High-Throughput Sequencing to Investigate Phytopathogenic Fungal Propagules Caught in Baited Insect Traps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Traps
2.2. Semiochemicals
2.3. Sample Processing and DNA Extraction
2.4. PCR, Purification and HTS
2.5. Bioinformatics
2.6. Species Subtraction
3. Results
3.1. Trees Associated with the Samples Processed
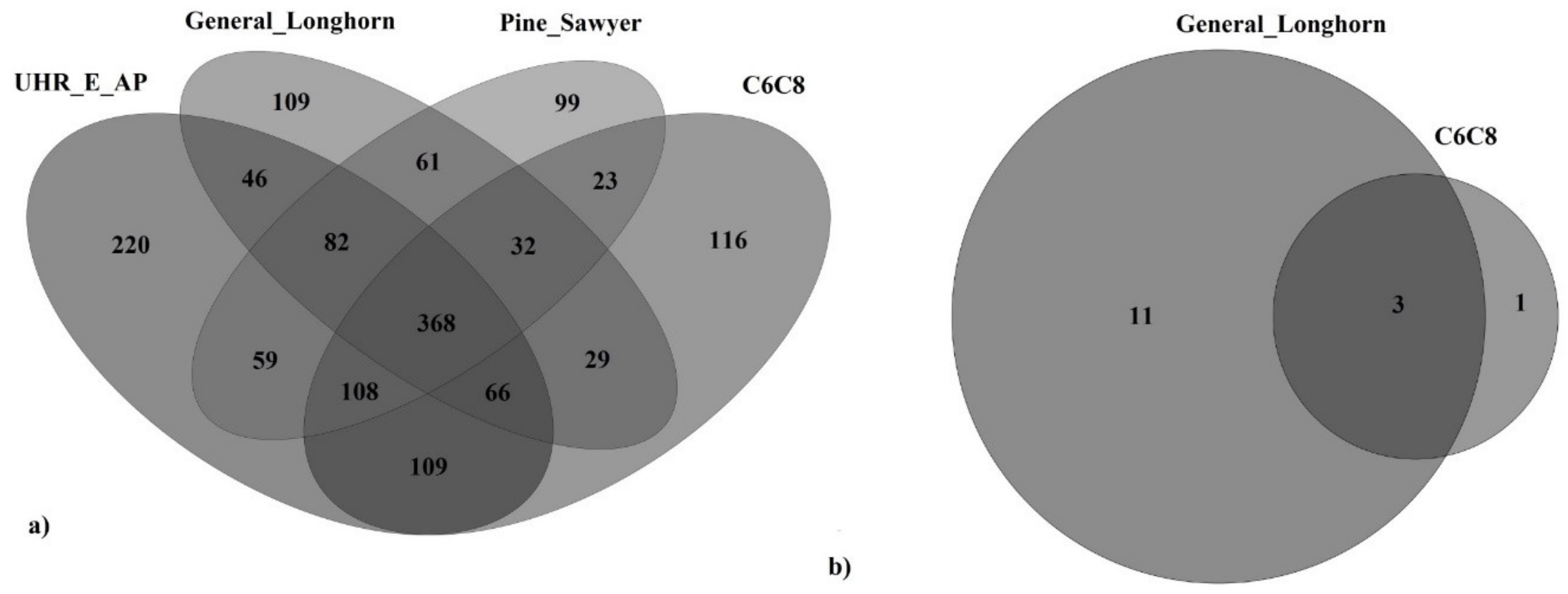
3.2. Fungal Diversity Associated with the Four Respective Semiochemicals Following Species Subtraction
3.3. Oomycete Diversity Recovered from Three of the Four Semiochemicals after Species Subtraction
3.4. Comparison of Fungal Diversity by Semiochemical
3.5. Comparison of Oomycete Diversity by Semiochemical
3.6. Variance of Fungal Diversity (PERMANOVA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Malacrinò, A.; Rassati, D.; Schena, L.; Mehzabin, R.; Battisti, A.; Palmeri, V. Fungal communities associated with bark and ambrosia beetles trapped at international harbours. Fungal Ecol. 2017, 28, 44–52. [Google Scholar] [CrossRef]
- Haack, R.A. Intercepted scolytidae (coleoptera) at us ports of entry: 1985–2000. Int.Pest Manag. Rev. 2001, 6, 253–282. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Bain, J.; Kimberley, M.; Knížek, M. Interception frequency of exotic bark and ambrosia beetles (coleoptera: Scolytinae) and relationship with establishment in new zealand and worldwide. Can. J. For. Res. 2006, 36, 289–298. [Google Scholar] [CrossRef]
- Haack, R.A. Exotic bark-and wood-boring coleoptera in the united states: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Gerson, H.; Illson-Skinner, B.; Turgeon, J. Live insects found in wood packaging materials after implementation of ispm 15. In Proceedings of the Forest Pest Management Forum, Ottawa, ON, Canada, 4–6 December 2012. [Google Scholar]
- Haack, R.A.; Britton, K.O.; Brockerhoff, E.G.; Cavey, J.F.; Garrett, L.J.; Kimberley, M.; Lowenstein, F.; Nuding, A.; Olson, L.J.; Turner, J. Effectiveness of the international phytosanitary standard ispm no. 15 on reducing wood borer infestation rates in wood packaging material entering the united states. PLoS ONE 2014, 9, e96611. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O. Invasive alien species in canadian forests. In Alien Invaders in Canada’s Waters, Wetlands, and Forests; Claudi, R., Nantel, P., Muckle-Jeffs, E., Eds.; Canadian Forest Service, Natural Resources Canada: Ottawa, ON, Canada, 2002; p. 320. [Google Scholar]
- Smith, G.; Hurley, J.; Sweeney, J.; Harrison, K.; MacKay, A. First North American record of the Palearctic species Tetropium fuscum (Fabricius) (coleoptera: Cerambycidae). In Proceedings of the U.S. Department of Agriculture Interagency Research Forum on Gypsy Moth and Other Invasive Species, Annapolis, MD, USA, 15–18 January 2002. [Google Scholar]
- Ryan, K.; de Groot, P.; Smith, S.M. Evidence of interaction between sirex noctilio and other species inhabiting the bole of pinus. Agric. For. Entomol. 2012, 14, 187–195. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef]
- Li, D.; Shi, J.; Lu, M.; Ren, L.; Zhen, C.; Luo, Y. Detection and identification of the invasive sirex noctilio (hymenoptera: Siricidae) fungal symbiont, amylostereum areolatum (russulales: Amylostereacea), in China and the stimulating effect of insect venom on laccase production by a. Areolatum YQL03. J. Econ. Entomol. 2015, 108, 1136–1147. [Google Scholar] [CrossRef]
- Natural Resources Canada: Mountain pine Beetle. Available online: http://www.nrcan.gc.ca/forests/fire-insects-disturbances/top-insects/13381 (accessed on 19 September 2018).
- Krokene, P.; Solheim, H. Pathogenicity of four blue-stain fungi associated with aggressive and nonaggressive bark beetles. Phytopathology 1998, 88, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Levieux, J.; Lieutier, F.; Moser, J.C.; Perry, T.J. Transportation of phytopathogenic fungi by the bark beetle ips sexdentatus boerner and associated mites. J. Appl. Entomol. 1989, 108, 1–11. [Google Scholar] [CrossRef]
