.; Nacher, M. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: A modelling study. Lancet Infect. Dis. 2018, 18, 1150–1159. [Google Scholar] [CrossRef]
- Adenis, A.; Nacher, M.; Hanf, M.; Basurko, C.; Dufour, J.; Huber, F.; Aznar, C.; Carme, B.; Couppie, P. Tuberculosis and Histoplasmosis among Human Immunodeficiency Virus–Infected Patients: A Comparative Study. Am. J. Trop. Med. Hyg. 2014, 90, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.H.; Tobón, Á.M.; Restrepo, Á.; Chiller, T.; Gómez, B.L. The important role of co-infections in patients with AIDS and progressive disseminated histoplasmosis (PDH): A cohort from Colombia. Med Mycol. Case Rep. 2018, 19, 41–44. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Denning, D.W. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [PubMed]
- Azar, M.M.; Hage, C.A. Clinical Perspectives in the Diagnosis and Management of Histoplasmosis. Clin. Chest Med. 2017, 38, 403–415. [Google Scholar] [CrossRef]
- Hage, C.A.; Azar, M.M.; Bahr, N.; Loyd, J.; Wheat, L.J. Histoplasmosis: Up-to-Date Evidence-Based Approach to Diagnosis and Management. Semin. Respir. Crit. Care Med. 2015, 36, 729–745. [Google Scholar]
- Azar, M.M.; Hage, C.A. Laboratory Diagnostics for Histoplasmosis. J. Clin. Microbiol. 2017, 55, 1612–1620. [Google Scholar] [CrossRef]
- Haynes, R.B.; Wilczynski, N.L. Optimal search strategies for retrieving scientifically strong studies of diagnosis from Medline: Analytical survey. BMJ 2004, 328, 1040. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Whiting, P. Metandi: Meta-analysis of Diagnostic Accuracy Using Hierarchical Logistic Regression. Stata J. Promot. Commun. Stat. Stata 2009, 9, 211–229. [Google Scholar] [CrossRef]
- Fandino-Devia, E.; Rodriguez-Echeverri, C.; Cardona-Arias, J.; Gonzalez, A. Antigen Detection in the Diagnosis of Histoplasmosis: A Meta-analysis of Diagnostic Performance. Mycopathologia 2016, 181, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.A.; Corti, M.; Negroni, R.; Arechavala, A.; Bianchi, M.; Santiso, G. Histoplasmosis in AIDS patients without tegumentary manifestations. Rev. Chil. Infectol. 2018, 35, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Fde, M.; Fernandes, S.S.; Severo, C.B.; Guazzelli, L.S.; Severo, L.C. Histoplasma capsulatum fungemia in patients with acquired immunodeficiency syndrome: Detection by lysis-centrifugation blood-culturing technique. Rev. Inst. Med. Trop. Sao Paulo 2007, 49, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Unis, G.; da Silva, V.B.; Severo, L.C. Disseminated histoplasmosis and AIDS. The role of culture medium for the bronchoscopic clinical specimens. Rev. Soc. Bras. Med. Trop. 2004, 37, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Robles, A.M.; Vitale, R.; Helou, S.; Arechavala, A.; Negroni, R. The usefulness of blood culture in diagnosing HIV-related systemic mycoses: Evaluation of a manual lysis centrifugation method. Med. Mycol. 2000, 38, 77–80. [Google Scholar] [CrossRef][Green Version]
