Fungal Diseases in Taiwan—National Insurance Data and Estimation
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design and Population
2.2. Database
2.3. Patients and Definitions
2.4. Data Analysis
3. Results
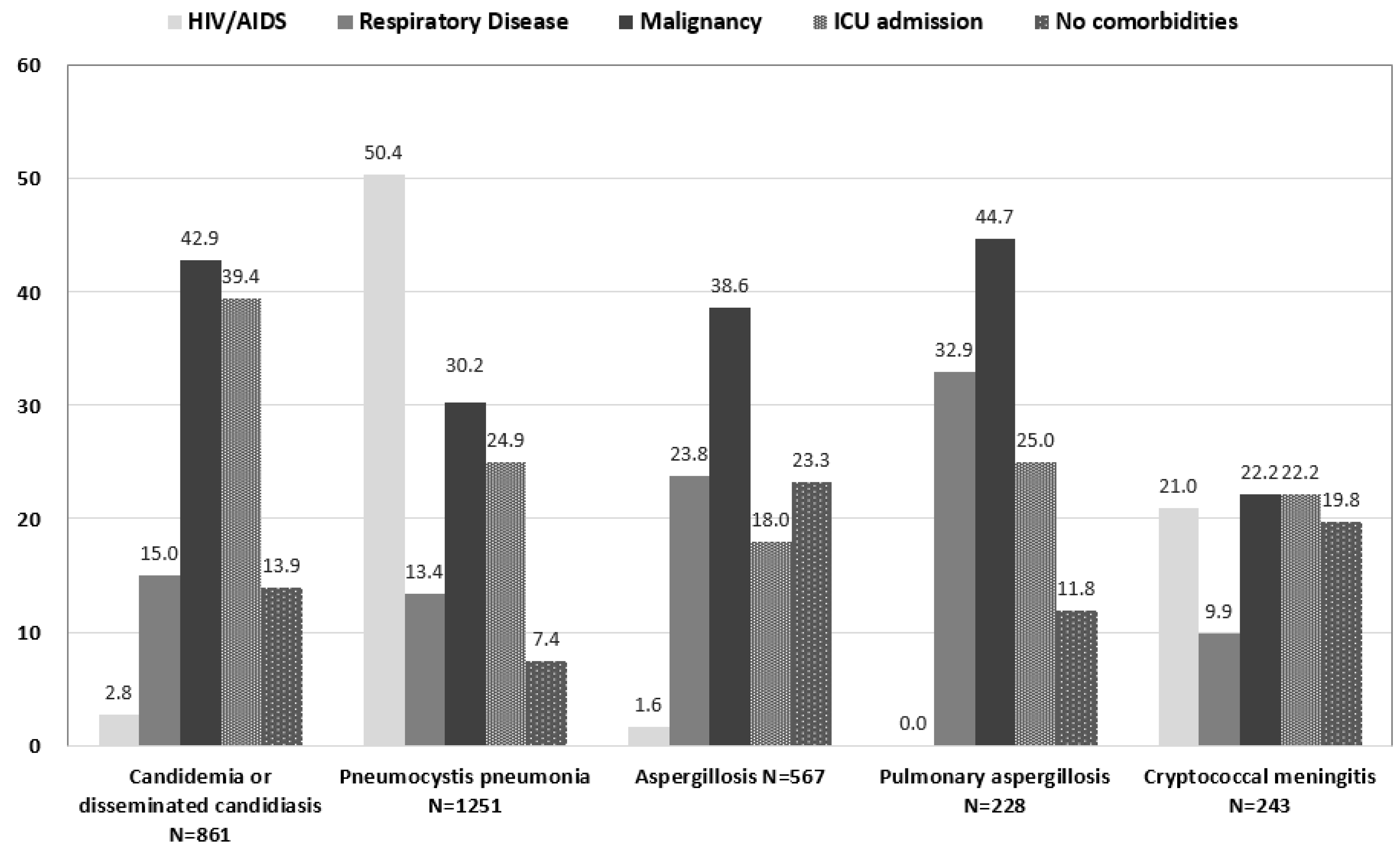
3.1. Candidiasis
3.2. Pneumocystis Pneumonia
3.3. Aspergillosis
3.4. Cryptococcal Meningitis
3.5. Other Fungal Diseases
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Mody, R.K.; Walker, T.; Chiller, T. The Global Burden of Fungal Diseases. Infect. Dis. Clin. N. Am. 2016, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Editorial [No authors listed] Stop neglecting fungi. Nat. Microbiol. 2017, 2, 17120. [CrossRef] [PubMed]
- Richardson, M.; Lass-Florl, C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008, 14, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.D.; Tsai, W.C.; Hsu, W.T.; Shih, M.C.; Chen, M.Y.; Sun, H.Y.; Hsieh, S.M.; Sheng, W.H.; Chuang, Y.C.; Cheng, A.; et al. Impact of initiation of combination antiretroviral therapy according to the WHO recommendations on the survival of HIV-positive patients in Taiwan. J. Microbiol Immunol Infect. 2019. [Google Scholar] [CrossRef] [PubMed]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Benedict, K.; Richardson, M.; Vallabhaneni, S.; Jackson, B.R.; Chiller, T. Emerging issues, challenges, and changing epidemiology of fungal disease outbreaks. Lancet Infect. Dis. 2017, 17, e403–e411. [Google Scholar] [CrossRef]
- National Statistics of Taiwan (R.O.C.). Available online: https://eng.stat.gov.tw/mp.asp?mp=5 (accessed on 29 May 2019).
