Candida Interactions with the Oral Bacterial Microbiota
Abstract
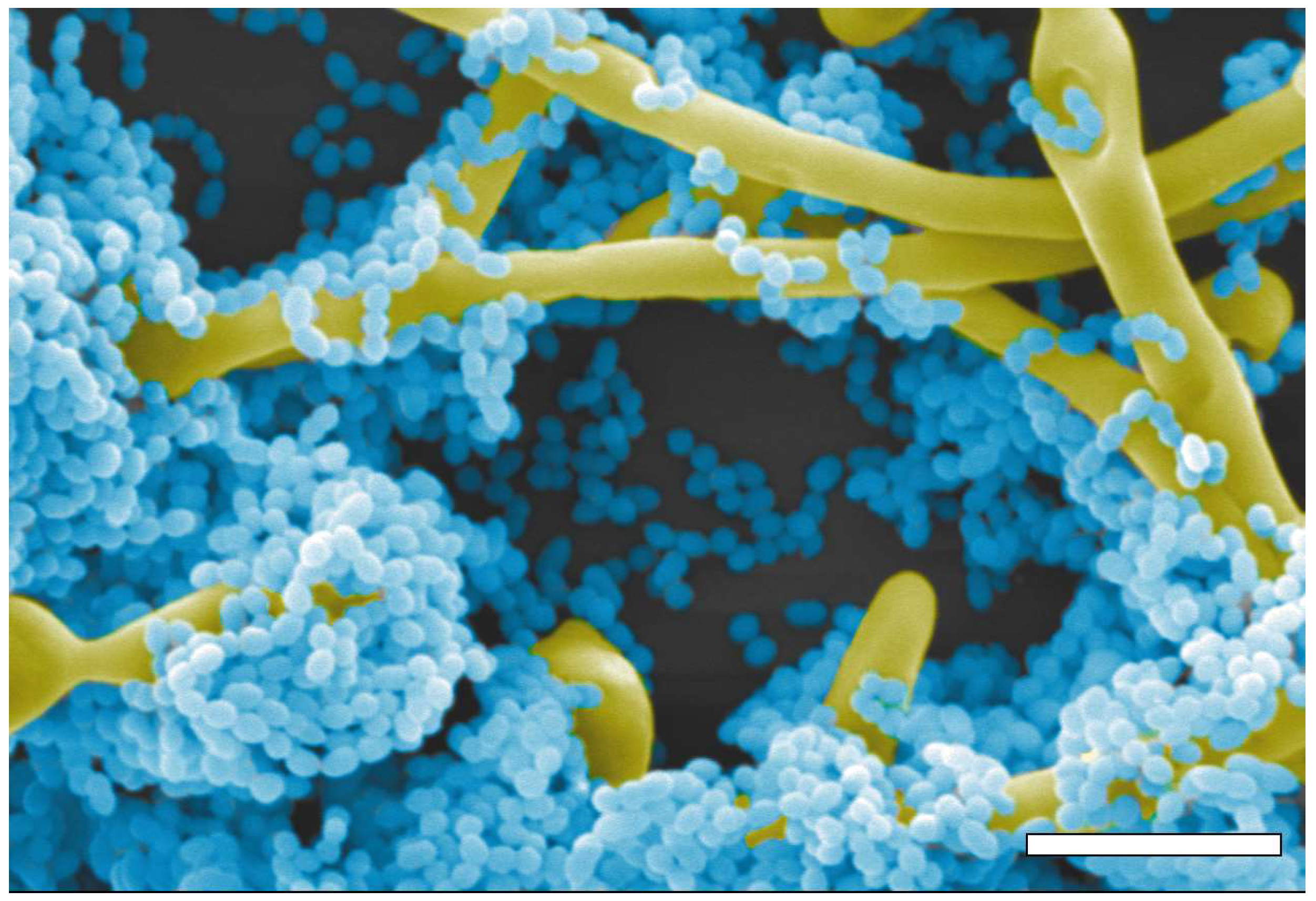
:1. Introduction
2. C. albicans Interactions with Oral Streptococci
2.1. Mutans Group Streptococci
Streptococcus mutans
2.2. Mitis Group Streptococci
2.2.1. Streptococcus gordonii
2.2.2. Streptococcus oralis
2.2.3. Streptococcus sanguinis, Streptococcus parasanguinis, and Streptococcus mitis
2.3. Salivarius Group Streptococci
Streptococcus salivarius
3. C. albicans Interactions with Other Oral Bacteria
3.1. Porphyromonas gingivalis
3.2. Actinomyces spp.
3.3. Fusobacterium nucleatum
3.4. Rothia dentocariosa
3.5. Aggregatibacter actinomycetemcomitans
3.6. Staphylococcus aureus
3.7. Enterococcus faecalis
4. Non-albicans Candida Species in the Oral Microbiome
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seminario, A.; Broukal, Z.; Ivancakova, R. Mutans streptococci and the development of dental plaque. Prague Med. Rep. 2005, 106, 349–358. [Google Scholar] [PubMed]
- Avila, M.; Ojcius, D.M.; Yilmaz, O. The oral microbiota: Living with a permanent guest. DNA Cell Biol. 2009, 28, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lof, M.; Janus, M.M.; Krom, B.P. Metabolic interactions between bacteria and fungi in commensal oral biofilms. J. Fungi 2017, 3, 40. [Google Scholar]
- Krom, B.P.; Kidwai, S.; Ten Cate, J.M. Candida and other fungal species: Forgotten players of healthy oral microbiota. J. Dent. Res. 2014, 93, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Silva, Y.; Vaz, C.; Carvalho-Pereira, J.; Carneiro, C.; Nogueira, E.; Correia, A.; Carreto, L.; Silva, S.; Faustino, A.; Pais, C.; et al. Participation of Candida albicans transcription factor RLM1 in cell wall biogenesis and virulence. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hezel, M.P.; Weitzberg, E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015, 21, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental plaque biofilm in oral health and disease. Chin. J. Dent. Res. 2011, 14, 87–94. [Google Scholar] [PubMed]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busscher, H.J.; van der Mei, H.C. Initial microbial adhesion events: Mechanisms and implications. In Community Structure and Co-Operation in Biofilms; Allison, D.G., Gilbert, P., Lappin-Scott, H.M., Wilson, M., Eds.; Cambridge University Press: Cambridge, UK, 2000; Volume 59, pp. 25–36. [Google Scholar]
- Busscher, H.J.; van der Mei, H.C. Physico-chemical interactions in initial microbial adhesion and relevance for biofilm formation. Adv. Dent. Res. 1997, 11, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Berg, E.A.; Costello, C.E.; Troxler, R.F.; Oppenheim, F.G. Identification of protein components in human acquired enamel pellicle and whole saliva using novel proteomics approaches. J. Biol. Chem. 2003, 278, 5300–5308. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C.; Gibbons, R.J. Inhibition of bacterial adherence by secretory immunoglobulin A: A mechanism of antigen disposal. Science 1972, 177, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Artacho, A.; Camelo-Castillo, A.; Garcia-Esteban, S.; Simon-Soro, A. Salivary immune and metabolic marker analysis (simma): A diagnostic test to predict caries risk. Diagnostics 2017, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Plaut, A.G.; Moellering, R.C., Jr. Evaluation of human oral organisms and pathogenic streptococcus for production of iga protease. J. Infect. Dis. 1975, 131, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Helmerhorst, E.J.; Leone, C.W.; Troxler, R.F.; Yaskell, T.; Haffajee, A.D.; Socransky, S.S.; Oppenheim, F.G. Identification of early microbial colonizers in human dental biofilm. J. Appl. Microbiol. 2004, 97, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, H.F.; Demuth, D.R. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol. Microbiol. 