Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision
Abstract
1. Introduction
2. Estimate precision
2.1. Candidaemia
2.1.1. 5142 Cases of Invasive Candidiasis in the UK
2.1.2. Discussion
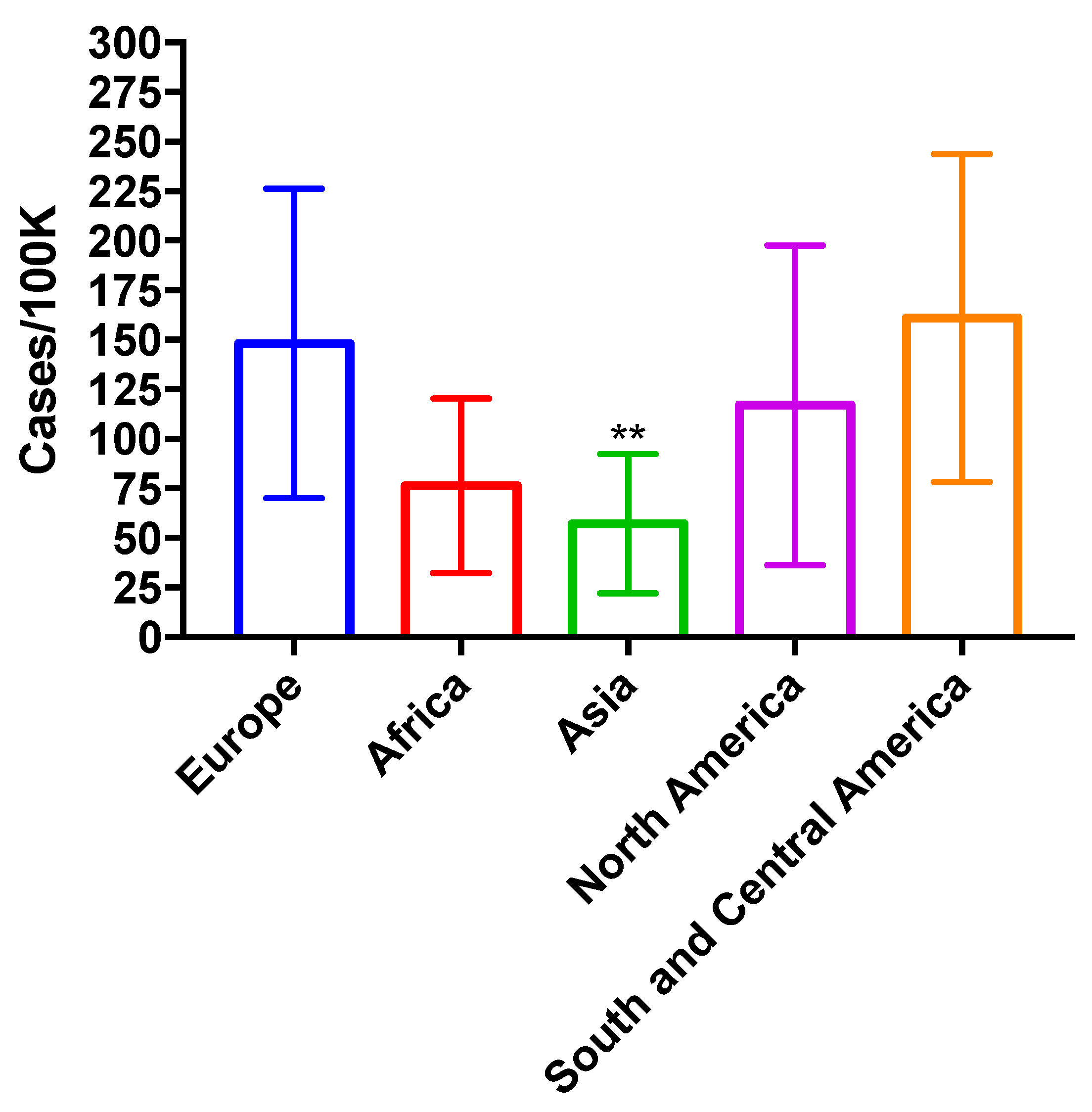
2.2. Invasive Aspergillosis
2.2.1. 294 Annual Cases of Invasive Aspergillosis in Denmark
2.2.2. Discussion
2.3. Pneumocystis jirovecii Pneumonia
2.3.1. Over 700 Cases of Pneumocystis jirovecii Pneumonia Per Year in Guatemala
2.3.2. Discussion
2.4. Chronic Pulmonary Aspergillosis (CPA)
2.4.1. About 120,754 Cases of CPA in Nigeria
2.4.2. Discussion
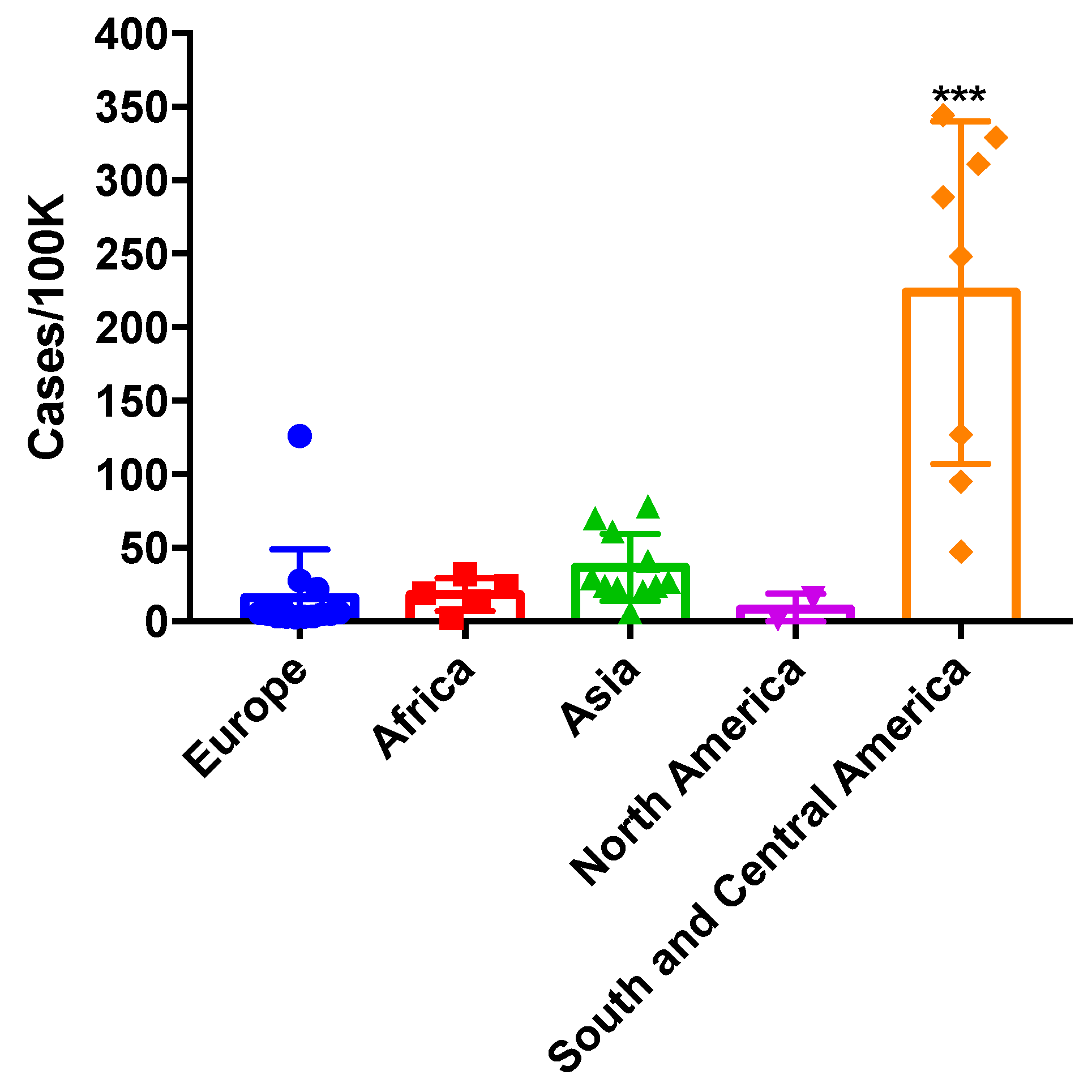
2.5. Allergic Bronchopulmonary Aspergillosis (ABPA) & Severe Asthma with Fungal Sensitisation (SAFS)
2.5.1. Up to 28,447 Patients with ABPA and 37,491 with SAFS in Ukraine
2.5.2. Discussion
2.6. Fungal Keratitis
2.6.1. 11,638 Cases of Fungal Keratitis in Mexico
2.6.2. Discussion
2.7. Recurrent Vulvovaginal Candidiasis
2.7.1. About 6% (443,237 of 7,380,000) of Nepalese Women Suffer from Recurrent Vulvovaginal Candidiasis
2.7.2. Discussion
2.8. Tinea Capitis
2.8.1. Over 15,500,000 Cases of Tinea Capitis among Nigerian Children
2.8.2. Discussion
3. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Minimizing fungal disease deaths will allow the UNAIDS target of reducing annual AIDS deaths below 500,000 by 2020 to be realized. Philos. Trans. R. Soc. B 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A neglected epidemic: Fungal infections in HIV/AIDS. Trends Microbiol. 2014, 22, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J.; Torres-Narbona, M.; Gijón, P.; Muñoz, P.; Pozo, F.; Peláez, T.; de Miguel, J.; Bouza, E. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome. Clin. Microbiol. Infect. 2010, 16, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Carter, R.A.; Boeckh, M.; Martin, P.; Corey, L. Invasive aspergillosis in allogeneic stem cell transplant recipients: Changes in epidemiology and risk factors. Blood 2002, 100, 4358–4366. [Google Scholar] [CrossRef] [PubMed]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017. [Google Scholar] [CrossRef]