- Ho Chan, W.S. Taiwan′s healthcare report 2010. EPMA J. 2010, 1, 563–585. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, J.; Denning, D.W.; Chau, N.V.; Yen, N.T.; Crump, J.A.; Day, J.N. Estimating the burden of fungal disease in Vietnam. Mycoses 2015, 58, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Velayuthan, R.D.; Samudi, C.; Lakhbeer Singh, H.K.; Ng, K.P.; Shankar, E.M.; Denning, D.W. Estimation of the Burden of Serious Human Fungal Infections in Malaysia. J. Fungi 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Khwakhali, U.S.; Denning, D.W. Burden of serious fungal infections in Nepal. Mycoses 2015, 58, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Chakrabarti, A.; Li, R.Y.; Patel, A.K.; Watcharananan, S.P.; Liu, Z.; Chindamporn, A.; Tan, A.L.; Sun, P.L.; Wu, U.I.; et al. Incidence and species distribution of candidaemia in Asia: A laboratory-based surveillance study. Clin. Microbiol. Infect. 2015, 21, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Kung, H.C.; Wang, J.L.; Chang, S.C.; Wang, J.T.; Sun, H.Y.; Hsueh, P.R.; Chen, Y.C. Community-onset candidemia at a university hospital, 1995–2005. J. Microbiol. Immunol. Infect. 2007, 40, 355–363. [Google Scholar] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Pan, S.C.; Yang, T.S.; Matsuda, K.; Kim, H.B.; Choi, Y.H.; Hori, S.; Wang, J.T.; Sheng, W.H.; Chen, Y.C.; et al. Healthcare-associated infections in intensive care units in Taiwan, South Korea, and Japan; Recent trends based on national surveillance reports. Antimicrob. Resist. Infect. Control. 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.; Leibovici, L.; Paul, M. PCR diagnosis of invasive candidiasis: Systematic review and meta-analysis. J. Clin. Microbiol. 2011, 49, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Buck, M.; Witebsky, F.; Stock, F.; Pizzo, P.A.; Walsh, T.J. Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis. Disseminated versus single-organ infection. Diagn. Microbiol. Infect. Dis. 1993, 17, 103–109. [Google Scholar] [CrossRef]
- Sun, K.S.; Tsai, C.F.; Chen, S.C.; Chen, Y.Y.; Huang, W.C. Galactomannan testing and the incidence of invasive pulmonary aspergillosis: A 10-year nationwide population-based study in Taiwan. PLoS ONE 2016, 11, e0149964. [Google Scholar]
- Chen, M.; Xu, Y.; Hong, N.; Yang, Y.; Lei, W.; Du, L.; Zhao, J.; Lei, X.; Xiong, L.; Cai, L.; et al. Epidemiology of fungal infections in China. Front. Med. 2018, 12, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chen, M.; Hartmann, T.; Yang, R.Y.; Liao, W.Q. Epidemiology of opportunistic invasive fungal infections in China: Review of literature. Chin. Med. J. 2013, 126, 361–368. [Google Scholar] [PubMed]
- Chen, C.Y.; Sheng, W.H.; Tien, F.M.; Lee, P.C.; Huang, S.Y.; Tang, J.L.; Tsay, W.; Tien, H.F.; Hsueh, P.R. Clinical characteristics and treatment outcomes of pulmonary invasive fungal infection among adult patients with hematological malignancy in a medical centre in Taiwan, 2008–2013. J. Microbiol. Immunol. Infect. 2018. [Google Scholar] [CrossRef]
- Tang, J.L.; Kung, H.C.; Lei, W.C.; Yao, M.; Wu, U.I.; Hsu, S.C.; Lin, C.T.; Li, C.C.; Wu, S.J.; Hou, H.A.; et al. High incidences of invasive fungal infections in acute myeloid leukemia patients receiving induction chemotherapy without systemic antifungal prophylaxis: A prospective observational study in Taiwan. PLoS ONE 2015, 10, e0128410. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Zhang, W.B.; Yu, B.; Chen, Y.W.; Mu, S.; Cui, Y.L. Prevalence of allergic bronchopulmonary aspergillosis in Chinese patients with bronchial asthma. Zhonghua Jie He He Hu Xi Za Zhi 2011, 34, 909–913. [Google Scholar]
