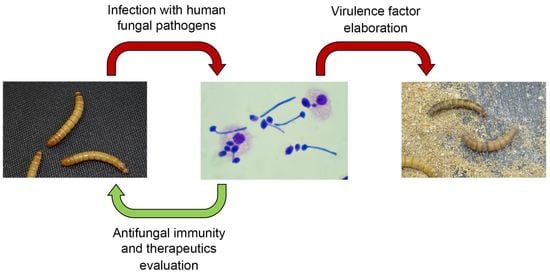
An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor
Abstract
1. Alternative Methods of Infection
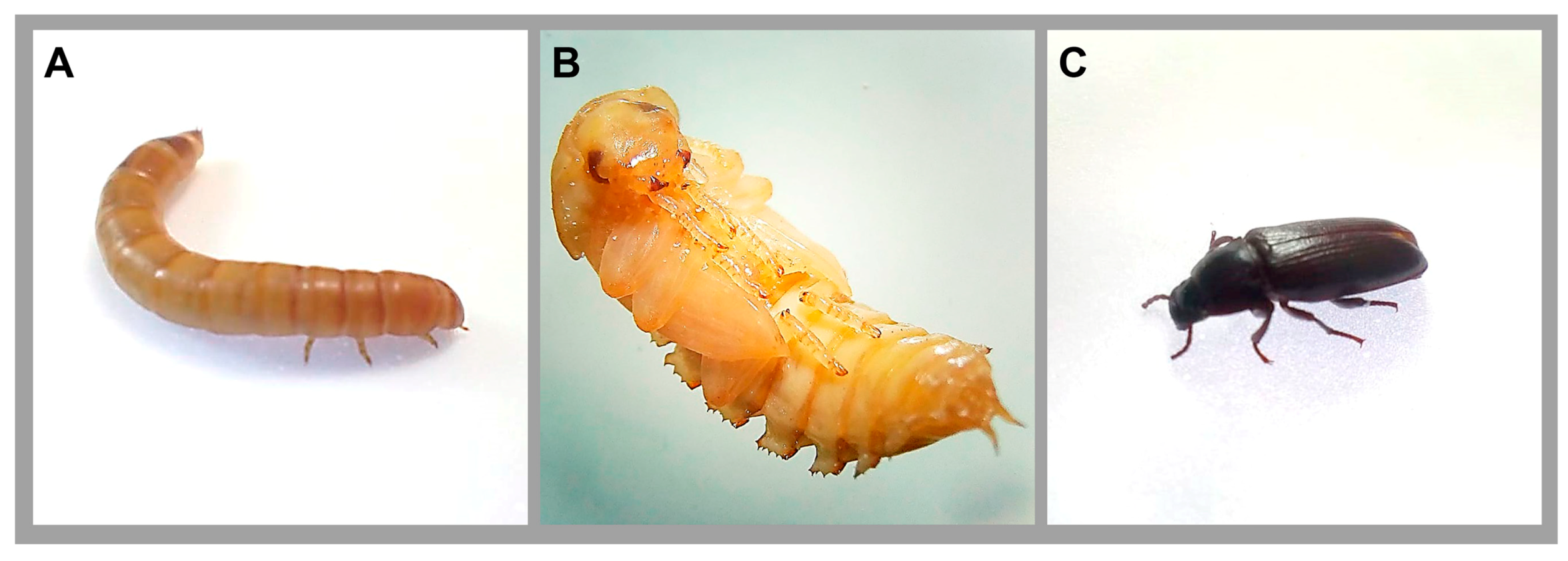
2. Tenebrio molitor
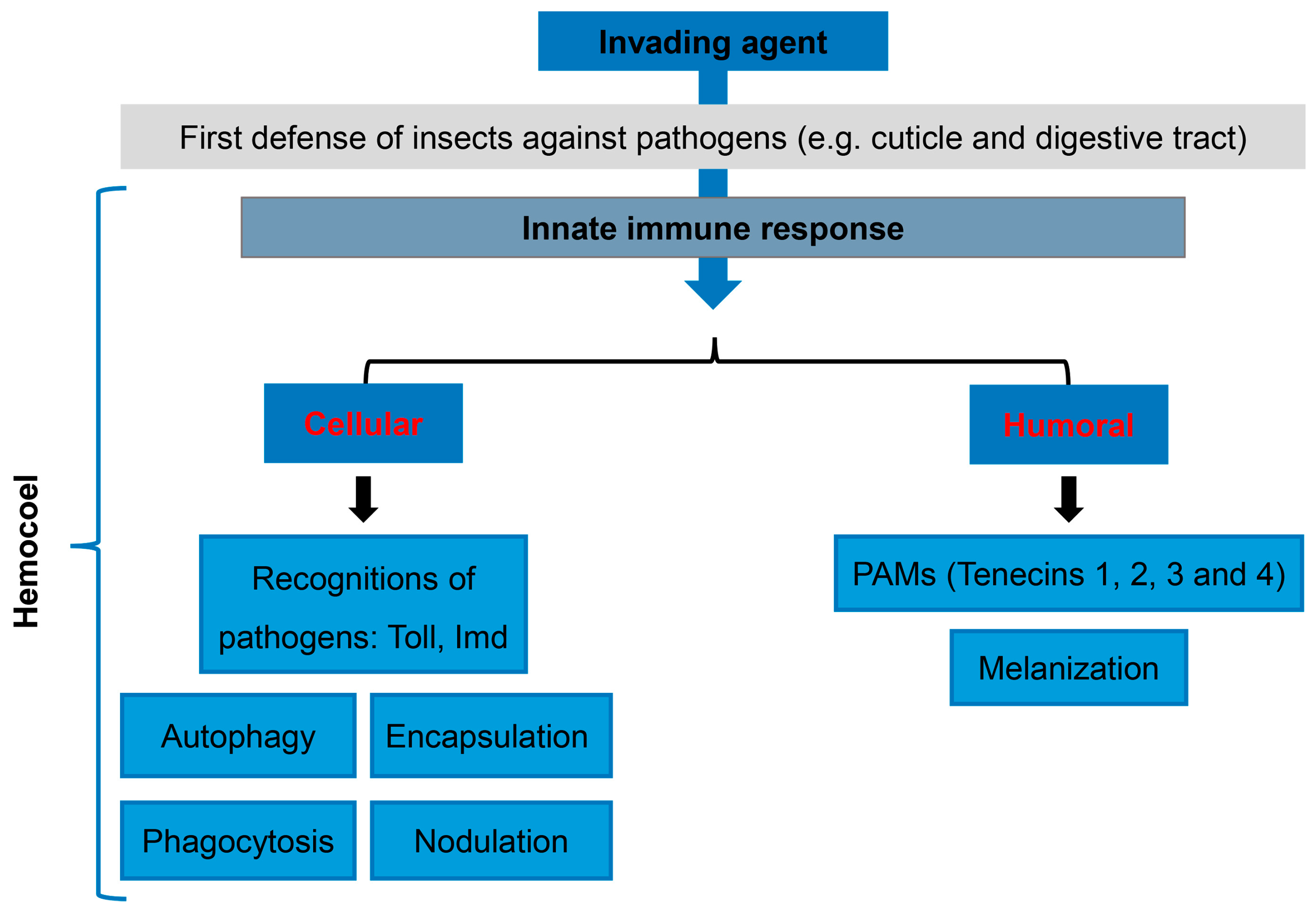
3. Cellular and Humoral Immunology of T. molitor as a Tool to Study Human Immunity
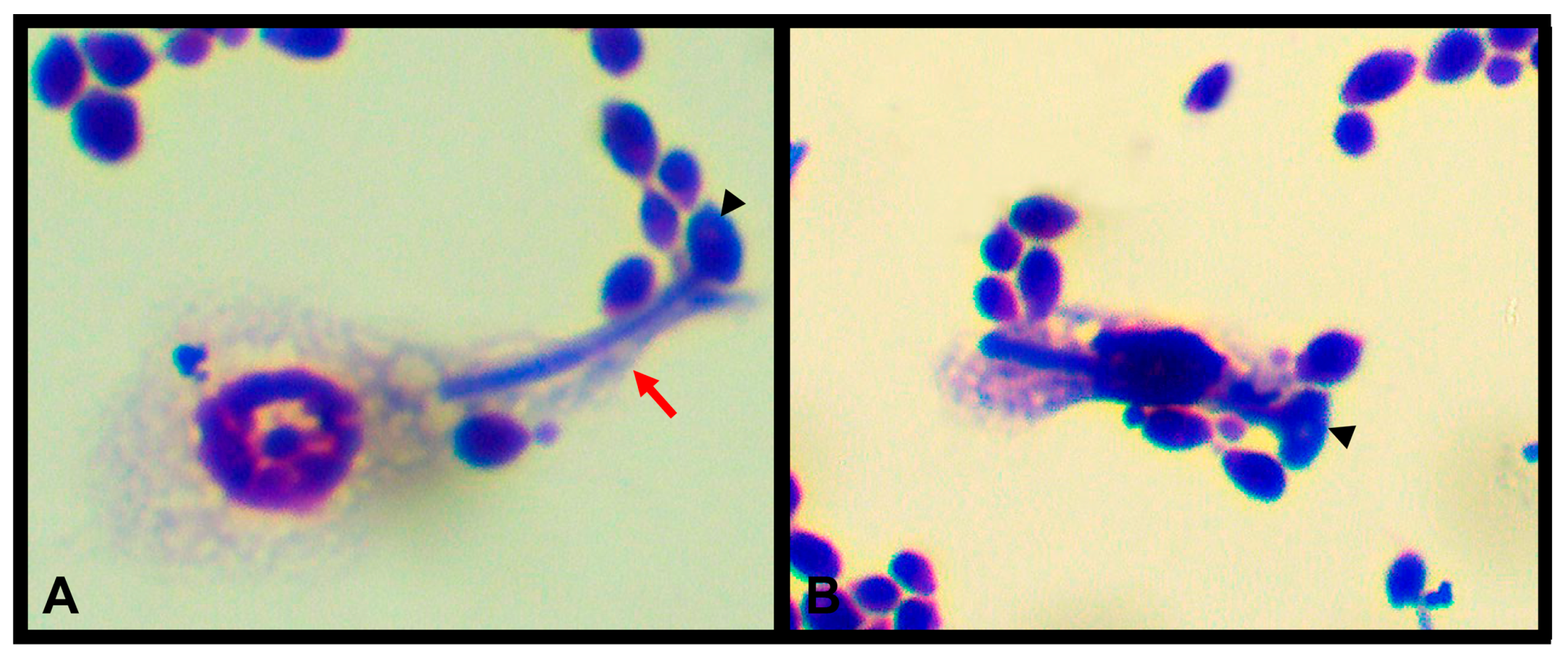
4. T. molitor as an Alternative Host in the Study of Human Pathogenic Fungi
5. Investigating Physiological Changes Due to Ingestion of Mycotoxins
6. Studying Infections Caused by Fungi of Clinical Interest and Evaluating the Antifungal Activity of Compounds
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hendriksen, C.F. Replacement, reduction and refinement alternatives to animal use in vaccine potency measurement. Expert Rev. Vaccines 2009, 8, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Giacomotto, J.; Segalat, L. High-throughput screening and small animal models, where are we? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Rollin, B.E. Toxicology and new social ethics for animals. Toxicol. Pathol. 2003, 31, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Franco, N.H. Animal experiments in biomedical research: A historical perspective. Animals (Basel) 2013, 19, 238–273. [Google Scholar] [CrossRef] [PubMed]
- Balls, M. Replacement of animal procedures: Alternatives in research, education and testing. Lab. Anim. 1994, 28, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Trevijano-Contador, N.; Zaragoza, O. Expanding the use of alternative models to investigate novel aspects of immunity to microbial pathogens. Virulence 2014, 15, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Vilmos, P.; Kurucz, E. Insect immunity: Evolutionary roots of the mammalian innate immune system. Immunol. Lett. 1998, 62, 59–66. [Google Scholar] [CrossRef]
- Browne, N.; Heelan, M.; Kavanagh, K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013, 1, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Tokura, A.; Fu, G.S.; Sakamoto, M.; Endo, H.; Tanaka, S.; Kikuta, S.; Tabunoki, H.; Sato, R. Factors functioning in nodule melanization of insects and their mechanisms of accumulation in nodules. J. Insect Physiol. 2014, 60, 40–49. [Google Scholar] [CrossRef]
- Dubovskii, I.M.; Grizanova, E.V.; Chertkova, E.A.; Slepneva, I.A.; Komarov, D.A.; Vorontsova, Y.L.; Glupov, V.V. Generation of reactive oxygen species and activity of antioxidants in hemolymph of the moth larvae Galleria mellonella (L.) (Lepidoptera: Piralidae) at development of the process of encapsulation. Zh. Evol. Biokhim. Fiziol. 2010, 46, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Lionakis, M.S. Drosophila and Galleria insect model hosts: New tools for the study of fungal virulence, pharmacology and immunology. Virulence 2011, 2, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Sanders, S.E. Invertebrate models for biomedical research, testing, and education. ILAR J. 2011, 52, 126–152. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.S. Pests: Class Insecta. Pests of Stored Foodstuffs and Their Control; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2003; pp. 135–315. ISBN 0-306-48131-6. [Google Scholar]