1997, 23, 183–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, L.J.; Maddocks, S.E.; Larson, M.R.; Forsgren, N.; Persson, K.; Deivanayagam, C.C.; Jenkinson, H.F. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol. Microbiol. 2010, 77, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.S.; Stromberg, N.; van Dolleweerd, C.J.; Kelly, C.G.; Jenkinson, H.F. Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands. Mol. Microbiol. 2005, 55, 1591–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daep, C.A.; James, D.M.; Lamont, R.J.; Demuth, D.R. Structural characterization of peptide-mediated inhibition of Porphyromonas gingivalis biofilm formation. Infect. Immun. 2006, 74, 5756–5762. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.J.; Nobbs, A.H.; Vickerman, M.M.; Barbour, M.E.; Jenkinson, H.F. Interaction of Candida albicans cell wall ALS3 protein with Streptococcus gordonii ssp adhesin promotes development of mixed-species communities. Infect. Immun. 2010, 78, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, M.; Mylonakis, E. Fungal-bacterial interactions and their relevance in health. Cell Microbiol. 2015, 17, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Bowen, W.H. Candida albicans and Streptococcus mutans: A potential synergistic alliance to cause virulent tooth decay in children. Future Microbiol. 2014, 9, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Elving, G.J.; van der Mei, H.C.; Busscher, H.J.; van Weissenbruch, R.; Albers, F.W. Comparison of the microbial composition of voice prosthesis biofilms from patients requiring frequent versus infrequent replacement. Ann. Otol. Rhinol. Laryngol. 2002, 111, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Radford, D.R.; Challacombe, S.J.; Walter, J.D. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit. Rev. Oral Biol. Med. 1999, 10, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Xie, Z.; Sobue, T.; Thompson, A.; Biyikoglu, B.; Ricker, A.; Ikonomou, L.; Dongari-Bagtzoglou, A. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect. Immun. 2012, 80, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.; Nagao, J.; Imayoshi, R.; Tanaka, Y. Importance of diversity in the oral microbiota including candida species revealed by high-throughput technologies. Int. J. Dent. 2014, 2014, 454391. [Google Scholar] [CrossRef] [PubMed]
- Pidamale, R.; Sowmya, B.; Thomas, A.; Jose, T.; Madhusudan, K.K.; Prasad, G. Association between early childhood caries, Streptococcus mutans level and genetic sensitivity levels to the bitter taste of, 6-N propylthiouracil among the children below 71 months of age. Dent. Res. J. 2012, 9, 730–734. [Google Scholar]
- Rugg-Gunn, A. Dental caries: Strategies to control this preventable disease. Acta Med. Acad. 2013, 42, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Dennis, D.A.; Gawronski, T.H.; Sudo, S.Z.; Harris, R.S.; Folke, L.E. Variations in microbial and biochemical components of four-day plaque during a four-week controlled diet period. J. Dent. Res. 1975, 54, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Staat, R.H.; Gawronski, T.H.; Cressey, D.E.; Harris, R.S.; Folke, L.E. Effects of dietary sucrose levels on the quantity and microbial composition of human dental plaque. J. Dent. Res. 1975, 54, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Ajdic, D.; Chen, Z. A novel phosphotransferase system of Streptococcus mutans is responsible for transport of carbohydrates with alpha-1,3 linkage. Mol. Oral Microbiol. 2013, 28, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Branting, C.; Sund, M.L.; Linder, L.E. The influence of Streptococcus mutans on adhesion of Candida albicans to acrylic surfaces in vitro. Arch. Oral Biol. 1989, 34, 347–353. [Google Scholar] [CrossRef]