- Allen, E.; Humble, L. Nonindigenous species introductions: A threat to Canada’s forests and forest economy. Can. J. Plant Pathol. 2002, 24, 103–110. [Google Scholar] [CrossRef]
- Negrón, J.F.; Witcosky, J.J.; Cain, R.J.; LaBonte, J.R.; Duerr, D.A.; McElwey, S.J.; Lee, J.C.; Seybold, S.J. The banded elm bark beetle: A new threat to the elms in North America. Am. Entomol. 2005, 51, 84–94. [Google Scholar] [CrossRef]
- Kubátová, A.; Kolařík, M.; Pažoutová, S. Phaeoacremonium rubrigenum—hyphomycete associated with bark beetles found in Czechia. Folia Microbiol. 2004, 49, 99–104. [Google Scholar] [CrossRef]
- Epstein, L.; Sukhwinder, K.; VanderGheynst, J. Botryosphaeria-related dieback and control investigated in noncoastal California grapevines. Calif. Agric. 2008, 62, 161–166. [Google Scholar] [CrossRef]
- Moyo, P. The Role of Arthropods in The Dispersal of Trunk Disease Pathogens Associated with Petri Disease and Esca. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2013. [Google Scholar]
- Callaham, R.; Shifrine, M. The yeasts associated with bark beetles. Forest Sci. 1960, 6, 146–154. [Google Scholar]
- Klimaszewski, J.; Morency, M.-J.; Labrie, P.; Seguin, A.; Langor, D.; Work, T.; Bourdon, C.; Thiffault, E.; Pare, D.; Newton, A.F.; et al. Molecular and microscopic analysis of the gut contents of abundant rove beetle species (coleoptera, staphylinidae) in the boreal balsam fir forest of Quebec, Canada. Zookeys 2013, 353, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Waalberg, M.E. Fungi Associated with Three Common Bark Beetle Species in Norwegian Scots Pine Forest. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2015. [Google Scholar]
- Nguyen, N.H.; Suh, S.-O.; Erbil, C.K.; Blackwell, M. Metschnikowia noctiluminum sp. Nov., metschnikowia corniflorae sp. Nov., and candida chrysomelidarum sp. Nov., isolated from green lacewings and beetles. Mycol. Res. 2006, 110, 346–356. [Google Scholar] [CrossRef]
- Zhu, X.-f.; Zhang, D.-p.; Yang, S.; Zhang, Q.-w. Candida xinjiangensis sp. Nov., a new anamorphic yeast species isolated from scolytus scheryrewi semenov in China. Arch. Microbiol. 2017, 199, 377–383. [Google Scholar] [CrossRef]
- Mecteau, M.R.; Joseph, A.; Tweddell, R.J. Effect of organic and inorganic salts on the growth and development of fusarium sambucinum, a causal agent of potato dry rot. Mycol. Res. 2002, 106, 688–696. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Roques, A.; Yart, A.; Fan, J.-T.; Sun, J.-H. Sentinel trees as a tool to forecast invasions of alien plant pathogens. PLoS 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.A. Ecological impacts of non-indigenous invasive fungi as forest pathogens. Biol. Invasions 2009, 11, 81–96. [Google Scholar] [CrossRef]
- Bilodeau, G.J.; Martin, F.N.; Coffey, M.D.; Blomquist, C.L. Development of a multiplex assay for genus- and species-specific detection of phytophthora based on differences in mitochondrial gene order. Phytopathology 2014, 104, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Hyder, N.; Coffey, M.D.; Stanghellini, M.E. Viability of oomycete propagules following ingestion and excretion by fungus gnats, shore flies, and snails. Plant Dis. 2009, 93, 720–726. [Google Scholar] [CrossRef]
- Webber, J.; Gibbs, J. Insect dissemination of fungal pathogens of trees. In Proceedings of the Insect-Fungus Interactions 14th Symposium of the Royal Entomological Society of London in Collaboration with the British Mycological Society, London, UK, 16–17 September 1987. [Google Scholar]