- Shantakumar, S.; Pwu, R.F.; D’Silva, L.; Wurst, K.; Kuo, Y.W.; Yang, Y.Y.; Juan, Y.C.; Chan, K.A. Burden of asthma and COPD overlap (ACO) in Taiwan: A nationwide population-based study. BMC Pulm. Med. 2018, 18, 16. [Google Scholar] [CrossRef]
- Xu, H.; Li, L.; Huang, W.J.; Wang, L.X.; Li, W.F.; Yuan, W.F. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: A case control study from China. Clin. Microbiol. Infect. 2012, 18, 403–408. [Google Scholar] [CrossRef]
- Chindamporn, A.; Chakrabarti, A.; Li, R.; Sun, P.L.; Tan, B.H.; Chua, M.; Wahyuningsih, R.; Patel, A.; Liu, Z.; Chen, Y.C.; et al. Survey of laboratory practices for diagnosis of fungal infection in seven Asian countries: An Asia Fungal Working Group (AFWG) initiative. Med. Mycol. 2018, 56, 416–425. [Google Scholar] [CrossRef]
- Sun, K.S.; Tsai, C.F.; Chen, S.C.; Huang, W.C. Clinical outcome and prognostic factors associated with invasive pulmonary aspergillosis: An 11-year follow-up report from Taiwan. PLoS ONE 2017, 12, e0186422. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Chen, M.Y.; Hsieh, S.M.; Sheng, W.H.; Chang, S.Y.; Hsiao, C.F.; Hung, C.C.; Chang, S.C. Changes in the clinical spectrum of opportunistic illnesses in persons with HIV infection in Taiwan in the era of highly active antiretroviral therapy. Jpn. J. Infect. Dis. 2006, 59, 311–316. [Google Scholar] [PubMed]
- Huang, Y.T.; Hung, C.C.; Liao, C.H.; Sun, H.Y.; Chang, S.C.; Chen, Y.C. Detection of circulating galactomannan in serum samples for diagnosis of Penicillium marneffei infection and cryptococcosis among patients infected with human immunodeficiency virus. J. Clin. Microbiol. 2007, 45, 2858–2862. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Cheng, C.Y.; Li, C.W.; Yang, C.J.; Tsai, M.S.; Liu, C.E.; Lee, Y.T.; Tang, H.J.; Wang, N.C.; Lin, T.Y.; et al. Trends and outcomes of late initiation of combination antiretroviral therapy driven by late presentation among Hiv-positive Taiwanese patients in the era of treatment scale-up. PLoS ONE 2017, 12, e0179870. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Lai, L.C.; Shih, S.M.; Hsiung, C.A.; Chen, Y.C. The incidences and outcomes of cryptococcosis among different risk populations in Taiwan: A nationwide, population-based study during 2003–2012. Abstracts of the 30th International Congress of Chemotherapy and Infection 2017 (ICC 2017). Int. J. Antimicrob. Agents 2017, 50 (Suppl. 2), S157. [Google Scholar]
- Tseng, H.K.; Liu, C.P.; Ho, M.W.; Lu, P.L.; Lo, H.J.; Lin, Y.H.; Cho, W.L.; Chen, Y.C. Microbiological, epidemiological, and clinical characteristics and outcomes of patients with cryptococcosis in Taiwan, 1997–2010. PLoS ONE 2013, 8, e61921. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lai, C.H. Nationwide population-based epidemiologic study of cryptococcal meningitis in Taiwan. Neuroepidemiology 2011, 36, 79–84. [Google Scholar] [CrossRef]
- Bratton, E.W.; El Husseini, N.; Chastain, C.A.; Lee, M.S.; Poole, C.; Stürmer, T.; Juliano, J.J.; Weber, D.J.; Perfect, J.R. Comparison and temporal trends of three groups with cryptococcosis: Hiv-infected, solid organ transplant, and Hiv-negative/non-transplant. PLoS ONE 2012, 7, e43582. [Google Scholar] [CrossRef]
