Histoplasma Responses to Nutritional Immunity Imposed by Macrophage Activation
Abstract
1. Introduction
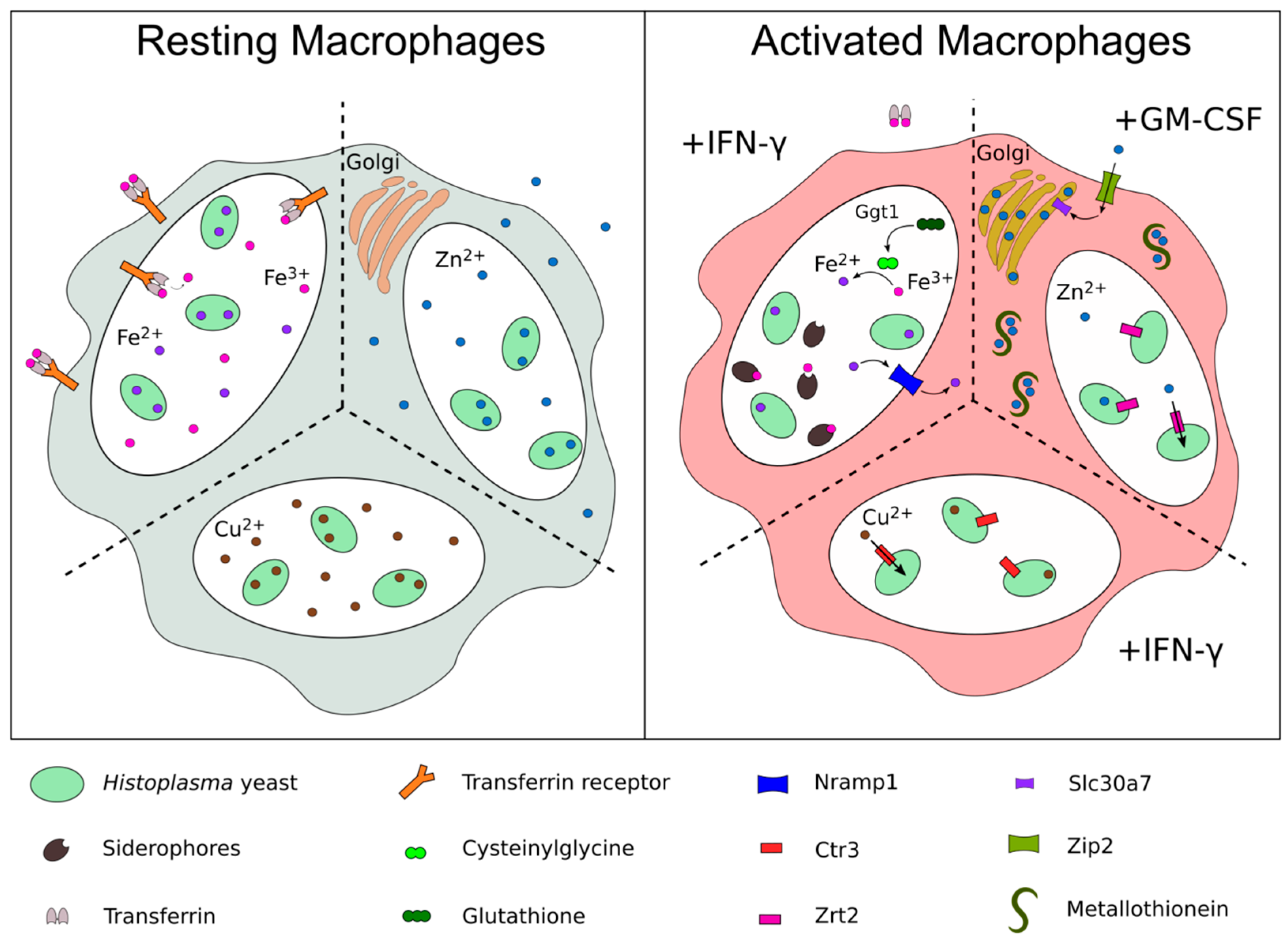
2. Acquisition of Zinc
3. Acquisition of Copper
4. Acquisition of Iron
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ajello, L. The medical mycological iceberg. HSMHA Health Rep. 1971, 86, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Garfoot, A.L.; Shen, Q.; Wüthrich, M.; Klein, B.S.; Rappleye, C.A. The Eng1 β-Glucanase Enhances Histoplasma Virulence by Reducing β-Glucan Exposure. MBio 2016, 7, e01388-15. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, E.D.; Smolnycki, K.A.; Youseff, B.H.; Rappleye, C.A. Redundant catalases detoxify phagocyte reactive oxygen and facilitate Histoplasma capsulatum pathogenesis. Infect. Immun. 2013, 81, 2334–2346. [Google Scholar] [CrossRef] [PubMed]
- Youseff, B.H.; Holbrook, E.D.; Smolnycki, K.A.; Rappleye, C.A. Extracellular superoxide dismutase protects Histoplasma yeast cells from host-derived oxidative stress. PLoS Pathog. 2012, 8, e1002713. [Google Scholar] [CrossRef]
- Garfoot, A.L.; Zemska, O.; Rappleye, C.A. Histoplasma capsulatum depends on de novo vitamin biosynthesis for intraphagosomal proliferation. Infect. Immun. 2014, 82, 393–404. [Google Scholar] [CrossRef]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Divanovic, S.; Caruso, J.A.; Deepe, G.S. IL-4 Induces Metallothionein 3- and SLC30A4-Dependent Increase in Intracellular Zn(2+) that Promotes Pathogen Persistence in Macrophages. Cell Rep. 2016, 16, 3232–3246. [Google Scholar] [CrossRef]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in fungal virulence. FEMS Microbiol. Rev. 2017, 42, fux050. [Google Scholar] [CrossRef]
- Winters, M.S.; Chan, Q.; Caruso, J.A.; Deepe, G.S. Metallomic analysis of macrophages infected with Histoplasma capsulatum reveals a fundamental role for zinc in host defenses. J. Infect. Dis. 2010, 202, 1136–1145. [Google Scholar] [CrossRef]
- Botella, H.; Peyron, P.; Levillain, F.; Poincloux, R.; Poquet, Y.; Brandli, I.; Wang, C.; Tailleux, L.; Tilleul, S.; Charrière, G.M.; et al. Mycobacterial P1-Type ATPases Mediate Resistance to Zinc Poisoning in Human Macrophages. Cell Host Microbe 2011, 10, 248–259. [Google Scholar] [CrossRef]
- Lane, T.E.; Wu-Hsieh, B.A.; Howard, D.H. Iron limitation and the gamma interferon-mediated antiHistoplasma state of murine macrophages. Infect. Immun. 1991, 59, 2274–2278. [Google Scholar]
- Newman, S.L.; Gootee, L. Colony-stimulating factors activate human macrophages to inhibit intracellular growth of Histoplasma capsulatum yeasts. Infect. Immun. 1992, 60, 4593–4597. [Google Scholar] [PubMed]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Caruso, J.A.; Deepe, G.S. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 2013, 39, 697–710. [Google Scholar] [CrossRef]