- Franco, F.P.; Moura, D.S.; Vivanco, J.M.; Silva-Filho, M.C. Plant–insect–pathogen interactions: A naturally complex ménage à trois. Curr. Opin. Microbiol. 2017, 37, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, J.; Tibboel, M.; Dicke, M.; Loon, J.J. Inoculation of susceptible and resistant potato plants with the late blight pathogen phytophthora infestans: Effects on an aphid and its parasitoid. Entomol. Exp. Appl. 2017, 163, 305–314. [Google Scholar] [CrossRef]
- Ellsbury, M.; Pratt, R.; Knight, W. Effects of single and combined infection of arrowleaf clover with bean yellow mosaic virus and a phytophthora sp. On reproduction and colonization by pea aphids (homoptera: Aphididae). Environ. Entomol. 1985, 14, 356–359. [Google Scholar] [CrossRef]
- Pratt, R.; Ellsbury, M.; Barnett, O.; Knight, W. Interactions of bean yellow mosaic virus and an aphid vector with phytophthora root diseases in arrowleaf clover. Phytopathology 1982, 72, 1189–1192. [Google Scholar] [CrossRef]
- Hulcr, J.; Dunn, R.R. The sudden emergence of pathogenicity in insect–fungus symbioses threatens naive forest ecosystems. Proc. R. Soc. Lond. B Biol. Sci. 2011, 278, 2866–2873. [Google Scholar] [CrossRef]
- Kanzaki, N.; Giblin-Davis, R.M. Pine wilt and red ring, lethal plant diseases caused by insect-mediated bursaphelenchus nematodes. In Vector-Mediated Transmission of Plant Pathogens; Brown, J.K., Ed.; APS Press: Saint-Paul, MN, USA, 2016. [Google Scholar]
- Barroso, V.M.; Rocha, L.O.; Reis, T.A.; Reis, G.M.; Duarte, A.P.; Michelotto, M.D.; Correa, B. Fusarium verticillioides and fumonisin contamination in bt and non-bt maize cultivated in brazil. Mycotoxin Res. 2017, 33, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hell, K.; Cardwell, K.; Setamou, M.; Schulthess, F. Influence of insect infestation on aflatoxin contamination of stored maize in four agroecological regions in benin. Afr. Entomol. 2000, 8, 169–177. [Google Scholar]
- Miller, S.S.; Reid, L.M.; Harris, L.J. Colonization of maize silks by fusarium graminearum, the causative organism of gibberella ear rot. Botany 2007, 85, 369–376. [Google Scholar] [CrossRef]
- Kirisits, T. Fungal associates of european bark beetles with special emphasis on the ophiostomatoid fungi. In Bark and Wood Boring Insects in Living Trees in Europe, A Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.-C., Evans, H.F., Eds.; Springer: Berlin, Germany, 2007; pp. 181–236. [Google Scholar]
- Adams, I.P.; Glover, R.H.; Monger, W.A.; Mumford, R.; Jackeviciene, E.; Navalinskiene, M.; Samuitiene, M.; Boonham, N. Next-generation sequencing and metagenomic analysis: A universal diagnostic tool in plant virology. Mol. Plant Pathol. 2009, 10, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Tolin, S.A.; Langham, M.A.C.; Gergerich, R.C. Beetle transmission: A unique alliance of virus, vector, and host. In Vector-Mediated Transmission of Plant Pathogens; Brown, J.K., Ed.; The American Phytopathological Society: Saint-Paul, MN, USA, 2016; pp. 131–146. [Google Scholar]
- Pinheiro, P.V.; Kliot, A.; Ghanim, M.; Cilia, M. Is there a role for symbiotic bacteria in plant virus transmission by insects? Curr. Opin. Insect Sci. 2015, 8, 69–78. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Oshima, K.; AMMAR, E.-D.; Kakizawa, S.; Kingdom, H.N.; Namba, S. Phytoplasmas: Bacteria that manipulate plants and insects. Mol. Plant Pathol. 2008, 9, 403–423. [Google Scholar] [CrossRef]
- Orlovskis, Z.; Canale, M.C.; Thole, V.; Pecher, P.; Lopes, J.R.; Hogenhout, S.A. Insect-borne plant pathogenic bacteria: Getting a ride goes beyond physical contact. Curr. Opin. Insect Sci. 2015, 9, 16–23. [Google Scholar] [CrossRef]
- Teale, S.A.; Wickham, J.D.; Zhang, F.; Su, J.; Chen, Y.; Xiao, W.; Hanks, L.M.; Millar, J.G. A male-produced aggregation pheromone of monochamus alternatus (coleoptera: Cerambycidae), a major vector of pine wood nematode. J. Econ. Entomol. 2011, 104, 1592–1598. [Google Scholar] [CrossRef]