- Pappas, P.G. Cryptococcal infections in non-HIV-infected patients. Trans. Am. Clin. Climatol. Assoc. 2013, 124, 61–79. [Google Scholar]
- Yuchong, C.; Fubin, C.; Jianghan, C.; Fenglian, W.; Nan, X.; Minghui, Y.; Yalin, S.; Zhizhong, Z. Cryptococcosis in China (1985–2010): Review of cases from Chinese database. Mycopathologia 2012, 173, 329–335. [Google Scholar] [CrossRef]
| Fungal Disease | Taiwan, This Study ** | Vietnam [12] | Malaysia [13] | Nepal [14] | |||||
|---|---|---|---|---|---|---|---|---|---|
| ICD-9 Code | Incident Case Number | Incidence Rate | Incident Case Number | Incidence Rate | Incident Case Number | Incidence Rate | Incident Case Number | Incidence Rate | |
| Yeasts | |||||||||
| Candida vulvovaginitis | 112.1 | 45,291 | 477.05 + | - | - | - | - | - | - |
| Recurrent (>4 times/year) | 112.1 | 9363 | 98.62 + | 1,767,581 | 3893 * | 501,138 | 4800 + | 443,237 | 2908 + |
| Oral candidiasis | 112 | 21,066 | 90.13 | - | - | - | - | - | - |
| Candida esophagitis | 112.84 | 1440 | 6.16 | 33,107 | 36 | 5850 | 19 | 2950 | 10.8 |
| Systemic candidiasis | 112.5 | 861 | 3.68 | 4540 | 5 | 1533 | 5 | - | - |
| Candida peritonitis | 112.85 | 27 | 0.12 | - | - | 230 | 0.8 | - | - |
| Pneumocystis pneumonia | 136.3 | 1251 | 5.35 | 608 | 0.67 | 1286 | 4.2 | 990 | 3.6 |
| Cryptococcal meningitis | 321.0 | 243 | 1.04 | 140 | 0.15 | 885 | 2.8 | 164 | 0.6 |
| Molds | |||||||||
| Aspergillosis | 117.3, 484.6 | 567 | 2.43 | 14,523 | 15.99 | 1018 | 3.3 | 1119 | 4 |
| Pulmonary aspergillosis (PA) | 484.6 | 228 | 0.98 | ||||||
| PA post Tuberculosis (incidence) | 484.6, 010–0.18 | 60 | 0.26 | - | - | - | - | - | - |
| PA post Tuberculosis (prevalence) | 484.6, 010–0.18 | 75 * | 0.32 * | - | - | - | - | - | - |
| Chronic pulmonary aspergillosis | None # | - | - | 55,509 | 61 | - | - | 6611 | 24.2 |
| Allergic bronchopulmonary aspergillosis (ABPA) | 518.6 | 45 * | 0.19 * | 23,607 | 26 * | 30,062 | 98 | 9546 | 35 |
| Severe asthma with fungal sensitization | - | - | 31,161 | 34 | 39,682 | 130 | 12,600 | 46.1 | |
| Mucormycosis | 117.7 | 66 | 0.28 | 109 | 0.12 | - | - | 55 | 0.2 |
| Strengths | Limitations | |
|---|---|---|
| National health insurance database |
|
|
| Hospital-based epidemiological studies |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-S.; Denning, D.W.; Shih, S.-M.; Hsiung, C.A.; Wu, U.-I.; Sun, H.-Y.; Chen, P.-Y.; Chen, Y.-C.; Chang, S.-C. Fungal Diseases in Taiwan—National Insurance Data and Estimation. J. Fungi 2019, 5, 78. https://doi.org/10.3390/jof5030078
Huang Y-S, Denning DW, Shih S-M, Hsiung CA, Wu U-I, Sun H-Y, Chen P-Y, Chen Y-C, Chang S-C. Fungal Diseases in Taiwan—National Insurance Data and Estimation. Journal of Fungi. 2019; 5(3):78. https://doi.org/10.3390/jof5030078
Chicago/Turabian StyleHuang, Yu-Shan, David W. Denning, Shu-Man Shih, Chao A. Hsiung, Un-In Wu, Hsin-Yun Sun, Pao-Yu Chen, Yee-Chun Chen, and Shan-Chwen Chang. 2019. "Fungal Diseases in Taiwan—National Insurance Data and Estimation" Journal of Fungi 5, no. 3: 78. https://doi.org/10.3390/jof5030078
APA StyleHuang, Y.-S., Denning, D. W., Shih, S.-M., Hsiung, C. A., Wu, U.-I., Sun, H.-Y., Chen, P.-Y., Chen, Y.-C., & Chang, S.-C. (2019). Fungal Diseases in Taiwan—National Insurance Data and Estimation. Journal of Fungi, 5(3), 78. https://doi.org/10.3390/jof5030078