- Suhy, D.A.; Simon, K.D.; Linzer, D.I.; O’Halloran, T.V. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J. Biol. Chem. 1999, 274, 9183–9192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maret, W. Human metallothionein metallomics. J. Anal. At. Spectrom. 2008, 23, 1055–1062. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Stout, B.A.; Bates, M.E.; Bertics, P.J.; Malter, J.S. Granulocyte macrophage colony-stimulating factor and interleukin-5 activate STAT5 and induce CIS1 mRNA in human peripheral blood eosinophils. Am. J. Respir. Cell Mol. Biol. 2001, 24, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Huang, L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004, 447, 744–751. [Google Scholar] [CrossRef] [PubMed]
- LeVine, A.M.; Reed, J.A.; Kurak, K.E.; Cianciolo, E.; Whitsett, J.A. GM-CSF–deficient mice are susceptible to pulmonary group B streptococcal infection. J. Clin. Investig. 1999, 103, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R.; Sozzani, S.; Stine, J.T.; Luini, W.; D’Amico, G.; Allavena, P.; Chantry, D.; Mantovani, A. Divergent effects of interleukin-4 and interferon-gamma on macrophage-derived chemokine production: An amplification circuit of polarized T helper 2 responses. Blood 1998, 92, 2668–2671. [Google Scholar] [PubMed]
- Szymczak, W.A.; Deepe, G.S. The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J. Immunol. 2009, 183, 1964–1974. [Google Scholar] [CrossRef]
- Verma, A.; Kroetz, D.N.; Tweedle, J.L.; Deepe, G.S. Type II cytokines impair host defense against an intracellular fungal pathogen by amplifying macrophage generation of IL-33. Mucosal. Immunol. 2015, 8, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Dade, J.; DuBois, J.C.; Pasula, R.; Donnell, A.M.; Caruso, J.A.; Smulian, A.G.; Deepe, G.S. HcZrt2, a zinc responsive gene, is indispensable for the survival of Histoplasma capsulatum in vivo. Med. Mycol. 2016, 54, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Beucler, M.J.; Ray, S.C.; Rappleye, C.A. Macrophage activation by IFN-γ triggers restriction of phagosomal copper from intracellular pathogens. PLoS Pathog. 2018, 14, e1007444. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.R.; Waterman, S.R.; Qiu, J.; Bleher, R.; Williamson, P.R.; O’Halloran, T.V. A Copper Hyperaccumulation Phenotype Correlates with Pathogenesis in Cryptococcus neoformans. Metallomics 2013, 5, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [PubMed]
- García-Santamarina, S.; Thiele, D.J. Copper at the Fungal Pathogen-Host Axis. J. Biol. Chem. 2015, 290, 18945–18953. [Google Scholar] [CrossRef]
- Silva, M.B.D.; Thomaz, L.; Marques, A.F.; Svidzinski, A.E.; Nosanchuk, J.D.; Casadevall, A.; Travassos, L.R.; Taborda, C.P. Resistance of melanized yeast cells of Paracoccidioides brasiliensis to antimicrobial oxidants and inhibition of phagocytosis using carbohydrates and monoclonal antibody to CD18. Mem. Inst. Oswaldo Cruz 2009, 104, 644–648. [Google Scholar] [CrossRef]