- Ghaly, A.E.; Alkoaik, F.N. The Yellow Mealworm as a Novel Source of Protein. Am. J. Agric. Biol. Sci. 2009, 4, 319–331. [Google Scholar] [CrossRef]
- Park, J.B.; Choi, W.H.; Kim, S.H.; Jin, H.J.; Han, Y.S.; Lee, Y.S.; Kim, N.J. Developmental characteristics of Tenebrio molitor larvae (Coleoptera: Tenebrionidae) in different instars. Int. J. Ind. Entomol. 2014, 28, 5–9. [Google Scholar] [CrossRef]
- Cotton, R.T. Notes on the biology of the meal worms Tenebrio molitor Linne and T. obscures Fab. Ann. Entomol. Soc. Am. 1927, 20, 81–86. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Kay, S.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Morphometric Analysis of Instar Variation in Tenebrio molitor (Coleoptera: Tenebrionidae). Ann. Entomol. Soc. Am. 2015, 108, 146–159. [Google Scholar] [CrossRef]
- Ludwig, D. Effect of temperature and parental age in the life cycle of the mealworm, Tenebrio molitor Linnaeus (Coleoptera, Tenebrionidae). Ann. Entomol. Soc. Am. 1956, 49, 12–15. [Google Scholar] [CrossRef]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Urs, K.C.D.; Hopkins, T.L. Effect of moisture on growth rate and development of two strains of Tenebrio molitor L. (Coleoptera, Tenebrionidae). J. Stored Prod. Res. 1973, 8, 291–297. [Google Scholar] [CrossRef]
- Loudon, C. Development of Tenebrio molitor in low oxygen levels. J. Insect Physiol. 1988, 34, 97–103. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Developmental plasticity in Tenebrio molitor (Coleoptera: Tenebrionidae): Analysis of instar variation in number and development time under different diets. J. Entomol. Sci. 2010, 45, 75–90. [Google Scholar] [CrossRef]
- Connat, J.L.; Delbecque, J.P.; Glitho, I.; Delachambre, J. The onset of metamorphosis on Tenebrio molitor larvae (Insecta, Coleoptera) under grouped, isolated, and starved conditions. J. Insect Physiol. 1991, 37, 653–662. [Google Scholar] [CrossRef]
- Cotton, R.T. Pests of Stored Grain and Grain Products; Burgess Publishing Company: Minneapolis, MN, USA, 1963; p. 318. [Google Scholar]
- Philips, T.W.; Throne, J.E. Biorational Approaches to Managing Stored-Product Insect. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Naturforsch. 2017, 72, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.K.; Liew, F.L.; Ang, L.P.; Wong, K.W. Potential of mealworm (Tenebrio molitor) as an alternative protein source in practical diets for African catfish, Clarias gariepinus. Aquac. Res. 2001, 32, 273–280. [Google Scholar] [CrossRef]