- Roe, A.; Torson, A.; Bilodeau, G.; Bilodeau, P.; Blackburn, G.; Cui, M.; Cusson, M.; Doucet, D.; Griess, V.; Lafond, V.; et al. Biosurveillance of forest insects: Part I—Integration and application of genomic tools to the surveillance of non-native forest insects. J. Pest Sci. 2018, 92, 1–20. [Google Scholar]
- Douglas, H.; Bouchard, P.; Anderson, R.S.; de Tonnancour, P.; Vigneault, R.; Webster, R.P. New curculionoidea (coleoptera) records for Canada. Zookeys 2013. [Google Scholar] [CrossRef]
- Bullas-Appleton, E.; Kimoto, T.; Turgeon, J.J. Discovery of trichoferus campestris (coleoptera: Cerambycidae) in Ontario, Canada and first host record in North America. Can. Entomol. 2014, 146, 111–116. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency. CFIA Deploys Traps to Detect Emerald Ash Borer. Available online: http://www.inspection.gc.ca/about-the-cfia/newsroom/news-releases/emerald-ash-borer/eng/1323652437105/1323652437106 (accessed on 20 September 2018).
- Canadian Food Inspection Agency. Emerald Ash Borer – Agrilus Planipennis. Available online: http://www.inspection.gc.ca/plants/plant-pests-invasive-species/insects/emerald-ash-borer/eng/1337273882117/1337273975030 (accessed on 20 September 2018).
- Gullan, P.; Cranston, P. Semiochemicals: Kairomones, allomones, and synomones. In The Insects: An Outline of Entomology, Fifth Edition; John Wiley & Sons, Ltd.: Canberra, Australia, 2014; Volume 370. [Google Scholar]
- Ray, A.M.; Millar, J.G.; Moreira, J.A.; McElfresh, J.S.; Mitchell, R.F.; Barbour, J.D.; Hanks, L.M. North American species of cerambycid beetles in the genus neoclytus share a common hydroxyhexanone-hexanediol pheromone structural motif. J. Econ. Entomol. 2015, 108, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Petrice, T.R.; Haack, R.A.; Poland, T.M. Evaluation of three trap types and five lures for monitoring hylurgus ligniperda (coleoptera: Scolytidae) and other local scolytids in new york. Great Lakes Entomol. 2018, 37, 1. [Google Scholar]
- Brockerhoff, E.G.; Jones, D.C.; Kimberley, M.O.; Suckling, D.M.; Donaldson, T. Nationwide survey for invasive wood-boring and bark beetles (coleoptera) using traps baited with pheromones and kairomones. Forest Ecol.Manag. 2006, 228, 234–240. [Google Scholar] [CrossRef]
- Silk, P.J.; Sweeney, J.; Wu, J.; Price, J.; Gutowski, J.M.; Kettela, E.G. Evidence for a male-produced pheromone in tetropium fuscum (f.) and tetropium cinnamopterum (kirby) (coleoptera: Cerambycidae). Naturwissenschaften 2007, 94, 697. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.D.; Silk, P.; Grebennikov, V.; Mandelshtam, M. Efficacy of semiochemical-baited traps for detection of scolytinae species (coleoptera: Curculionidae) in the russian far east. Eur. J. Entomol. 2016, 113, 84. [Google Scholar] [CrossRef]
- Pajares, J.A.; Álvarez, G.; Ibeas, F.; Gallego, D.; Hall, D.R.; Farman, D.I. Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, monochamus galloprovincialis. J. Chem. Ecol. 2010, 36, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Ryall, K.; Silk, P.; Webster, R.P.; Gutowski, J.M.; Meng, Q.; Li, Y.; Gao, W.; Fidgen, J.; Kimoto, T.; Scarr, T. Further evidence that monochamol is attractive to monochamus (coleoptera: Cerambycidae) species, with attraction synergised by host plant volatiles and bark beetle (coleoptera: Curculionidae) pheromones. Can. Entomol. 2015, 147, 564–579. [Google Scholar] [CrossRef]
- Tremblay, É.D.; Duceppe, M.-O.; Bérubé, J.A.; Kimoto, T.; Bilodeau, G.J. Screening for exotic forest pathogens to increase survey capacity using metagenomics. Phytopathology 2018, 108, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, J.A.; Nicolas, G.G. Alien fungal species on asymptomatic live woody plant material imported into Canada. Can. J. Plant Pathol. 2015, 37, 67–81. [Google Scholar] [CrossRef]