- Salas, S.D.; Bennett, J.E.; Kwon-Chung, K.J.; Perfect, J.R.; Williamson, P.R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Gómez, B.L.; Youngchim, S.; Díez, S.; Aisen, P.; Zancopé-Oliveira, R.M.; Restrepo, A.; Casadevall, A.; Hamilton, A.J. Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect. Immun. 2002, 70, 5124–5131. [Google Scholar] [CrossRef]
- Ladomersky, E.; Khan, A.; Shanbhag, V.; Cavet, J.S.; Chan, J.; Weisman, G.A.; Petris, M.J. Host and Pathogen Copper-Transporting P-Type ATPases Function Antagonistically during Salmonella Infection. Infect. Immun. 2017, 85, e00351-17. [Google Scholar] [CrossRef]
- White, C.; Lee, J.; Kambe, T.; Fritsche, K.; Petris, M.J. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 2009, 284, 33949–33956. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Festa, R.A.; Ioerger, T.R.; Butler-Wu, S.; Sacchettini, J.C.; Darwin, K.H.; Samanovic, M.I. The copper-responsive RicR regulon contributes to Mycobacterium tuberculosis virulence. MBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Wolschendorf, F.; Ackart, D.; Shrestha, T.B.; Hascall-Dove, L.; Nolan, S.; Lamichhane, G.; Wang, Y.; Bossmann, S.H.; Basaraba, R.J.; Niederweis, M. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2011, 108, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Festa, R.A.; Chen, Y.-L.; Espart, A.; Palacios, Ò.; Espín, J.; Capdevila, M.; Atrian, S.; Heitman, J.; Thiele, D.J. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe 2013, 13, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Waterman, S.R.; Park, Y.-D.; Raja, M.; Qiu, J.; Hammoud, D.A.; O’Halloran, T.V.; Williamson, P.R. Role of CTR4 in the Virulence of Cryptococcus neoformans. MBio 2012, 3, e00285-12. [Google Scholar] [CrossRef] [PubMed]
- Waterman, S.R.; Hacham, M.; Hu, G.; Zhu, X.; Park, Y.-D.; Shin, S.; Panepinto, J.; Valyi-Nagy, T.; Beam, C.; Husain, S.; et al. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J. Clin. Investig. 2007, 117, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Byrd, T.F.; Horwitz, M.A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J. Clin. Investig. 1989, 83, 1457–1465. [Google Scholar] [CrossRef]
- Howard, D.H. Iron gathering by zoopathogenic fungi. FEMS Immunol. Med. Microbiol. 2004, 40, 95–100. [Google Scholar] [CrossRef][Green Version]
- Martínez-Garay, C.A.; de Llanos, R.; Romero, A.M.; Martínez-Pastor, M.T.; Puig, S. Responses of Saccharomyces cerevisiae Strains from Different Origins to Elevated Iron Concentrations. Appl. Environ. Microbiol. 2016, 82, 1906–1916. [Google Scholar] [CrossRef]
- Newman, S.L.; Gootee, L.; Brunner, G.; Deepe, G.S. Chloroquine induces human macrophage killing of Histoplasma capsulatum by limiting the availability of intracellular iron and is therapeutic in a murine model of histoplasmosis. J. Clin. Investig. 1994, 93, 1422–1429. [Google Scholar] [CrossRef]