- Denning, D.W. Global fungal Burden. Mycoses 2013, 56, 13. [Google Scholar]
- Rajasingham, R.; Rachel, M.S.; Benjamin, J.P.; Joseph, N.J.; Nelesh, P.G.; Tom, M.C.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global Burden of Disease of HIV-Associated Cryptococcal Meningitis: An Updated Analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Denning, D.W. The ambitious “95-95 by 2025” roadmap for the diagnosis and management of fungal diseases. Thorax 2015, 70, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Armstead, J.; Morris, J.; Denning, D.W. Multi-country estimate of different manifestations of aspergillosis in cystic fibrosis. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- The Fungal Infection Trust. How common are fungal diseases? In Proceedings of the Fungal Research Trust 20th Anniversary Meeting, London, UK, 18 June 2011; updated August 2017, London, UK. 2017. Available online: http://www.fungalinfectiontrust.org/wp-content/uploads/2015/12/How-Common-are-Fungal-Diseases-v12.2.pdf (accessed on 12 September 2017).
- Van de Sande, W.W.J. Global Burden of Human Mycetoma: A Systematic Review and Meta-analysis. PLoS Negl. Trop. Dis. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Calling upon all public health mycologists. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 923–924. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur. Respir. J. 2013, 41, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med. Mycol. 2013, 51, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Kneale, M.; Rautemaa-Richardson, R.; Sobel, J.D. Global burden of recurrent vulvovaginal candidiasis. Lancet Infect. Dis. 2017, in press. [Google Scholar]
- Pegorie, M.; Denning, D.W.; Welfare, W. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J. Infect. 2017, 74, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Global Action Fund for Fungal Infections (GAFFI). Priority Fungal Infections. Available online: http://www.gaffi.org/media/fact-sheets/ (accessed on 14 August 2017).