- Aylward, J.; Steenkamp, E.T.; Dreyer, L.L.; Roets, F.; Wingfield, B.D.; Wingfield, M.J. A plant pathology perspective of fungal genome sequencing. IMA Fungus 2017, 8, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Barba, M.; Czosnek, H.; Hadidi, A. Historical perspective, development and applications of next-generation sequencing in plant virology. Viruses 2014, 6, 106–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ding, S.-W.; Zhang, Y.; Zhu, S. Identification of viruses and viroids by next-generation sequencing and homology-dependent and homology-independent algorithms. Annu. Rev. Phytopathol. 2015, 53, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Malacrinò, A.; Wisniewski, M.; Cacciola, S.O.; Schena, L. Metabarcoding: A powerful tool to investigate microbial communities and shape future plant protection strategies. Biol. Control 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Barnes, C.; Szabo, L.; Johnson, J.; Nguyen, K.; Floyd, C.; Kurle, J. Detection of phakopsora pachyrhizi DNA in rain using qpcr and a portable rain collector. Phytopathology 2006, 96, S9. [Google Scholar]
- Hambleton, S.; Tenuta, A.; Anderson, T.; Tropiano, R.; Bergeron, J.; Van Herk, C. Asian soybean rust monitoring program pays off in 2007 with first detections in Canada. In Proceedings of the 2007 National Soybean Rust Symposium, The American Phytopathological Society, Louisville, KY, USA, 12–14 December 2007. [Google Scholar]
- Szabo, L.J. Spore trapping: Technologies and results from 2007. In Proceedings of the 2007 National Soybean Rust Symposium, The American Phytopathological Society, Louisville, KY, USA, 12–14 December 2007. [Google Scholar]
- Barnes, C.W.; Szabo, L.J.; Bowersox, V. Identifying and quantifying phakopsora pachyrhizi spores in rain. Phytopathology 2009, 99, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.F.; Graham, E.E.; Wong, J.C.; Reagel, P.F.; Striman, B.L.; Hughes, G.P.; Paschen, M.A.; Ginzel, M.D.; Millar, J.G.; Hanks, L.M. Fuscumol and fuscumol acetate are general attractants for many species of cerambycid beetles in the subfamily lamiinae. Entomol. Exp. Appl. 2011, 141, 71–77. [Google Scholar] [CrossRef]
- Hayes, C.J.; DeGomez, T.E.; Clancy, K.M.; Williams, K.K.; McMillin, J.D.; Anhold, J.A. Evaluation of funnel traps for characterizing the bark beetle (coleoptera: Scolytidae) communities in ponderosa pine forests of north-central Arizona. J. Econ. Entomol. 2008, 101, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Government of British Columbia. Lindgren Funnel Traps. Available online: https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/forestry/forest-health/forest-health-docs/spruce-beetle-docs/spruce_beetle_funnel__traps.pdf (accessed on 10 February 2019).
- Kox, L.F.F.; van Brouwershaven, I.R.; van de Vossenberg, B.; van den Beld, H.E.; Bonants, P.J.M.; de Gruyter, J. Diagnostic values and utility of immunological, morphological, and molecular methods for in planta detection of phytophthora ramorum. Phytopathology 2007, 97, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Miles, T.D.; Martin, F.N.; Robideau, G.; Bilodeau, G.; Coffey, M. Systematic development of phytophthora species-specific mitochondrial diagnostic markers for economically important members of the genus. Plant Dis. 2017, 101, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.N.; Abad, Z.G.; Balci, Y.; Ivors, K. Identification and detection of phytophthora: Reviewing our progress, identifying our needs. Plant Dis. 2012, 96, 1080–1103. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D. PCR Purification: Ampure and Simple. Available online: http://www.keatslab.org/blog/pcrpurificationampureandsimple (accessed on 5 July 2017).
