Burden of Fungal Infections in Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature and Data Search
2.2. Frequency Estimates
2.3. Data Estimation Sources
3. Results
3.1. Population and Risk Factors
3.2. Candidiasis
3.3. Aspergillosis
3.4. Opportunistic Infections Related to HIV and Other Conditions Involving Immunosuppression
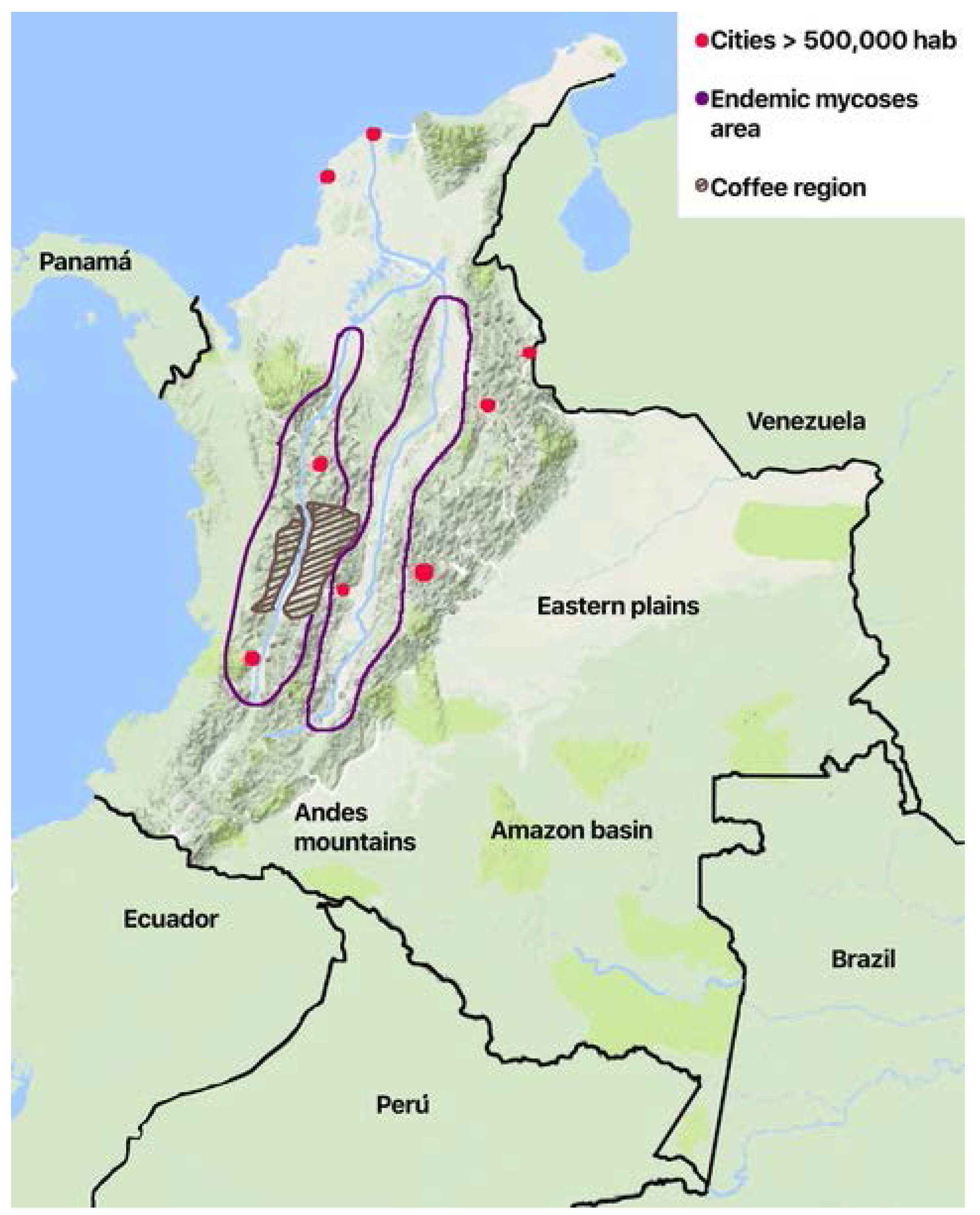
3.5. Endemic Mycoses and Other Fungal Infections
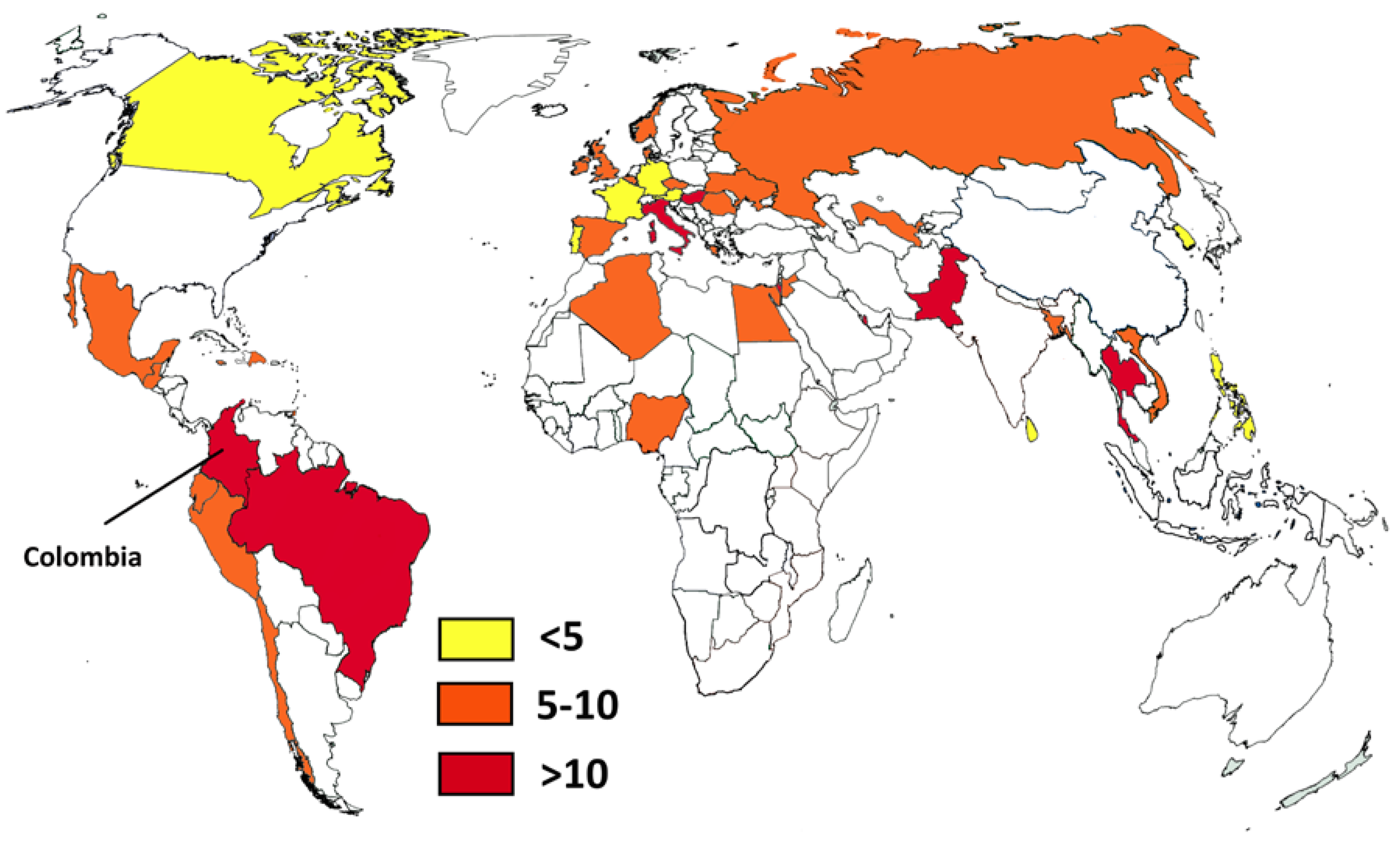
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denning, D.W. The ambitious ‘95-95 by 2025’ roadmap for the diagnosis and management of fungal diseases. Thorax 2015, 70, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M.; Talhari, C.; Talhari, S. Advances in paracoccidioidomycosis. Clin. Exp. Dermatol. 2010, 35, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Tobon, A.; Restrepo, A.; Queiroz-Telles, F.; Nucci, M. Epidemiology of endemic systemic fungal infections in Latin America. Med. Mycol. 2011, 49, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of candidemia in Latin America: A laboratory-based survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Tobon, A.M.; Restrepo, A.; Colombo, A.L. Epidemiology of opportunistic fungal infections in Latin America. Clin. Infect. Dis. 2010, 51, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Leading International Fungal Education (LIFE). Available online: http://www.life-worldwide.org/ (accessed on 15 January 2018).