- Font, E.; Molina-Borja, M. Ultraviolet reflectance of color patches in Gallotia galloti lizards from Tenerife, Canary Islands. In The Biology of Lacertid Lizards: Evolutionary and Ecological Perspectives; Mellado, V.P., Ed.; Institut Menorquí d’Estudis: Balearic Islands, Spain, 2004; Volume 8, pp. 201–221. ISBN 978-8495718204. [Google Scholar]
- Martinson, T.J.; Flaspohler, D.J. Winter bird feeding and localized predation on simulated bark-dwelling arth. Wildl. Soc. Bull. 2003, 31, 510–516. [Google Scholar]
- McCartney, J.; Stringer, I.A.N.; Potter, M.A. Feeding activity in captive New Zealand lesser short-tailed bats (Mystacina tuberculata). N. Z. J. Zool. 2007, 34, 227–238. [Google Scholar] [CrossRef]
- Kim, H.W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Adámková, A.; Mlček, J.; Kouřimská, L.; Borkovcová, M.; Bušina, T.; Adámek, M.; Bednářová, M.; Krajsa, J. Nutritional Potential of Selected Insect Species Reared on the Island of Sumatra. Int. J. Environ. Res. Public Health 2017, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Anggraeni, T.; Ratcliffe, N.A. Studies on cell-cell co-operation during phagocytosis by purified haemocyte populations of the wax moth, Galleria mellonella. J. Insect Physiol. 1991, 37, 453–460. [Google Scholar] [CrossRef]
- Urbański, A.; Adamski, Z.; Rosiński, G. Developmental changes in haemocyte morphology in response to Staphylococcus aureus and latex beads in the beetle Tenebrio molitor L. Micron 2018, 104, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.A. Cellular defense responses of insects: Unresolved problems. In Parasites and Pathogens of Insects; Beckage, N.E., Thompson, S.N., Federici, B.A., Eds.; Academic Press: San Diego, CA, USA, 1993; Volume 1, pp. 267–304. ISBN 9780080916491. [Google Scholar]
- Strand, M.R.; Pech, L.L. Immunological basis for compatibility in parasitoid–host relationships. Annu. Rev. Entomol. 1995, 40, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Pech, L.L.; Strand, M.R. Plasmatocyte from the moth Pseudoplusia includens induce apoptosis of granular cells. J. Insect Physiol. 2000, 42, 1565–1573. [Google Scholar] [CrossRef]
- Ribeiro, C.; Brehelin, M. Insect hemocytes: What type of cell is that? J. Insect Physiol. 2006, 52, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Oczypok, E.A.; Oury, T.D.; Chu, C.T. It’s a cell-eat-cell world, Autophagy and phagocytosis. Am. J. Pathol. 2013, 182, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- .Tindwa, H.; Jo, Y.H.; Patnaik, B.B.; Lee, Y.S.; Kang, S.S.; Han, Y.S. Molecular cloning and characterization of autophagy-related gene TmATG8 in Listeria-invaded hemocytes of Tenebrio molitor. Dev. Comp. Immunol. 2015, 51, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.-M.; Yoshimori, T.; JO, E.-K. Autophagy and bacterial infectious diseases. Exp. Mol. Med. 2012, 44, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Dubovskiy, I.M.; Kryukova, N.A.; Glupov, V.V.; Ratcliffe, N.A. Encapsulation and nodulation in insects. Invertebr. Surviv. J. 2016, 13, 229–246. [Google Scholar]