- Thermofisher. Ion Amplicon Library Preparation (Fusion Method). Available online: http://tools.thermofisher.com/content/sfs/manuals/4468326_IonAmpliconLibraryPrep_FusionMethod_UG.pdf (accessed on 13 April 2017).
- Breese, M.R.; Liu, Y. Ngsutils: A software suite for analyzing and manipulating next-generation sequencing datasets. Bioinformatics 2013, 29, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; Wit, P.; Sánchez-García, M.; Ebersberger, I.; Sousa, F. Improved software detection and extraction of its1 and its2 from ribosomal its sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Henrik Nilsson, R.; Tedersoo, L.; Lindahl, B.D.; Kjøller, R.; Carlsen, T.; Quince, C.; Abarenkov, K.; Pennanen, T.; Stenlid, J.; Bruns, T. Towards standardization of the description and publication of next-generation sequencing datasets of fungal communities. New Phytol. 2011, 191, 314–318. [Google Scholar] [CrossRef]
- Mundry, M.; Bornberg-Bauer, E.; Sammeth, M.; Feulner, P.G. Evaluating characteristics of de novo assembly software on 454 transcriptome data: A simulation approach. PLoS ONE 2012, 7, e31410. [Google Scholar] [CrossRef]
- Kõljalg, U.; Larsson, K.-H.; Abarenkov, K.; Nilsson, R.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; et al. Unite: A database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol 2005, 166, 1063–1068. [Google Scholar] [CrossRef]
- Kang, S. The Phytophthora Database Project. Available online: http://www.Phytophthoradb.Org (accessed on 17 February 2016).
- R Core Team (2013). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org/ (accessed on 20 December 2018).
- Chen, W.; Simpson, J.; Levesque, C. Ram: R for Amplicon-Sequencing-Based Microbial-Ecology R Package Version 1.2.1.3. Available online: http://cran.R-project.Org/package=ram (accessed on 17 February 2016).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-3. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 4 February 2019).
- Conway, J.R.; Lex, A.; Gehlenborg, N. Upsetr: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency. 2016-2017 Plant Protection Survey Report Executive Summary. Available online: http://www.inspection.gc.ca/plants/plant-pests-invasive-species/plant-pest-surveillance/2016-2017-plant-protection-survey-report/eng/1501889533057/1501889533572 (accessed on 10 February 2019).
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Jost, L. The New Synthesis of Diversity Indices and Similarity Measures. Available online: http://www.loujost.com/StatisticsandPhysics/DiversityandSimilarity/DiversitySimilarityHome.htm (accessed on 9 January 2017).
- Kéfi, S. Diversité. Available online: http://mon.univ-montp2.fr/claroline/backends/download.php?url=L0R5bmFtaXF1ZV9Db21tdW5hdXRlc19FY29zeXN06G1lcy9FY29sb2dpZUNvbW11bmF1dGVzX29ubGluZS5wZGY%3D&cidReset=true&cidReq=M2EFP (accessed on 13 February 2017).
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Zhan, J.; McDonald, B. Population structure of mycosphaerella graminicola: From lesions to continents. Phytopathology 2002, 92, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.B.; Kema, G.H. The genomes of mycosphaerella graminicola and m. Fijiensis. In Genomics of Plant-Associated Fungi: Monocot Pathogens; Dean, R.A., Lichens-Park, A., Kole, C., Eds.; Springer: Berlin, Germany, 2014; pp. 123–140. [Google Scholar]
- Gossen, B.D.; Carisse, O.; Kawchuk, L.M.; Van Der Heyden, H.; McDonald, M.R. Recent changes in fungicide use and the fungicide insensitivity of plant pathogens in Canada. Can. J. Plant Pathol. 2014, 36, 327–340. [Google Scholar] [CrossRef]
- Kennedy, R.; Wakeham, A.; Cullington, J. Production and immunodetection of ascospores of mycosphaerella brassicicola: Ringspot of vegetable crucifers. Plant Pathol. 1999, 48, 297–307. [Google Scholar] [CrossRef]
- Noms des Maladies des Plantes au Canada, Fourth ed.; SPPQ: Sainte-Anne-de-Bellevue QC, Canada, 2003; p. 340.