- Arechavala, A.I.; Robles, A.M.; Negroni, R.; Bianchi, M.H.; Taborda, A. Value of direct and indirect diagnostic methods in systemic mycoses associated with AIDS. Rev. Inst. Med. Trop. São Paulo 1993, 35, 163–169. [Google Scholar] [CrossRef]
- Zarabi, C.M.; Thomas, R.; Adesokan, A. Diagnosis of Systemic Histoplasmosis in Patients With AIDS. South. Med J. 1992, 85, 1171–1175. [Google Scholar] [CrossRef]
- Nightingale, S.D.; Parks, J.M.; Pounders, S.M.; Burns, D.K.; Reynolds, J.; Hernandez, J.A. Disseminated Histoplasmosis in Patients With AIDS. South. Med. J. 1990, 83, 624–630. [Google Scholar] [CrossRef]
- Almeida, M.A.; Damasceno, L.S.; Pizzini, C.V.; Muniz, M.D.M.; Almeida-Paes, R.; Zancopé-Oliveira, R.M. Role of western blot assay for the diagnosis of histoplasmosis in AIDS patients from a National Institute of Infectious Diseases in Rio de Janeiro, Brazil. Mycoses 2019, 62, 261–267. [Google Scholar] [CrossRef]
- Caceres, D.H.; Scheel, C.M.; Tobón, Á.M.; Cleveland, A.A.; Restrepo, Á.; Brandt, M.E.; Chiller, T.; Gómez, B.L. Validation of an Enzyme-Linked Immunosorbent Assay That Detects Histoplasma capsulatum Antigenuria in Colombian Patients with AIDS for Diagnosis and Follow-Up during Therapy. Clin. Vaccine Immunol. 2014, 21, 1364–1368. [Google Scholar] [CrossRef]
- Guimaraes, A.J.; Pizzini, C.V.; Almeida, M.D.A.; Peralta, J.M.; Nosanchuk, J.D.; Zancope-Oliveira, R.M. Evaluation of an enzyme-linked immunosorbent assay using purified, deglycosylated histoplasmin for different clinical manifestations of histoplasmosis. Microbiol. Res. 2010, 1, e2. [Google Scholar] [CrossRef]
- Scheel, C.M.; Samayoa, B.; Herrera, A.; Lindsley, M.D.; Benjamin, L.; Reed, Y. Development and evaluation of an enzyme-linked immunosorbent assay to detect Histoplasma capsulatum antigenuria in immunocompromised patients. Clin. Vaccine Immunol. 2009, 16, 852–858. [Google Scholar] [CrossRef]
- Negroni, R.; Iovannitti, C.; Arechavala, A.I.; Carnovale, S.; Euguchi, K. Preparation and study of an exoantigen of Histoplasma capsulatum for serological reactions. Rev. Iberoam. Micol. 1998, 15, 282–285. [Google Scholar]
- Cáceres, D.H.; Samayoa, B.E.; Medina, N.G.; Tobón, Á.M.; Guzmán, B.J.; Mercado, D.; Restrepo, Á.; Chiller, T.; Arathoon, E.E.; Gómez, B.L. Multicenter Validation of Commercial Antigenuria Reagents to Diagnose Progressive Disseminated Histoplasmosis in People Living with HIV/AIDS in Two Latin American Countries. J. Clin. Microbiol. 2018, 56, e01959-17. [Google Scholar] [CrossRef]
- Caceres, D.H.; Minderman, M.C.; Chaney, L.; Gomez, B.L.; Wheat, L.J.; Chiller, T.M. Evaluation of a Histoplasma Antigen Lateral Flow Assay for the Rapid Diagnosis of Progressive Disseminated Histoplasmosis in AIDS Patie. ISHAM 2018. Available online: https://www.morressier.com/article/evaluation-histoplasma-antigen-lateral-flow-assay-rapid-diagnosis-progressive-disseminated-histoplasmosis-aids-patients/5ac39997d462b8028d899d7d (accessed on 14 August 2019).