- Sugumaran, H. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 2002, 15, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Armitage, S.A.O.; Siva-Jothy, M.T. Immune function responds to selection for cuticular colour in Tenebrio molitor. Heredity (Edinb.) 2005, 94, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.I.; Siva-Jothy, M.T. Density-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): Cuticular melanization is an indicator of investment in immunity. Proc. Biol. Sci. 2000, 22, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Auvynet, C.; Rosenstein, Y. Multifunctional host defense peptides: Antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J. 2009, 276, 6497–6508. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Moon, H.J.; Kurata, S.; Kurama, T.; Natori, S.; Lee, B.L. Purification and molecular cloning of cDNA for an inducible antibacterial protein of larvae of a coleopteran insect, Holotrichia diomphalia. J. Biochem. 1994, 115, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.B.; Kim, C.H.; Lee, H.; Kwon, H.M.; Park, J.W.; Ryu, J.H.; Kurokawa, K.; Ha, N.C.; Lee, W.J.; Lemaitre, B.; et al. Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. J. Biol. Chem. 2009, 284, 19474–19481. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Chung, T.J.; Park, C.W.; Hahn, Y.; Chung, J.H.; Lee, B.L.; Han, D.M.; Jung, Y.H.; Kim, S.; Lee, Y. Structure and expression of the tenecin 3 gene in Tenebrio molitor. Biochem. Biophys. Res. Commun. 1996, 218, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.; Kim, D.H.; Suh, J.Y.; Chung, J.H.; Lee, B.L.; Lee, Y.; Choi, B.S. Structural characteristics of tenecin 3, an insect antifungal protein. Biochem. Mol. Biol. Int. 1999, 47, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, D.G.; Kim, K.L.; Lee, Y. Internalization of tenecin 3 by a fungal cellular process is essential for its fungicidal effect on Candida albicans. Eur. J. Biochem. 2001, 268, 4449–4458. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.H.; Kurokawa, K.; So, Y.I.; Hwang, H.O.; Kim, M.S.; Park, J.W.; Jo, Y.H.; Lee, Y.S.; Lee, B.L. Purification and characterization of tenecin 4, a new anti-Gram-negative bacterial peptide, from the beetle Tenebrio molitor. Dev. Comp. Immunol. 2012, 36, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Lee, S.Y.; Kurata, S.; Natori, S.; Lee, B.L. Purification Antibacterial molitorl and Molecular Protein from Cloning Larvae of cDNA for an Inducible of the Coleopteran, Tenebrio molitor. J. Biochem. 1994, 116, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Patnaik, B.B.; Seo, G.W.; Kang, S.M.; Lee, Y.S.; Lee, B.L.; Han, Y.S. Identification and expression analysis of a novel R-type lectin from the coleopteran beetle, Tenebrio molitor. J. Invertebr. Pathol. 2013, 114, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Role of mini-host models in the study of medically important fungi. Lancet Infect. Dis. 2007, 7, 42–55. [Google Scholar] [CrossRef]