- French, J.R.; Roeper, R.A. Observations on trypodendron rufitarsis (coleoptera: Scolytidae) and its primary symbiotic fungus, ambrosiella ferruginea. Ann. Entomol. Soc. Am. 1972, 65, 282. [Google Scholar] [CrossRef]
- Jacobs, K.; Wingfield, M.J. Leptographium Species: Tree Pathogens, Insect Associates, and Agents of Blue-Stain; American Phytopathological Society: Saint-Paul, MN, USA, 2001. [Google Scholar]
- Kim, S.; Harrington, T.C.; Lee, J.C.; Seybold, S.J. Leptographium tereforme sp. Nov. And other ophiostomatales isolated from the root-feeding bark beetle hylurgus ligniperda in California. Mycologia 2011, 103, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.; Bergdahl, D.R.; Wingfield, M.J.; Halik, S.; Seifert, K.A.; Bright, D.E.; Wingfield, B.D. Leptographium wingfieldii introduced into North America and found associated with exotic tomicus piniperda and native bark beetles. Mycol. Res. 2004, 108, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Greif, M.D.; Gibas, C.F.C.; Currah, R.S. Leptographium piriforme sp. Nov., from a taxonomically diverse collection of arthropods collected in an aspen-dominated forest in western Canada. Mycologia 2006, 98, 771–780. [Google Scholar] [CrossRef]
- Paciura, D.; De Beer, Z.; Jacobs, K.; Zhou, X.; Ye, H.; Wingfield, M.J. Eight new leptographium species associated with tree-infesting bark beetles in China. Persoonia 2010, 25, 94. [Google Scholar] [CrossRef]
- McArt, S.H.; Koch, H.; Irwin, R.E.; Adler, L.S. Arranging the bouquet of disease: Floral traits and the transmission of plant and animal pathogens. Ecol. Lett. 2014, 17, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Pattemore, D.; Goodwin, R.; McBrydie, H.; Hoyte, S.; Vanneste, J. Evidence of the role of honey bees (apis mellifera) as vectors of the bacterial plant pathogen pseudomonas syringae. Australas. Plant Path. 2014, 43, 571–575. [Google Scholar] [CrossRef]
- Johnson, K.; Stockwell, V.; Burgett, D.; Sugar, D.; Loper, J. Dispersal of erwinia amylovora and pseudomonas fluorescens by honey bees from hives to apple and pear blossoms. Phytopathology 1993, 83, 478–484. [Google Scholar] [CrossRef]
- Card, S.; Pearson, M.; Clover, G. Plant pathogens transmitted by pollen. Australas. Plant Path. 2007, 36, 455–461. [Google Scholar] [CrossRef]
- Stelfox, D.; Williams, J.; Soehngen, U.; Topping, R. Transport of sclerotinia sclerotiorum ascospores by rapeseed pollen in Alberta. Plant Disease Reporter 1978, 62, 576–579. [Google Scholar]
- Hau, B.; De Vallavieille-Pope, C. Chapter 15: Wind-dispersed diseases. In The Epidemiology of Plant Diseases; Cooke, B.M., Jones, D.G., Kaye, B., Eds.; Springer: Dordrecht, Netherlands, 2006. [Google Scholar]
- Rassati, D.; Faccoli, M.; Petrucco Toffolo, E.; Battisti, A.; Marini, L. Improving the early detection of alien wood-boring beetles in ports and surrounding forests. J. Appl. Ecol. 2015, 52, 50–58. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Leger, R.J.S.; Stuart, A.; Gowanlock, K. Nuclear rdna phylogeny in the fungal genus verticillium and its relationship to insect and plant virulence, extracellular proteases and carbohydrases. Microbiology 1999, 145, 955–963. [Google Scholar] [CrossRef]
- Tiberi, R.; Panzavolta, T.; Bracalini, M.; Ragazzi, A.; Ginetti, B.; Moricca, S. Interactions between insects and fungal pathogens of forest and ornamental trees. Ital. J. Entomol. 2016, 45, 54–65. [Google Scholar]
- Inderbitzin, P.; Bostock, R.M.; Davis, R.M.; Usami, T.; Platt, H.W.; Subbarao, K.V. Phylogenetics and taxonomy of the fungal vascular wilt pathogen verticillium, with the descriptions of five new species. PLoS ONE 2011, 6, e28341. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Subbarao, K.V. Verticillium systematics and evolution: How confusion impedes verticillium wilt management and how to resolve it. Phytopathology 2014, 104, 564–574. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Rossman, A.Y. Where are all the undescribed fungi? Phytopathology 1997, 87, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Rossman, A.; Palm, M. Why are Phytophthora and Other Oomycota Not True Fungi? The American Phytopathological Society, 2006. Available online: https://www.apsnet.org/edcenter/intropp/pathogengroups/pages/oomycetes.aspx (accessed on 10 February 2019).