- Timmerman, M.M.; Woods, J.P. Potential role for extracellular glutathione-dependent ferric reductase in utilization of environmental and host ferric compounds by Histoplasma capsulatum. Infect. Immun. 2001, 69, 7671–7678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cassat, J.E.; Skaar, E.P. Iron in Infection and Immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.R.; Gros, P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001, 9, 397–403. [Google Scholar] [CrossRef]
- Weiss, G.; Werner-Felmayer, G.; Werner, E.R.; Grünewald, K.; Wachter, H.; Hentze, M.W. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J. Exp. Med. 1994, 180, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, G.; Dlaska, M.; Barton, H.; Theurl, I.; Garimorth, K.; Weiss, G. Nramp1 Functionality Increases Inducible Nitric Oxide Synthase Transcription Via Stimulation of IFN Regulatory Factor 1 Expression. J. Immunol. 2003, 171, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, G.; Nairz, M.; Theurl, I.; Mair, S.; Bellmann-Weiler, R.; Barton, H.C.; Weiss, G. Modulation of macrophage iron transport by Nramp1 (Slc11a1). Immunobiology 2008, 212, 751–757. [Google Scholar] [CrossRef]
- Wu-Hsieh, B.A.; Howard, D.H. Inhibition of the intracellular growth of Histoplasma capsulatum by recombinant murine gamma interferon. Infect. Immun. 1987, 55, 1014–1016. [Google Scholar] [PubMed]
- Newman, S.L.; Smulian, A.G. Iron uptake and virulence in Histoplasma capsulatum. Curr. Opin. Microbiol. 2013, 16, 700–707. [Google Scholar] [CrossRef]
- Hilty, J.; George Smulian, A.; Newman, S.L. Histoplasma capsulatum utilizes siderophores for intracellular iron acquisition in macrophages. Med. Mycol. 2011, 49, 633–642. [Google Scholar]
- Hwang, L.H.; Seth, E.; Gilmore, S.A.; Sil, A. SRE1 regulates iron-dependent and -independent pathways in the fungal pathogen Histoplasma capsulatum. Eukaryotic Cell 2012, 11, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Burt, W.R. Identification of coprogen B and its breakdown products from Histoplasma capsulatum. Infect. Immun. 1982, 35, 990–996. [Google Scholar] [PubMed]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, J.R.; Skaar, E.P. Metals as phagocyte antimicrobial effectors. Curr. Opin. Immunol. 2019, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.H.; Rafie, R.; Tiwari, A.; Faull, K.F. Hydroxamate siderophores of Histoplasma capsulatum. Infect. Immun. 2000, 68, 2338–2343. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.H.; Mayfield, J.A.; Rine, J.; Sil, A. Histoplasma requires SID1, a member of an iron-regulated siderophore gene cluster, for host colonization. PLoS Pathog. 2008, 4, e1000044. [Google Scholar] [CrossRef] [PubMed]
- Zarnowski, R.; Cooper, K.G.; Brunold, L.S.; Calaycay, J.; Woods, J.P. Histoplasma capsulatum secreted gamma-glutamyltransferase reduces iron by generating an efficient ferric reductant. Mol. Microbiol. 2008, 70, 352–368. [Google Scholar] [CrossRef]