- Maistrou, S.; Paris, V.; Jensen, A.B.; Rolff, J.; Meyling, N.V.; Zanchi, C. A constitutively expressed antifungal peptide protects Tenebrio molitor during a natural infection by the entomopathogenic fungus Beauveria bassiana. Dev. Comp. Immunol. 2018, 86, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Jo, Y.H.; Seong, J.H.; Park, K.B.; Noh, M.Y.; Cho, J.H.; Ko, H.J.; Kim, C.E.; Tindwa, H.; Patnaik, B.B.; et al. TmSR-C, scavenger receptor class C, plays a pivotal role in antifungal and antibacterial immunity in the coleopteran insect Tenebrio molitor. Insect Biochem. Mol. Biol. 2017, 89, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Moret, Y.; Siva-Jothy, M.T. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc. Biol. Sci. 2003, 270, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Krams, I.; Daukste, J.; Kivleniece, I.; Krama, T.; Rantala, M.J. Previous encapsulation response enhances within individual protection against fungal parasite in the mealworm beetle Tenebrio molitor. Insect Sci. 2013, 20, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.M.; Rivas, G.A.; Hernández-Quintero, A.; Hernández, A.G.; Guzmán, J.C.T.; Mendoza, H.L.; Contreras-Garduño, J. The occurrence of immune priming can be species-specific in entomopathogens. Microb Pathog. 2018, 118, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Dhinaut, J.; Chogne, M.; Moret, Y. Trans-generational immune priming in the mealworm beetle protects eggs through pathogen-dependent mechanisms imposing no immediate fitness cost for the offspring. Dev. Comp. Immunol. 2018, 79, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Desalermos, A.; Fuchs, B.B.; Mylonakis, E. Selecting an invertebrate model host for the study of fungal pathogenesis. PLoS Pathog. 2012, 8, e1002451. [Google Scholar] [CrossRef] [PubMed]
- Piyankarage, S.C.; Augustin, H.; Grosjean, Y.; Featherstone, D.E.; Shippy, S.A. Hemolymph amino acid analysis of individual Drosophila larvae. Anal. Chem. 2008, 80, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Oppert, B.; Dowd, S.E.; Bouffard, P.; Li, L.; Conesa, A.; Lorenzen, M.D.; Toutges, M.; Marshall, J.; Huestis, D.L.; Fabrick, J.; et al. Transcriptome profiling of the intoxication response of Tenebrio molitor larvae to Bacillus thuringiensis Cry3Aa protoxin. PLoS ONE 2012, 7, e34624. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J. Toxicity of molds to the larvae of Tenebrio molitor. J. Invertebr. Pathol. 1973, 21, 112–113. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Cardwell, K.; Hounsa, A.; Egal, S.; Turner, P.C.; Hall, A.J.; Wild, C.P. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. BMJ 2002, 325, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hounsa, A.; Egal, S.; Turner, P.C.; Sutcliffe, A.E.; Hall, A.J.; Cardwell, K.; Wild, C.P. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environ. Health Perspect. 2004, 112, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Helferich, W.G.; Garrett, W.N.; Hsieh, D.P.H.; Baldwin, R.L. Feedlot performance and tissue residues of cattle consuming diets containing aflatoxins. J. Anim. Sci. 1986, 62, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Panangala, V.S.; Giambrone, J.J.; Diener, U.L.; Davis, N.D.; Hoerr, F.J.; Mitra, A.; Schultz, R.D.; Wilt, G.R. Effects of aflatoxin on the growth, performance, and imune responses of weanling swine. Am. J. Vet. Res. 1986, 47, 2062–2067. [Google Scholar] [PubMed]
- Choudhary, P.L.R.; Sharmn, S.; Borkhatarin, V.N.; Desaii, M.C. Effect of feeding aflatoxin B1 on feed consumption through naturally contaminated feeds. Indian J. Anim. Sci. 1998, 68, 400–401. [Google Scholar]
- Davis, G.R.F.; Smith, J.D.; Schiefer, B.; Loew, F.M. Screening for mycotoxins with larvae of Tenebrio molitor. J. Invertebr. Pathol. 1975, 26, 299–303. [Google Scholar] [CrossRef]
- Guo, Z.; Döll, K.; Dastjerdi, R.; Karlovsky, P.; Dehne, H.W.; Altincicek, B. Effect of fungal colonization of wheat grains with Fusarium spp. on food choice, weight gain and mortality of meal beetle larvae (Tenebrio molitor). PLoS ONE 2014, 16, e100112. [Google Scholar] [CrossRef] [PubMed]
- Dilkin, P.; Zorzete, P.; Mallmann, C.A.; Gomes, J.D.; Utiyama, C.E.; Oetting, L.L.; Corrêa, B. Toxicological effects of chronic low doses of aflatoxin b-1 and fumonisin b-1 containing Fusarium mondiforme culture material in weaned piglets. Food Chem. Toxicol. 2003, 41, 1345–1353. [Google Scholar] [CrossRef]
- Kimanya, M.E.; De Meulenaer, B.; Roberfroid, D.; Lachat, C.; Kolsteren, P. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol. Nutr. Food Res. 2010, 54, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; van der Fels-Klerx, H.J.; Rijk, T.C.D.; Oonincx, D.G. Aflatoxin B1 Tolerance and Accumulation in Black Soldier Fly Larvae (Hermetia illucens) and Yellow Mealworms (Tenebrio molitor). Toxins 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, Y.C.; Bomfim, M.R.; Melônio, L.C.; Ribeiro, P.C.; Cosme, L.M.; Rhoden, C.R.; Marques, S.G. Clinical significance of the isolation of Candida species from hospitalized patients. Braz. J. Microbiol. 2015, 31, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Jones, R.N.; Castanheira, M. Antifungal susceptibilities of Candida, Cryptococcus neoformans and Aspergillus fumigatus from the Asia and Western Pacific region: Data from the SENTRY antifungal surveillance program (2010–2012). J. Antibiot. 