- Torres-González, P.; Niembro-Ortega, M.D.; Martínez-Gamboa, A.; Ahumada-Topete, V.H.; Andrade-Villanueva, J.; Araujo-Meléndez, J.; Chaparro-Sánchez, A.; Crabtree-Ramírez, B.; Cruz-Martínez, S.; Gamboa-Domínguez, A.; et al. Diagnostic accuracy cohort study and clinical value of the Histoplasma urine antigen (ALPHA Histoplasma EIA) for disseminated histoplasmosis among HIV infected patients: A multicenter study. PLoS Negl. Trop. Dis. 2018, 12, e0006872. [Google Scholar] [CrossRef]
- Hoffmann, E.R.; Daboit, T.C.; Paskulin, D.D.; Monteiro, A.A.; Falci, D.R.; Linhares, T. Disseminated histoplasmosis and AIDS: A prospective and multicentre study to evaluate the performance of different diagnostic tests. Mycoses 2017, 60, 20–24. [Google Scholar] [CrossRef]
- Hage, C.A.; Ribes, J.A.; Wengenack, N.L.; Baddour, L.M.; Assi, M.; McKinsey, D.S.; Hammoud, K.; Alapat, D.; Babady, N.E.; Parker, M.; et al. A Multicenter Evaluation of Tests for Diagnosis of Histoplasmosis. Clin. Infect. Dis. 2011, 53, 448–454. [Google Scholar] [CrossRef]
- Swartzentruber, S.; Lemonte, A.; Witt, J.; Fuller, D.; Davis, T.; Hage, C.; Connolly, P.; Durkin, M.; Wheat, L.J. Improved Detection of Histoplasma Antigenemia following Dissociation of Immune Complexes. Clin. Vaccine Immunol. 2009, 16, 320–322. [Google Scholar] [CrossRef]
- Connolly, P.A.; Durkin, M.M.; Lemonte, A.M.; Hackett, E.J.; Wheat, L.J. Detection of Histoplasma Antigen by a Quantitative Enzyme Immunoassay. Clin. Vaccine Immunol. 2007, 14, 1587–1591. [Google Scholar] [CrossRef]
- Gomez, B.L.; Figueroa, J.I.; Hamilton, A.J.; Ortiz, B.L.; Robledo, M.A.; Restrepo, A.; Hay, R.J. Development of a novel antigen detection test for histoplasmosis. J. Clin. Microbiol. 1997, 35, 2618–2622. [Google Scholar]
- Durkin, M.M.; Connolly, P.A.; Wheat, L.J. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J. Clin. Microbiol. 1997, 35, 2252–2255. [Google Scholar]
- Wheat, L.J.; Connolly-Stringfield, P.; Williams, B.; Connolly, K.; Blair, R.; Bartlett, M.; Durkin, M. Diagnosis of Histoplasmosis in Patients with the Acquired Immunodeficiency Syndrome by Detection of Histoplasma capsulatum Polysaccharide Antigen in Bronchoalveolar Lavage Fluid. Am. Rev. Respir. Dis. 1992, 145, 1421–1424. [Google Scholar] [CrossRef]
- Wheat, L.J.; Connolly-Stringfield, P.; Kohler, R.B.; Frame, P.T.; Gupta, M.R. Histoplasma capsulatum polysaccharide antigen detection in diagnosis and management of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome. Am. J. Med. 1989, 87, 396–400. [Google Scholar] [CrossRef]
- Gago, S.; Esteban, C.; Valero, C.; Zaragoza, Ó.; De La Bellacasa, J.P.; Buitrago, M.J. A Multiplex Real-Time PCR Assay for Identification of Pneumocystis jirovecii, Histoplasma capsulatum, and Cryptococcus neoformans/Cryptococcus gattii in Samples from AIDS Patients with Opportunistic Pneumonia. J. Clin. Microbiol. 2014, 52, 1168–1176. [Google Scholar] [CrossRef]
- Toranzo, A.I.; Tiraboschi, I.N.; Fernández, N.; Ibarra-Camou, B.; Rivas, M.C.; Lee, W.; Davel, G.; Canteros, C.E. Molecular diagnosis of human histoplasmosis in whole blood samples. Rev. Argent. Microbiol. 2009, 41, 20–26. [Google Scholar]
- Maubon, D.; Simon, S.; Aznar, C. Histoplasmosis diagnosis using a polymerase chain reaction method. Application on human samples in French Guiana, South America. Diagn. Microbiol. Infect. Dis. 2007, 58, 441–444. [Google Scholar] [CrossRef]
- Buitrago, M.J.; Gómez-López, A.; Monzón, A.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M. Assessment of a quantitative PCR method for clinical diagnosis of imported histoplasmosis. Enferm. Infect. Microbiol. Clín. 2007, 25, 16–24. [Google Scholar]