- Pereira-Cenci, T.; Deng, D.M.; Kraneveld, E.A.; Manders, E.M.; Del Bel Cury, A.A.; Ten Cate, J.M.; Crielaard, W. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch. Oral Biol. 2008, 53, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Marsh, G.; Gao, L.; Waugh, R.; Koo, H. Binding force dynamics of Streptococcus mutans-glucosyltransferase B to Candida albicans. J. Dent. Res. 2015, 94, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Ellepola, K.; Liu, Y.; Cao, T.; Koo, H.; Seneviratne, C.J. Bacterial gtfb augments Candida albicans accumulation in cross-kingdom biofilms. J. Dent. Res. 2017, 96, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Kim, D.; Zhou, X.; Ahn, S.J.; Burne, R.A.; Richards, V.P.; Koo, H. RNA-seq reveals enhanced sugar metabolism in Streptococcus mutans co-cultured with Candida albicans within mixed-species biofilms. Front. Microbiol. 2017, 8, 1036. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GTFB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Liu, Y.; Benhamou, R.I.; Sanchez, H.; Simon-Soro, A.; Li, Y.; Hwang, G.; Fridman, M.; Andes, D.R.; Koo, H. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018, 12, 1427–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzysciak, W.; Koscielniak, D.; Papiez, M.; Vyhouskaya, P.; Zagorska-Swiezy, K.; Kolodziej, I.; Bystrowska, B.; Jurczak, A. Effect of a lactobacillus salivarius probiotic on a double-species Streptococcus mutans and Candida albicans caries biofilm. Nutrients 2017, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Pestova, E.V.; Havarstein, L.S.; Morrison, D.A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 1996, 21, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, R.; Lemme, A.; Ballhausen, B.; Thiel, V.; Schulz, S.; Jansen, R.; Sztajer, H.; Wagner-Dobler, I. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). Chembiochem 2010, 11, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Joyner, P.M.; Liu, J.; Zhang, Z.; Merritt, J.; Qi, F.; Cichewicz, R.H. Mutanobactin a from the human oral pathogen Streptococcus mutans is a cross-kingdom regulator of the yeast-mycelium transition. Org. Biomol. Chem. 2010, 8, 5486–5489. [Google Scholar] [CrossRef] [PubMed]
- Sztajer, H.; Szafranski, S.P.; Tomasch, J.; Reck, M.; Nimtz, M.; Rohde, M.; Wagner-Dobler, I. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 2014, 8, 2256–2271. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Sengupta, A.; Niepa, T.H.; Lee, B.H.; Weljie, A.; Freitas-Blanco, V.S.; Murata, R.M.; Stebe, K.J.; Lee, D.; Koo, H. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017, 7, 41332. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Hayacibara, M.F.; Schobel, B.D.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Vacca-Smith, A.M.; Bowen, W.H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003, 52, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.W.; Bergmeier, L.A.; Zanders, E.D.; Lehner, T. Protein antigens of Streptococcus mutans: Purification and properties of a double antigen and its protease-resistant component. Infect. Immun. 1980, 28, 486–493. [Google Scholar] [PubMed]
- Yang, C.; Scoffield, J.; Wu, R.; Deivanayagam, C.; Zou, J.; Wu, H. Antigen I/II mediates interactions between Streptococcus mutans and Candida albicans. Mol. Oral Microbiol. 2018, 33, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Tan, T.K.; Paterson, I.C.; Mutha, N.V.; Siow, C.C.; Tan, S.Y.; Old, L.A.; Jakubovics, N.S.; Choo, S.W. Streptobase: An oral Streptococcus mitis group genomic resource and analysis platform. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, H.F.; Lala, H.C.; Shepherd, M.G. Coaggregation of Streptococcus sanguis and other streptococci with Candida albicans. Infect. Immun. 1990, 58, 1429–1436. [Google Scholar] [PubMed]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R.J., Jr.; Kolenbrander, P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 2006, 72, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Bamford, C.V.; d’Mello, A.; Nobbs, A.H.; Dutton, L.C.; Vickerman, M.M.; Jenkinson, H.F. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect. Immun. 2009, 77, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Montelongo-Jauregui, D.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. An in vitro model for oral mixed biofilms of Candida albicans and Streptococcus gordonii in synthetic saliva. Front. Microbiol 2016, 7, 686. [Google Scholar] [CrossRef] [PubMed]