2015, 68, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, Y.; Hong, N.; Yang, Y.; Lei, W.; Du, L.; Zhao, J.; Lei, X.; Xiong, L.; Cai, L.; et al. Epidemiology of fungal infections in China. Front. Med. 2018, 12, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Klingspor, L.; Ullberg, M.; Rydberg, J.; Kondori, N.; Serrander, L.; Swanberg, J.; Nilsson, K.; Jendle Bengtén, C.; Johansson, M.; Granlund, M.; et al. Epidemiology of fungaemia in Sweden: A nationwide retrospective observational survey. Mycoses 2018, 61, 777–785. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.C.; Morey, A.T.; Castanheira, G.M.; Bocate, K.P.; Panagio, L.A.; Ito, F.A.; Furlaneto, M.C.; Yamada-Ogatta, S.F.; Costa, I.N.; Mora-Montes, H.M.; et al. Tenebrio molitor (Coleoptera: Tenebrionidae) as an alternative host to study fungal infections. J. Microbiol. Methods 2015, 118, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.B.; O’Brien, E.; Khoury, J.B.; Mylonakis, E.; Mylonakis, E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010, 1, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Thewes, S.; Zakikhany, K.; Fradin, C.; Albrecht, A.; Almeida, R.; Brunke, S.; Grosse, K.; Martin, R.; Mayer, F.; et al. Identifying infection-associated genes of Candida albicans in the postgenomic era. FEMS Yeast Res. 2009, 9, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Thewes, S.; Reed, H.K.; Grosse-Siestrup, C.; Groneberg, D.A.; Meissler, M.; Schaller, M.; Hube, B. Haemoperfused liver as an ex vivo model for organ invasion of Candida albicans. J. Med. Microbiol. 2007, 56, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Grosse, K.; Berndt, A.; Hube, B. Pathogenesis of Candida albicans infections in the alternative chorio-allantoicmembrane chicken embryo model resembles systemic murine infections. PLoS ONE 2011, 6, e19741. [Google Scholar] [CrossRef]
- Eastman, A.J.; He, X.; Qiu, Y.; Davis, M.J.; Vedula, P.; Lyons, D.M.; Park, Y.D.; Hardison, S.E.; Malachowski, A.N.; Osterholzer, J.J.; et al. Cryptococcal Heat Shock Protein 70 Homolog Ssa1 Contributes to Pulmonary Expansion of Cryptococcus neoformans during the Afferent Phase of the Immune Response by Promoting Macrophage M2 Polarization. J. Immunol. 2015, 194, 5999–6010. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.R.; Valerio, A.D.; Viana, R.O.; Ricoy, A.C.S.; Johann, S.; Alves, V. Caenorhabditis elegans and Tenebrio molitor—New tools to investigate Malassezia species. Preprints 2018. [Google Scholar] [CrossRef]
- Brown, A.J.P.; Brown, G.D.; Netea, M.G.; Gow, N.A.R. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014, 22, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Fornari, G.; Gomes, R.R.; Degenhardt-Goldbach, J.; dos Santos, S.S.; de Almeida, S.R.; dos Santos, G.D.; Muro, M.D.; Bona, C.; Scola, R.H.; Trindade, E.S.; et al. A Model for Trans-Kingdom Pathogenicity in Fonsecaea Agents of Human Chromoblastomycosis. Front. Microbiol. 2018, 9, 2211. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.R.; Vicente, V.A.; de Azevedo, C.M.; Salgado, C.G.; da Silva, M.B.; Queiroz-Telles, F.; Marques, S.G.; Santos, D.W.; de Andrade, T.S.; Takagi, E.H.; et al. Molecular epidemiology of agents of human chromoblastomycosis in Brazil with the description of two novel species. PLoS Negl. Trop. Dis. 2016, 10, e0005102. [Google Scholar] [CrossRef] [PubMed]