- Timmerman, M.M.; Woods, J.P. Ferric reduction is a potential iron acquisition mechanism for Histoplasma capsulatum. Infect. Immun. 1999, 67, 6403–6408. [Google Scholar]
- Ricci, V.; Giannouli, M.; Romano, M.; Zarrilli, R. Helicobacter pylori gamma-glutamyl transpeptidase and its pathogenic role. World J. Gastroenterol. 2014, 20, 630–638. [Google Scholar] [CrossRef]
- Morris, D.; Nguyen, T.; Kim, J.; Kassissa, C.; Khurasany, M.; Luong, J.; Kasko, S.; Pandya, S.; Chu, M.; Chi, P.-T.; et al. An Elucidation of Neutrophil Functions against Mycobacterium tuberculosis Infection. Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef]
- Rahman, I.; MacNee, W. Lung glutathione and oxidative stress: Implications in cigarette smoke-induced airway disease. Am. J. Physiol. 1999, 277, L1067–L1088. [Google Scholar] [CrossRef] [PubMed]
- Kosman, D.J. Redox Cycling in Iron Uptake, Efflux, and Trafficking. J. Biol. Chem. 2010, 285, 26729–26735. [Google Scholar] [CrossRef] [PubMed]
- Eissenberg, L.G.; Goldman, W.E.; Schlesinger, P.H. Histoplasma capsulatum modulates the acidification of phagolysosomes. J. Exp. Med. 1993, 177, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.L.; Gootee, L.; Hilty, J.; Morris, R.E. Human macrophages do not require phagosome acidification to mediate fungistatic/fungicidal activity against Histoplasma capsulatum. J. Immunol. 2006, 176, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Princiotto, J.V.; Zapolski, E.J. Difference between the two iron-binding sites of transferrin. Nature 1975, 255, 87. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.-A.A. Utilization and cell-surface binding of hemin by Histoplasma capsulatum. Can. J. Microbiol. 2002, 48, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Hilty, J.; Smulian, A.G.; Newman, S.L. The Histoplasma capsulatum vacuolar ATPase is required for iron homeostasis, intracellular replication in macrophages and virulence in a murine model of histoplasmosis. Mol. Microbiol. 2008, 70, 127–139. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brechting, P.J.; Rappleye, C.A. Histoplasma Responses to Nutritional Immunity Imposed by Macrophage Activation. J. Fungi 2019, 5, 45. https://doi.org/10.3390/jof5020045
Brechting PJ, Rappleye CA. Histoplasma Responses to Nutritional Immunity Imposed by Macrophage Activation. Journal of Fungi. 2019; 5(2):45. https://doi.org/10.3390/jof5020045
Chicago/Turabian StyleBrechting, Peter J., and Chad A. Rappleye. 2019. "Histoplasma Responses to Nutritional Immunity Imposed by Macrophage Activation" Journal of Fungi 5, no. 2: 45. https://doi.org/10.3390/jof5020045
APA StyleBrechting, P. J., & Rappleye, C. A. (2019). Histoplasma Responses to Nutritional Immunity Imposed by Macrophage Activation. Journal of Fungi, 5(2), 45. https://doi.org/10.3390/jof5020045