- Montelongo-Jauregui, D.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. An in vitro model for Candida albicans (-)Streptococcus gordonii biofilms on titanium surfaces. J. Fungi 2018, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Vickerman, M.M.; Jenkinson, H.F. Heterologous expression of Candida albicans cell wall-associated adhesins in Saccharomyces cerevisiae reveals differential specificities in adherence and biofilm formation and in binding oral Streptococcus gordonii. Eukaryot. Cell 2010, 9, 1622–1634. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.R.; McNab, R.; Jenkinson, H.F. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect. Immun. 1996, 64, 4680–4685. [Google Scholar] [PubMed]
- Dutton, L.C.; Nobbs, A.H.; Jepson, K.; Jepson, M.A.; Vickerman, M.M.; Aqeel Alawfi, S.; Munro, C.A.; Lamont, R.J.; Jenkinson, H.F. O-mannosylation in Candida albicans enables development of interkingdom biofilm communities. MBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Dutton, L.C.; Jenkinson, H.F.; Lamont, R.J.; Nobbs, A.H. Role of Candida albicans secreted aspartyl protease sap9 in interkingdom biofilm formation. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.H.; Palmer, R.J., Jr.; Blehert, D.S.; Campagna, S.R.; Semmelhack, M.F.; Egland, P.G.; Bassler, B.L.; Kolenbrander, P.E. Autoinducer 2: A concentration-dependent signal for mutualistic bacterial biofilm growth. Mol. Microbiol. 2006, 60, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Federle, M.J. Autoinducer-2-based chemical communication in bacteria: Complexities of interspecies signaling. Contrib. Microbiol. 2009, 16, 18–32. [Google Scholar] [PubMed]
- Ricker, A.; Vickerman, M.; Dongari-Bagtzoglou, A. Streptococcus gordonii glucosyltransferase promotes biofilm interactions with Candida albicans. J. Oral Microbiol. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.A.; Daniels, D.E.; Jepson, M.A.; Vickerman, M.M.; Lamont, R.J.; Jenkinson, H.F.; Nobbs, A.H. Streptococcus gordonii comcde (competence) operon modulates biofilm formation with Candida albicans. Microbiology 2015, 161, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Vickerman, M.; Nobile, C.J.; Dongari-Bagtzoglou, A.S. Oralis activates the EFG1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence 2017, 8, 1602–1617. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Sobue, T.; Thompson, A.; Dongari-Bagtzoglou, A. Chemotherapy induces oral mucositis in mice without additional noxious stimuli. Transl. Oncol. 2017, 10, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Sobue, T.; Bertolini, M.; Thompson, A.; Peterson, D.E.; Diaz, P.I.; Dongari-Bagtzoglou, A. Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Mol. Oral Microbiol. 2018, 33, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Bagg, J.; Silverwood, R.W. Coagglutination reactions between Candida albicans and oral bacteria. J. Med. Microbiol. 1986, 22, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sobue, T.; Thompson, A.; Xie, Z.; Poon, K.; Ricker, A.; Cervantes, J.; Diaz, P.I.; Dongari-Bagtzoglou, A. Streptococcal co-infection augments candida pathogenicity by amplifying the mucosal inflammatory response. Cell. Microbiol. 2014, 16, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.M.; Xu, H.; Sobue, T.; Nobile, C.J.; Del Bel Cury, A.A.; Dongari-Bagtzoglou, A. Candida-streptococcal mucosal biofilms display distinct structural and virulence characteristics depending on growth conditions and hyphal morphotypes. Mol. Oral Microbiol. 