- Cuenta de Alto Costo. Sitación del VIH en Colombia 2016; Fondo Colombiano de Enfermedades de Alto Costo: Bogota, Colombia, 2017; ISSN 2344-7702. [Google Scholar]
- Buchacz, K.; Baker, R.K.; Palella, F.J., Jr.; Chmiel, J.S.; Lichtenstein, K.A.; Novak, R.M.; Wood, K.C.; Brooks, J.T.; HOPS Investigators. AIDS-defining opportunistic illnesses in US patients, 1994–2007: A cohort study. AIDS 2010, 24, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Matee, M.I.; Scheutz, F.; Moshy, J. Occurrence of oral lesions in relation to clinical and immunological status among HIV-infected adult Tanzanians. Oral Dis. 2000, 6, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.A.; Jaimes, J.A.; Leal, A.L. Incidence and prevalence of candidemia in critically ill patients in Colombia. Rev. Chil. Infectol. 2013, 30, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Arteta, D.S. History of Critical Care Medicine in Colombia. AMCI. 2013. Available online: http://www.amci.org.co/20-institucional/151-historia-de-la-medicina-critica-en-colombia (accessed on 17 November 2017).
- Montravers, P.; Mira, J.P.; Gangneux, J.P.; Leroy, O.; Lortholary, O.; AmarCand Study Group. A multicentre study of antifungal strategies and outcome of Candida spp. peritonitis in intensive-care units. Clin. Microbiol. Infect. 2011, 17, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Muraglia, R.; Dietz, J.P.; Sobel, J.D.; Wagner, J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: Results from an internet panel survey. J. Low. Genit. Tract Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Orholm, M.K. Danish AIDS patients 1988–1993: A recent decline in Pneumocystis carinii pneumonia as AIDS-defining disease related to the period of known HIV positivity. Scand. J. Infect. Dis. 1994, 26, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Chakrabarti, A. Pulmonary and sinus fungal diseases in non-immunocompromised patients. Lancet Infect. Dis. 2017, 17, e357–e366. [Google Scholar] [CrossRef]
- World Health Organization. Tuberculosis Profile Colombia. 2016. Available online: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=CO&LAN=EN&outtype=html (accessed on 25 January 2018).
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2011, 37, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Colombian Ministry of Health. Global Data on Donation and Transplantation, 2014; Colombian Ministry of Health: Bogota, Colombia, 2015. [Google Scholar]
- Pardo, C.; Murillo, R.; Piñeros, M.; Castro, M.A. New cases of cancer at the National Insitute of Cancer, Colombia, 2002. Rev. Colomb. Cancerol. 2003, 7, 4–19. [Google Scholar]
- Pardo Ramos, C.; Cendales Duarte, R. Incidence, Mortality, and Prevalence of Cancer in COLOMBIA, 2007–2011; Instituto Nacional de Cancerologá: Bogotá, Colombia, 2015. [Google Scholar]
- Caballero, A.; Torres-Duque, C.A.; Jaramillo, C.; Bolivar, F.; Sanabria, F.; Osorio, P.; Orduz, C.; Guevara, D.P.; Maldonado, D. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study). Chest 2008, 133, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Gaffi. Available online: http://www.gaffi.org/media/academic-papers/ (accessed on 20 January 2018).