- Buitrago, M.J.; Berenguer, J.; Mellado, E.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Detection of imported histoplasmosis in serum of HIV-infected patients using a real-time PCR-based assay. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 665–668. [Google Scholar] [CrossRef]
- Caceres, D.H.; Zuluaga, A.; Arango-Bustamante, K.; De Bedout, C.; Tobón, Á.M.; Restrepo, Á.; Gómez, B.L.; Cano, L.E.; González, Á. Implementation of a Training Course Increased the Diagnosis of Histoplasmosis in Colombia. Am. J. Trop. Med. Hyg. 2015, 93, 662–667. [Google Scholar] [CrossRef]
- Bansal, N.; Sethuraman, N.; Gopalakrishnan, R.; Ramasubramanian, V.; Kumar, D.S.; Nambi, P.S. Can urinary histoplasma antigen test improve the diagnosis of histoplasmosis in a tuberculosis endemic region? Mycoses 2019, 62, 502–507. [Google Scholar] [CrossRef]
- Samayoa, B.; Roy, M.; Cleveland, A.A.; Medina, N.; Lau-Bonilla, D.; Scheel, C.M.; Gomez, B.L.; Chiller, T.; Arathoon, E. High Mortality and Coinfection in a Prospective Cohort of Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome Patients with Histoplasmosis in Guatemala. Am. J. Trop. Med. Hyg. 2017, 97, 42–48. [Google Scholar] [CrossRef]
- Falci, D.R.; Monteiro, A.A.; Caurio, C.F.B.; Magalhães, T.C.O.; Xavier, M.O.; Basso, R.P.; Melo, M.; Schwarzbold, A.V.; Ferreira, P.R.A.; Vidal, J.E.; et al. Histoplasmosis, An Underdiagnosed Disease Affecting People Living With HIV/AIDS in Brazil: Results of a Multicenter Prospective Cohort Study Using Both Classical Mycology Tests and Histoplasma Urine Antigen Detection. Open Forum Infect. Dis. 2019, 6, ofz073. [Google Scholar] [CrossRef]
- Buitrago, M.J.; Canteros, C.E.; De León, G.F.; González, Á.; De Oliveira, M.M.-E.; Muñoz, C.O.; Ramirez, J.A.; Toranzo, A.I.; Zancope-Oliveira, R.; Cuenca-Estrella, M. Comparison of PCR protocols for detecting Histoplasma capsulatum DNA through a multicenter study. Rev. Iberoam. Micol. 2013, 30, 256–260. [Google Scholar] [CrossRef]
- Scheel, C.M.; Gómez, B.L. Diagnostic Methods for Histoplasmosis: Focus on Endemic Countries with Variable Infrastructure Levels. Curr. Trop. Med. Rep. 2014, 1, 129–137. [Google Scholar] [CrossRef]
| Assay | Sensitivity% (95% CI) | Specificity% (95% CI) | Accuracy% (95% CI) | LR+/LR– | 1/LR– (95% CI) |
|---|---|---|---|---|---|
| Antibody detection assays | 58 (53–62) | 100 (99–100) | 89 (87–91) | 1146.3 (8.5–154326.2)/0.4 (0.2–0.7) | 2.3 (1.4–3.7) |
| Antigen detection assays | 95 (94–97) | 97 (97–98) | 95 (94–96) | 18.7 (11.7–30.1)/0.07 (0.04–0.13) | 13.2 (7.7–22.7) |
| Molecular assays | 95 (89–100) | 99 (96–100) | 96 (94–99) | 70.7 (7.2–691.9)/0.08 (0.02–0.27) | 12.3 (3.6–41.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caceres, D.H.; Knuth, M.; Derado, G.; Lindsley, M.D. Diagnosis of Progressive Disseminated Histoplasmosis in Advanced HIV: A Meta-Analysis of Assay Analytical Performance. J. Fungi 2019, 5, 76. https://doi.org/10.3390/jof5030076
Caceres DH, Knuth M, Derado G, Lindsley MD. Diagnosis of Progressive Disseminated Histoplasmosis in Advanced HIV: A Meta-Analysis of Assay Analytical Performance. Journal of Fungi. 2019; 5(3):76. https://doi.org/10.3390/jof5030076
Chicago/Turabian StyleCaceres, Diego H., Martha Knuth, Gordana Derado, and Mark D. Lindsley. 2019. "Diagnosis of Progressive Disseminated Histoplasmosis in Advanced HIV: A Meta-Analysis of Assay Analytical Performance" Journal of Fungi 5, no. 3: 76. https://doi.org/10.3390/jof5030076
APA StyleCaceres, D. H., Knuth, M., Derado, G., & Lindsley, M. D. (2019). Diagnosis of Progressive Disseminated Histoplasmosis in Advanced HIV: A Meta-Analysis of Assay Analytical Performance. Journal of Fungi, 5(3), 76. https://doi.org/10.3390/jof5030076