2015, 30, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Dongari-Bagtzoglou, A. Streptococcus oralis and Candida albicans synergistically activate mu-calpain to degrade E-cadherin from oral epithelial junctions. J. Infect. Dis. 2016, 214, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, H.; Yan, C.; Wang, D.; Li, H.; Xia, X.; Dong, X.; Zhao, Y.; Sun, T.; Hu, P.; et al. Antagonistic effect of protein extracts from Streptococcus sanguinis on pathogenic bacteria and fungi of the oral cavity. Exp. Ther. Med. 2014, 7, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, Y.W.; Morse, D.J.; da Silva, W.J.; Del-Bel-Cury, A.A.; Wei, X.; Wilson, M.; Milward, P.; Lewis, M.; Bradshaw, D.; Williams, D.W. Virulence and pathogenicity of Candida albicans is enhanced in biofilms containing oral bacteria. Biofouling 2015, 31, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, Y.W.; Wilson, M.; Lewis, M.; Del-Bel-Cury, A.A.; da Silva, W.J.; Williams, D.W. Modulation of Candida albicans virulence by bacterial biofilms on titanium surfaces. Biofouling 2016, 32, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, S.; Nakamura, T. Sanguicin, a bacteriocin of oral Streptococcus sanguis. Antimicrob. Agents Chemother. 1979, 16, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.; Ramos, L.P.; Domingues, N.; Back-Brito, G.N.; de Oliveira, L.D.; Pereira, C.A.; Jorge, A.O.C. Biofilms of Candida albicans and Streptococcus sanguinis and their susceptibility to antimicrobial effects of photodynamic inactivation. Photodiagn. Photodyn. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, S.A.; Hayama, K.; Burton, J.P.; Reid, G.; Okada, M.; Matsushita, Y.; Abe, S. Effect of Streptococcus salivarius K12 on the in vitro growth of Candida albicans and its protective effect in an oral candidiasis model. Appl. Environ. Microbiol. 2012, 78, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Sztukowska, M.N.; Dutton, L.C.; Delaney, C.; Ramsdale, M.; Ramage, G.; Jenkinson, H.F.; Nobbs, A.H.; Lamont, R.J. Community development between Porphyromonas gingivalis and Candida albicans mediated by inlj and als3. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Tamai, R.; Sugamata, M.; Kiyoura, Y. Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb. Pathog. 2011, 51, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Burns, L.H.; Jack, A.A.; Back, C.R.; Dutton, L.C.; Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Microbial interactions in building of communities. Mol. Oral Microbiol. 2013, 28, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Daep, C.A.; Novak, E.A.; Lamont, R.J.; Demuth, D.R. Structural dissection and in vivo effectiveness of a peptide inhibitor of Porphyromonas gingivalis adherence to Streptococcus gordonii. Infect. Immun. 2011, 79, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Dancing with the stars: How choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 2016, 24, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Haverman, T.M.; Laheij, A.; de Soet, J.J.; de Lange, J.; Rozema, F.R. Candida and Porphyromonas gingivalis: The effect on wound closure in vitro. J. Oral Microbiol. 2017, 9, 1328266. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Follo, M.; Selzer, A.C.; Hellwig, E.; Hannig, M.; Hannig, C. Bacterial colonization of enamel in situ investigated using fluorescence in situ hybridization. J. Med. Microbiol. 2009, 58, 1359–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, R.J., Jr.; Kazmerzak, K.; Hansen, M.C.; Kolenbrander, P.E. Mutualism versus independence: Strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source. Infect. Immun. 2001, 69, 5794–5804. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.J., Jr.; Gordon, S.M.; Cisar, J.O.; Kolenbrander, P.E. Coaggregation-mediated interactions of Streptococci and Actinomyces detected in initial human dental plaque. J. Bacteriol. 2003, 185, 3400–3409. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez de Annan, S.; Benito de Cardenas, L. Effect of metabolic substances of oral Actinomyces on Candida albicans. Rev. Iberoam. Micol. 2004, 21, 29–34. [Google Scholar] [PubMed]