- Vicente, V.A.; Najafzadeh, M.J.; Sun, J.; Gomes, R.R.; Robl, D.; Marques, S.G.; Azevedo, C.M.P.S.; De Hoog, G.S. Environmental siblings of black agents of human chromoblastomycosis. Fungal Divers. 2013, 65, 47–63. [Google Scholar] [CrossRef]
- Sousa Mda, G.; Reid, D.M.; Schweighoffer, E.; Tybulewicz, V.; Ruland, J.; Langhorne, J.; Yamasaki, S.; Taylor, P.R.; Almeida, S.R.; Brown, G.D. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe 2011, 9, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, I.M.; Castro, R.J.A.; Leonhardt, L.C.M.; Jeronimo, M.S.; Soares, A.C.; Raiol, T.; Nishibe, C.; Almeida, N.; Tavares, A.H.; Hoffmann, C.; et al. Modulation of the immune response by Fonsecaea pedrosoi morphotypes in the course of experimental chromoblastomycosis and their role on inflammatory response chronicity. PLoS Negl. Trop. Dis. 2017, 11, e0005461. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Meyling, N.V.; Williams, A.R.; Mueller-Harvey, I.; Fryganas, C.; Kapel, C.M.; Fredensborg, B.L. Efficacy of condensed tannins against larval Hymenolepis diminuta (Cestoda) in vitro and in the intermediate host Tenebrio molitor (Coleoptera) in vivo. Vet. Parasitol. 2015, 207, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Morey, A.T.; de Souza, F.C.; Santos, J.P.; Pereira, C.A.; Cardoso, J.D.; de Almeida, R.S.; Costa, M.A.; de Mello, J.C.; Nakamura, C.V. Pinge-Filho, P.; et al. Antifungal Activity of Condensed Tannins from Stryphnodendron adstringens: Effect on Candida tropicalis Growth and Adhesion Properties. Curr. Pharm. Biotechnol. 2016, 17, 365–375. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canteri de Souza, P.; Custódio Caloni, C.; Wilson, D.; Sergio Almeida, R. An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor. J. Fungi 2018, 4, 125. https://doi.org/10.3390/jof4040125
Canteri de Souza P, Custódio Caloni C, Wilson D, Sergio Almeida R. An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor. Journal of Fungi. 2018; 4(4):125. https://doi.org/10.3390/jof4040125
Chicago/Turabian StyleCanteri de Souza, Patrícia, Carla Custódio Caloni, Duncan Wilson, and Ricardo Sergio Almeida. 2018. "An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor" Journal of Fungi 4, no. 4: 125. https://doi.org/10.3390/jof4040125
APA StyleCanteri de Souza, P., Custódio Caloni, C., Wilson, D., & Sergio Almeida, R. (2018). An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor. Journal of Fungi, 4(4), 125. https://doi.org/10.3390/jof4040125