- Yan, X.; Li, M.; Jiang, M.; Zou, L.Q.; Luo, F.; Jiang, Y. Clinical characteristics of 45 patients with invasive pulmonary aspergillosis: Retrospective analysis of 1711 lung cancer cases. Cancer 2009, 115, 5018–5025. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.J.; Caraballo, L.; Garcia, E.; Rojas, M.X.; Rondon, M.A.; Perez, A.; Aristizabal, G.; Peñaranda, A.; Barragan, A.M.; Ahumada, V.; et al. Prevalence of asthma and other allergic conditions in Colombia 2009-2010: A cross-sectional study. BMC Pulm. Med. 2012, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med. Mycol. 2013, 51, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Vásquez Sagra, C.; Colmenares Betancourt, A.; Madero Oróstegui, D.; Jurado Hernández, J.R.; Aristizábal Duque, J.R.; Barón Puentes, O.; Ovalle Rodríguez, A. Clinical Practice Guidelines for the Prevention, Diagnosis, Treatment and Rehabilitation of Cystic Fibrosis; Ministry of Health of Colombia: Bogota, Colombia, 2014. [Google Scholar]
- Agarwal, R.; Aggarwal, A.N.; Gupta, D.; Jindal, S.K. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: Systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2009, 13, 936–944. [Google Scholar] [PubMed]
- Lizarazo, J.; Linares, M.; de Bedout, C.; Restrepo, A.; Agudelo, C.I.; Castaneda, E. Results of nine years of the clinical and epidemiological survey on cryptococcosis in Colombia, 1997–2005. Biomedica 2007, 27, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Escandon, P.; de Bedout, C.; Lizarazo, J.; Agudelo, C.I.; Tobon, A.; Bello, S.; Restrepo, Á.; Castañeda, E. Cryptococcosis in Colombia: Results of the national surveillance program for the years 2006–2010. Biomedica 2012, 32, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Arango, M.; Castaneda, E.; Agudelo, C.I.; De Bedout, C.; Agudelo, C.A.; Tobon, A.; Denning, D.W.; Loyse, A.; Boulware, D.R. Histoplasmosis: Results of the Colombian national survey, 1992–2008. Biomedica 2011, 31, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vega, F.; Botero, M.; Cortes, J.A.; Tobon, A. Pathological findings in patients with HIV infection and lymphadenopathies. Biomedica 2017, 37, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gómez Arias, B.; Zarco otero, L.A. Meningeal criptococcosis: Clinical and laboratory characteristics. Acta Neurol. Colomb. 2011, 27, 19–27. [Google Scholar]
- Velásquez Uribe, G.; Rueda, Z.V.; Vélez, L.A.; Aguirre, D.C.; Gómez Arias, R.D. Histoplasmosis in AIDS patients. A cohort study in Medellín, Colombia. Infectio 2010, 17, S99–S106. [Google Scholar]
- Miller, R.F.; Huang, L.; Walzer, P.D. Pneumocystis pneumonia associated with human immunodeficiency virus. Clin. Chest. Med. 2013, 34, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Rubio, G.; Sanchez, G.; Porras, L.; Alvarado, Z. Sporotrichosis: Prevalence, clinical and epidemiological features in a reference center in Colombia. Rev. Iberoam. Micol. 2010, 27, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Torrado, E.; Castaneda, E.; de la Hoz, F.; Restrepo, A. Paracoccidioidomicocic: Definición de las áreas endémicas de Colombia. Biomedica 2000, 20, 327–334. [Google Scholar] [CrossRef]
- Galvis, V.; Tello, A.; Guerra, A.; Acuna, M.F.; Villarreal, D. Antibiotic susceptibility patterns of bacteria isolated from keratitis and intraocular infections at Fundacion Oftalmologica de Santander (FOSCAL), Floridablanca, Colombia. Biomedica 2014, 34, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rigal, E.; Nourrisson, C.; Sciauvaud, J.; Pascal, J.; Texier, C.; Corbin, V.; Poirier, V.; Beytout, J.; Labbe, A.; Lesens, O. Skin diseases in internationally adopted children. Eur. J. Dermatol. 