- Grimaudo, N.J.; Nesbitt, W.E.; Clark, W.B. Coaggregation of Candida albicans with oral actinomyces species. Oral Microbiol. Immunol. 1996, 11, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Arzmi, M.H.; Dashper, S.; Catmull, D.; Cirillo, N.; Reynolds, E.C.; McCullough, M. Coaggregation of Candida albicans, Actinomyces naeslundii and Streptococcus mutans is Candida albicans strain dependent. FEMS Yeast Res. 2015, 15, fov038. [Google Scholar] [CrossRef] [PubMed]
- Arzmi, M.H.; Alnuaimi, A.D.; Dashper, S.; Cirillo, N.; Reynolds, E.C.; McCullough, M. Polymicrobial biofilm formation by Candida albicans, Actinomyces naeslundii, and Streptococcus mutans is Candida albicans strain and medium dependent. Med. Mycol. 2016, 54, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.M.; Nobbs, A.H.; Ricomini-Filho, A.P.; Jenkinson, H.F.; Del Bel Cury, A.A. Interkingdom cooperation between Candida albicans, Streptococcus oralis and Actinomyces oris modulates early biofilm development on denture material. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef]
- Cavalcanti, I.M.; Del Bel Cury, A.A.; Jenkinson, H.F.; Nobbs, A.H. Interactions between Streptococcus oralis, Actinomyces oris, and Candida albicans in the development of multispecies oral microbial biofilms on salivary pellicle. Mol. Oral Microbiol. 2017, 32, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wei, C.; Liu, C.; Li, D.; Sun, J.; Huang, H.; Zhou, H. Inhibitory effects of oral Actinomyces on the proliferation, virulence and biofilm formation of Candida albicans. Arch. Oral Biol. 2015, 60, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, N.J.; Nesbitt, W.E. Coaggregation of Candida albicans with oral Fusobacterium species. Oral Microbiol. Immunol. 1997, 12, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Falkler, W.A., Jr.; Merz, W.G.; Kelley, J.I.; Baqui, A.A.; Meiller, T.F. Coaggregation of Candida dubliniensis with Fusobacterium nucleatum. J. Clin. Microbiol. 1999, 37, 1464–1468. [Google Scholar] [PubMed]
- Wu, T.; Cen, L.; Kaplan, C.; Zhou, X.; Lux, R.; Shi, W.; He, X. Cellular components mediating coadherence of Candida albicans and Fusobacterium nucleatum. J. Dent. Res. 2015, 94, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.W.; Lux, R.; Haake, S.K.; Shi, W. The Fusobacterium nucleatum outer membrane protein RADD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol. Microbiol. 2009, 71, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Kaplan, C.W.; He, X.; McHardy, I.; Shi, W.; Lux, R. Characterization of aid1, a novel gene involved in Fusobacterium nucleatum interspecies interactions. Microb. Ecol. 2014, 68, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Bor, B.; Cen, L.; Agnello, M.; Shi, W.; He, X. Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum. Sci. Rep. 2016, 6, 27956. [Google Scholar] [CrossRef] [PubMed]
- Elving, G.J.; van Der Mei, H.C.; Busscher, H.J.; van Weissenbruch, R.; Albers, F.W. Air-flow resistances of silicone rubber voice prostheses after formation of bacterial and fungal biofilms. J. Biomed. Mater. Res. 2001, 58, 421–426. [Google Scholar] [CrossRef] [PubMed]