- Blackwell, M. The fungi: 1, 2, 3… 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Donahoo, R.; Blomquist, C.L.; Thomas, S.L.; Moulton, J.K.; Cooke, D.E.; Lamour, K.H. Phytophthora foliorum sp. Nov., a new species causing leaf blight of azalea. Mycol. Res. 2006, 110, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Canadian Food Inspection Agency. Pest Categorization Phytophthora foliorum Donahoo & Lamour Leaf Blight of Azalea. 2015. Available online: https://collab.cfia-acia.inspection.gc.ca/cfia-acia/inspection/PHRA/Shared%20Documents/PHRA%20Final%20Docs/2015/2015-79/Phytophthora%20foliorum%20-%20Pest%20Categorization%202015-79.pdf (accessed on 10 February 2019).
- Rahman, M.Z.; Uematsu, S.; Suga, H.; Kageyama, K. Diversity of phytophthora species newly reported from Japanese horticultural production. Mycoscience 2015, 56, 443–459. [Google Scholar] [CrossRef]
- McKeever, K.; Chastagner, G. A survey of phytophthora spp. Associated with abies in us christmas tree farms. Plant Dis. 2016, 100, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 10 February 2019).
- Gonzalez, F. Symbiosis between yeasts and insects. Crop Prod. Sci. 2014, 3, 1–52. [Google Scholar]
- El-Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Ultrastructural and cytochemical aspects of the biological control of botrytis cinerea by candida saitoana in apple fruit. Phytopathology 1998, 88, 282–291. [Google Scholar] [CrossRef]
- Droby, S.; Vinokur, V.; Weiss, B.; Cohen, L.; Daus, A.; Goldschmidt, E.; Porat, R. Induction of resistance to penicillium digitatum in grapefruit by the yeast biocontrol agent candida oleophila. Phytopathology 2002, 92, 393–399. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef]
- Malacrinò, A. Meta-omics tools in the world of insect-microorganism interactions. Biology 2018, 7, 50. [Google Scholar] [CrossRef]
- Augustyniuk-Kram, A.; Kram, K.J. Entomopathogenic fungi as an important natural regulator of insect outbreaks in forests. In Forest Ecosystems-More than Just Trees; Blanco, J.A., Ed.; InTechOpen: London, UK, 2012. [Google Scholar]
- Liu, H.; Bauer, L.S. Microbial control of emerald ash borer, agrilus planipennis (coleoptera: Buprestidae) with beauveria bassiana strain gha: Greenhouse and field trials. Biol. Control 2008, 45, 124–132. [Google Scholar] [CrossRef]
| Diversity | Evenness | |||
|---|---|---|---|---|
| Fungi | Oomycetes | Fungi | Oomycetes | |
| GL vs C6C8 | 0.242 | 0.046 * | 0.615 | 0.0361 * |
| PS vs C6C8 | 0.006 * | 0.112 | 0.615 | 0.0924 |
| UHR vs C6C8 | 0.586 | 0.504 | 0.065 | 0.8886 |
| PS vs GL | 0.104 | 0.504 | 0.932 | 0.5825 |
| UHR vs GL | 0.104 | 0.046 * | 0.017 * | 0.0076 * |
| PS vs UHR | 0.002 * | 0.125 | 0.017 * | 0.0293 * |
| Diversity | df | R2 | F | p Value |
|---|---|---|---|---|
| Area a | 2 | 0.02 | 2.204 | 0.055 |
| Lure b | 3 | 0.05 | 3.105 | 0.005 * |
| Lure Set c | 1 | 0.02 | 3.825 | 0.014 * |
| Province d | 4 | 0.10 | 4.712 | 0.001 * |
| Year e | 2 | 0.02 | 3.412 | 0.025 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremblay, É.D.; Kimoto, T.; Bérubé, J.A.; Bilodeau, G.J. High-Throughput Sequencing to Investigate Phytopathogenic Fungal Propagules Caught in Baited Insect Traps. J. Fungi 2019, 5, 15. https://doi.org/10.3390/jof5010015
Tremblay ÉD, Kimoto T, Bérubé JA, Bilodeau GJ. High-Throughput Sequencing to Investigate Phytopathogenic Fungal Propagules Caught in Baited Insect Traps. Journal of Fungi. 2019; 5(1):15. https://doi.org/10.3390/jof5010015
Chicago/Turabian StyleTremblay, Émilie D., Troy Kimoto, Jean A. Bérubé, and Guillaume J. Bilodeau. 2019. "High-Throughput Sequencing to Investigate Phytopathogenic Fungal Propagules Caught in Baited Insect Traps" Journal of Fungi 5, no. 1: 15. https://doi.org/10.3390/jof5010015
APA StyleTremblay, É. D., Kimoto, T., Bérubé, J. A., & Bilodeau, G. J. (2019). High-Throughput Sequencing to Investigate Phytopathogenic Fungal Propagules Caught in Baited Insect Traps. Journal of Fungi, 5(1), 15. https://doi.org/10.3390/jof5010015