2016, 26, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Toro, G.; Tellez, N. Lobomycosis in Colombian Amer Indian patients. Mycopathologia 1992, 120, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Tobon, A.M.; Arango, M.; Fernandez, D.; Restrepo, A. Mucormycosis (zygomycosis) in a heart-kidney transplant recipient: Recovery after posaconazole therapy. Clin. Infect. Dis. 2003, 36, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.Y.; Rodriguez, G.J.; Morales-Lopez, S.E.; Cantillo, C.E.; Le Pape, P.; Alvarez-Moreno, C.A. Saksenaea erythrospora infection after medical tourism for esthetic breast augmentation surgery. Int. J. Infect. Dis. 2016, 49, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 2010, 16, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.A.; Reyes, P.; Gomez, C.H.; Cuervo, S.I.; Rivas, P.; Casas, C.A.; Sánchez, R. Clinical and epidemiological characteristics and risk factors for mortality in patients with candidemia in hospitals from Bogota, Colombia. Braz. J. Infect. Dis. 2014, 18, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.A.; Reyes, P.; Gomez, C.; Buitrago, G.; Leal, A.L.; Group, G. Fungal bloodstream infections in tertiary care hospitals in Colombia. Rev. Iberoam. Micol. 2011, 28, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Motoa, G.; Munoz, J.S.; Onate, J.; Pallares, C.J.; Hernandez, C.; Villegas, M.V. Epidemiology of Candida isolates from Intensive Care Units in Colombia from 2010 to 2013. Rev. Iberoam. Micol. 2017, 34, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of Colombia. Global Data on Transplants and Donation, 2014; Ministry of Health of Colombia: Bogota, Colombia, 2015. [Google Scholar]
- Bustamante, B.; Denning, D.W.; Campos, P.E. Serious fungal infections in Peru. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Zurita, J.; Denning, D.W.; Paz, Y.M.A.; Solis, M.B.; Arias, L.M. Serious fungal infections in Ecuador. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Giacomazzi, J.; Baethgen, L.; Carneiro, L.C.; Millington, M.A.; Denning, D.W.; Colombo, A.L.; Pasqualotto, A.C. The burden of serious human fungal infections in Brazil. Mycoses 2016, 59, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Garbino, J.; Pittet, D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 2003, 3, 685–702. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Al-Abdely, H.M.; El-Kholy, A.A.; AlKhawaja, S.A.A.; Leblebicioglu, H.; Mehta, Y.; Rai, V.; Hung, N.V.; Kanj, S.S.; Salama, M.F.; et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010–2015: Device-associated module. Am. J. Infect. Control 2016, 44, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Morales-Lopez, S.E.; Parra-Giraldo, C.M.; Ceballos-Garzon, A.; Martinez, H.P.; Rodriguez, G.J.; Alvarez-Moreno, C.A.; Rodríguez, J.Y. Invasive Infections with Multidrug-Resistant Yeast Candida auris, Colombia. Emerg. Infect. Dis. 2017, 23, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, N.; Tobón, A.; Arango, M.; Tabares, A.; de Bedout, C.; Gomez, B.; Castañeda, E.; Restrepo, A. Histoplasmosis outbreaks registered in the Andean area. Biomedica 1997, 17, 105–111. [Google Scholar]
- Carmona-Fonseca, J. Statistical, ecological and epidemiological analysis of the sensitivity to histoplasme in Colombia 1950–1968. Antioq. Med. 1971, 21, 109–154. [Google Scholar]
- Vallabhaneni, S.; Chiller, T.M. Fungal Infections and New Biologic Therapies. Curr. Rheumatol. Rep. 2016, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Longley, N.; Jarvis, J.N.; Meintjes, G.; Boulle, A.; Cross, A.; Kelly, N.; Govender, N.P.; Bekker, L.G.; Wood, R.; Harrison, T.S. Cryptococcal Antigen Screening in Patients Initiating ART in South Africa: A Prospective Cohort Study. Clin. Infect. Dis. 2016, 62, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros Calderón, A.L.; Morales Múnera, O.L.; Pabón, R.; Liliana, C. Aspergilosis broncopulmonar alérgica, una complicación del paciente con fibrosis quística: Reporte de dos casos y revisión de la literatura. IAtreia 2012, 25, 65–74. [Google Scholar]
- Bissinger, I.; Bareno, J. Clinical profile of sensitization to fungi in Medellin, Colombia. Rev. Alerg. Mex. 2016, 63, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Moreno, C.; Lavergne, R.A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Azole-resistant Aspergillus fumigatus harboring TR34/L98H, TR46/Y121F/T289A and TR53 mutations related to flower fields in Colombia. Sci. Rep. 2017, 7, 45631. [Google Scholar] [CrossRef] [PubMed]
- Bogomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar]
- Mares, M.; Moroti-Constantinescu, V.R.; Denning, D.W. The Burden of Fungal Diseases in Romania. J. Fungi 2018, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Nordøy, I.; Hesstvedt, L.; Torp Andersen, C.; Mylvaganam, H.; Kols, N.I.; Falch, B.M.; Tofteland, S.; Müller, F.; Denning, D.W. An Estimate of the Burden of Fungal Disease in Norway. J. Fungi 2018, 4, 29. [Google Scholar] [CrossRef]
- Bassetti, M.; Carnelutti, A.; Peghin, M.; Aversa, F.; Barchiesi, F.; Girmenia, C.; Pagano, L.; Sanguinetti, M.; Tortorano, A.M.; Montagna, M.T.; et al. Estimated burden of fungal infections in Italy. J. Infect. 2018, 76, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
| Population | Number |
|---|---|
| Total population, 2017 | 49,291,609 |
| Total adults, 2017 | 33,843,324 |
| Adult women, 2017 | 17,403,058 |
| Women 18–50 years-old, 2017 | 11,832,865 |
| HIV population | 73,465 |
| HIV population receiving ART | 65,044 |
| Pulmonary tuberculosis, 2016 | 10,442 |
| Asthma in adults | 2,090,506 |
| COPD in adults over 40 years | 921,260 |
| COPD patients admitted to hospital | 189,117 |
| Lung cancer, 2011 | 3985 |
| Acute leukemia, 2017 | 1089 |
| Renal transplant recipients, 2014 | 732 |
| Liver transplant recipients, 2014 | 211 |
| Allogeneic stem cell transplant, recipients, 2014 | 172 |
| Heart transplant recipients, 2014 | 72 |
| Lung transplant recipients, 2014 | 10 |
| Critical care beds, 2013 | 2310 |
| Fungal Infection | None | HIV/AIDS | Respiratory Disease | Cancer and Immunodeficiency | Critical Care and Surgery | Total | Rate/100,000 Inhabitants |
|---|---|---|---|---|---|---|---|
| Candidemia | 4407 | 1889 | 6296 | 12.8 | |||
| Candida peritonitis | 944 | 944 | 1.9 | ||||
| Oral candidiasis | 9150 | 9150 | 18.6 | ||||
| Oesophageal candidiasis | 4269 | 638 | 4907 | 10 | |||
| Recurrent Candida vaginitis (>4×/year) | 591,643 | 591,643 | 2401 | ||||
| Invasive aspergillosis | 361 | 2459 | 2820 | 5.7 | |||
| Chronic pulmonary aspergillosis post TB * | 2106 | 2106 | 4.3 | ||||
| Chronic pulmonary aspergillosis—all | 8426 | 8426 | 49 | ||||
| Allergic bronchopulmonary aspergillosis (ABPA) | 52,268 | 52,268 | 106 | ||||
| Severe asthma with fungal sensitisation (SAFS) | 68,987 | 68,987 | 140 | ||||
| Cryptococcal meningitis | 65 | 719 | 54 | 838 | 1.7 | ||
| Pneumocystis pneumonia | 1525 | 1525 | 3.1 | ||||
| Mucormycosis | 99 | 99 | 0.2 | ||||
| Histoplasmosis | 39 | 225 | 22 | 286 | 0.6 | ||
| Sporothricosis | 55 | 55 | 0.1 | ||||
| Fungal keratitis | 182 | 182 | 0.4 | ||||
| Tinea capitis | 12,134 | 12,134 | 25 | ||||
| Lobomycoses | 2 | 2 | 0.01 | ||||
| Paracoccidiodomycoses | 246 | 246 | 0.5 | ||||
| Total fungal infection burden | 604,366 | 15,888 | 129,681 | 5581 | 5292 | 760,808 | 1543 |
| Fungal Infection | Colombian Incidence | Global Incidence | Rate/100,000 |
|---|---|---|---|
| Histoplasma infection | 286 | 500,000 | 0.6 |
| Invasive candidiasis | 6296 | 750,000 | 12.8 |
| Invasive aspergillosis | 2820 | 300,000 | 5.7 |
| P. jirovecii pneumonia | 1525 | 500,000 | 3.1 |
| Cryptococcosis in AIDS | 719 | 223,000 | 1.5 |
| Mucormycosis | 99 | >10,000 | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Moreno, C.A.; Cortes, J.A.; Denning, D.W. Burden of Fungal Infections in Colombia. J. Fungi 2018, 4, 41. https://doi.org/10.3390/jof4020041
Alvarez-Moreno CA, Cortes JA, Denning DW. Burden of Fungal Infections in Colombia. Journal of Fungi. 2018; 4(2):41. https://doi.org/10.3390/jof4020041
Chicago/Turabian StyleAlvarez-Moreno, Carlos Arturo, Jorge Alberto Cortes, and David W. Denning. 2018. "Burden of Fungal Infections in Colombia" Journal of Fungi 4, no. 2: 41. https://doi.org/10.3390/jof4020041
APA StyleAlvarez-Moreno, C. A., Cortes, J. A., & Denning, D. W. (2018). Burden of Fungal Infections in Colombia. Journal of Fungi, 4(2), 41. https://doi.org/10.3390/jof4020041