- van der Mei, H.C.; Buijssen, K.J.; van der Laan, B.F.; Ovchinnikova, E.; Geertsema-Doornbusch, G.I.; Atema-Smit, J.; van de Belt-Gritter, B.; Busscher, H.J. Voice prosthetic biofilm formation and Candida morphogenic conversions in absence and presence of different bacterial strains and species on silicone-rubber. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Millsap, K.W.; Bos, R.; van der Mei, H.C.; Busscher, H.J. Adhesive interactions between voice prosthetic yeast and bacteria on silicone rubber in the absence and presence of saliva. Antonie Van Leeuwenhoek 2001, 79, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Elving, G.J.; van der Mei, H.; Busscher, H.; van Weissenbruch, R.; Albers, F. Influence of different combinations of bacteria and yeasts in voice prosthesis biofilms on air flow resistance. Antonie Van Leeuwenhoek 2003, 83, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Busscher, H.J.; Chakladar, J.; van der Mei, H.C.; Chaffin, W.L. Transcriptional profiling of C. albicans in a two species biofilm with Rothia dentocariosa. Front. Cell. Infect. Microbiol. 2017, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.; Ummer, F.; Dhivakar, C.P. Aggregatibacter actinomycetemcomitans—A tooth killer? J. Clin. Diagn. Res. 2014, 8, ZE13–ZE16. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, E.W.; Bachtiar, B.M.; Jarosz, L.M.; Amir, L.R.; Sunarto, H.; Ganin, H.; Meijler, M.M.; Krom, B.P. Ai-2 of Aggregatibacter actinomycetemcomitans inhibits Candida albicans biofilm formation. Front. Cell. Infect. Microbiol. 2014, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Lamont, R.J.; Demuth, D.R. Autoinducer 2 is required for biofilm growth of aggregatibacter (actinobacillus) actinomycetemcomitans. Infect. Immun. 2007, 75, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.G.; Smith, A.J.; Akram, A.N.; Jackson, M.; Robertson, D.; Edwards, G. Staphylococcus aureus and the oral cavity: An overlooked source of carriage and infection? Am. J. Infect. Control 2015, 43, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Ohara-Nemoto, Y.; Haraga, H.; Kimura, S.; Nemoto, T.K. Occurrence of staphylococci in the oral cavities of healthy adults and nasal oral trafficking of the bacteria. J. Med. Microbiol. 2008, 57, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Baena-Monroy, T.; Moreno-Maldonado, V.; Franco-Martinez, F.; Aldape-Barrios, B.; Quindos, G.; Sanchez-Vargas, L.O. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med. Oral Patol. Oral Cir. Bucal. 2005, 10, E27–E39. [Google Scholar] [PubMed]
- Samaranayake, L.P.; Cheung, L.K.; Samaranayake, Y.H. Candidiasis and other fungal diseases of the mouth. Dermatol. Ther. 2002, 15, 251–269. [Google Scholar] [CrossRef]
- MacFarlane, T.W.; Helnarska, S.J. The microbiology of angular cheilitis. Br. Dent. J. 1976, 140, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Oza, N.; Doshi, J.J. Angular cheilitis: A clinical and microbial study. Indian J. Dent. Res. 2017, 28, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Terai, H.; Shimahara, M. Cheilitis as a variation of Candida-associated lesions. Oral Dis. 2006, 12, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Byadarahally Raju, S.; Rajappa, S. Isolation and identification of candida from the oral cavity. ISRN Dent. 2011, 2011, 487921. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Ovchinnikova, E.S.; Krom, B.P.; Schlecht, L.M.; Zhou, H.; Hoyer, L.L.; Busscher, H.J.; van der Mei, H.C.; Jabra-Rizk, M.A.; Shirtliff, M.E. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 2012, 158, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, L.M.; Peters, B.M.; Krom, B.P.; Freiberg, J.A.; Hansch, G.M.; Filler, S.G.; Jabra-Rizk, M.A.; Shirtliff, M.E. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 2015, 161, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.; Rajendran, R.; Haggarty, J.; Townsend, E.M.; Short, B.; Burgess, K.E.; Lang, S.; Millington, O.; Mackay, W.G.; Williams, C.; et al. Candida albicans mycofilms support Staphylococcus aureus colonization and enhances miconazole resistance in dual-species interactions. Front. Microbiol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Kucharikova, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. MBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Hermann, C.; Hermann, J.; Munzel, U.; Ruchel, R. Bacterial flora accompanying Candida yeasts in clinical specimens. Mycoses 1999, 42, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.; Kovac, D.; Slobodnikova, L.; Kotulova, D. Enterococcus faecalis and Candida albicans in the dental root canal and periapical infections. Bratisl. Lek. Listy 2013, 114, 716–720. [Google Scholar] [PubMed]
- Dahlen, G.; Blomqvist, S.; Almstahl, A.; Carlen, A. Virulence factors and antibiotic susceptibility in Enterococci isolated from oral mucosal and deep infections. J. Oral Microbiol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.R.; Graham, C.E.; Gagliano, B.C.; Lorenz, M.C.; Garsin, D.A. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect. Immun. 2013, 81, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.E.; Cruz, M.R.; Garsin, D.A.; Lorenz, M.C. Enterococcus faecalis bacteriocin entv inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 2017, 114, 4507–4512. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Castellote, L.; Jimenez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, e279–e286. [Google Scholar] [CrossRef] [PubMed]
- Urzua, B.; Hermosilla, G.; Gamonal, J.; Morales-Bozo, I.; Canals, M.; Barahona, S.; Coccola, C.; Cifuentes, V. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Med. Mycol. 2008, 46, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; Barbosa, J.O.; Vilela, S.F.; dos Santos, J.D.; de Barros, P.P.; Prata, M.C.; Anbinder, A.L.; Fuchs, B.B.; Jorge, A.O.; Mylonakis, E.; et al. Competitive interactions between C. albicans, C. glabrata and C. krusei during biofilm formation and development of experimental candidiasis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Barros, P.P.; Ribeiro, F.C.; Rossoni, R.D.; Junqueira, J.C.; Jorge, A.O. Influence of Candida krusei and Candida glabrata on Candida albicans gene expression in in vitro biofilms. Arch. Oral Biol. 2016, 64, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.; Piva, E.; Vilela, S.F.; Jorge, A.O.; Junqueira, J.C. Mixed biofilms formed by C. albicans and non-albicans species: A study of microbial interactions. Braz. Oral Res. 2016, 30. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; Barros, P.P.; Freire, F.; Santos, J.D.D.; Jorge, A.O.C.; Junqueira, J.C. Study of microbial interaction formed by “Candida krusei” and “Candida glabrata”: “In vitro” and “in vivo” studies. Braz. Dent. J. 2017, 28, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Coco, B.J.; Bagg, J.; Cross, L.J.; Jose, A.; Cross, J.; Ramage, G. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral Microbiol. Immunol. 2008, 23, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Vipulanandan, G.; Herrera, M.; Wiederhold, N.P.; Li, X.; Mintz, J.; Wickes, B.L.; Kadosh, D. Dynamics of mixed-Candida species biofilms in response to antifungals. J. Dent. Res. 2018, 97, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.Y.; Minah, G.E.; Peterson, D.E.; Wingard, J.R.; Merz, W.G.; Altomonte, V.; Tylenda, C.A. Coaggregation of oral Candida isolates with bacteria from bone marrow transplant recipients. J. Clin. Microbiol. 1990, 28, 2621–2626. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montelongo-Jauregui, D.; Lopez-Ribot, J.L. Candida Interactions with the Oral Bacterial Microbiota. J. Fungi 2018, 4, 122. https://doi.org/10.3390/jof4040122
Montelongo-Jauregui D, Lopez-Ribot JL. Candida Interactions with the Oral Bacterial Microbiota. Journal of Fungi. 2018; 4(4):122. https://doi.org/10.3390/jof4040122
Chicago/Turabian StyleMontelongo-Jauregui, Daniel, and Jose L. Lopez-Ribot. 2018. "Candida Interactions with the Oral Bacterial Microbiota" Journal of Fungi 4, no. 4: 122. https://doi.org/10.3390/jof4040122
APA StyleMontelongo-Jauregui, D., & Lopez-Ribot, J. L. (2018). Candida Interactions with the Oral Bacterial Microbiota. Journal of Fungi, 4(4), 122. https://doi.org/10.3390/jof4040122