- Zijlstra, E.E.; van de Sande, W.W.J.; Welsh, O.; Mahgoub, E.S.; Goodfellow, M.; Fahal, A.H. Mycetoma: A unique neglected tropical disease. Lancet. Infect. Dis. 2016, 16, 100–112. [Google Scholar] [CrossRef]
- WHO Executive Board Recommends Mycetoma Resolution to World Health Assembly. 2016. Available online: http://www.who.int/neglected_diseases/news/EB_recommends_mycetoma_to_WHA/en/ (accessed on 21 September 2017).
- Queiroz-Telles, F.; de Hoog, S.; Santos, D.W.C.L.; Salgado, C.G.; Vicente, V.A.; Bonifaz, A.; Roilides, E.; Xi, L.; Azevedo, C.M.; da Silva, M.B.; et al. Chromoblastomycosis. Clin. Microbiol. Rev. 2017, 30, 233–276. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.; Leibovici, L.; Paul, M. PCR diagnosis of invasive candidiasis: Systematic review and meta-analysis. J. Clin. Microbiol. 2011, 49, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Finding the missing 50% of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Buck, M.; Witebsky, F.; Stock, F.; Pizzo, P.A.; Walsh, T.J. Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis disseminated versus single-organ infection. Diagn Microbiol. Infect. Dis. 1993, 17, 103–109. [Google Scholar] [CrossRef]
- Odds, F.C.; Hanson, M.F.; Davidson, A.D.; Jacobsen, M.D.; Wright, P.; Whyte, J.A.; Gow, N.A.; Jones, B.L. One year prospective survey of Candida bloodstream infections in Scotland. J. Med. Microbiol. 2007, 56, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Lass-Flörl, C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008, 14, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Slavin, M.; Marriott, D.; Halliday, C.; Kidd, S.; Arthur, I.; Bak, N.; Heath, C.H.; Kennedy, K.; Morrissey, C.O.; et al. Changing epidemiology of candidaemia in Australia. J. Antimicrob. Chemother. 2017, 72, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C. Candida and Candidaemia: Susceptibility and Epidemiology. Dan. Med. J. 2013, 60, 1–32. [Google Scholar]
- Fridkin, S.K.; Jarvis, W.R. Epidemiology of nosocomial fungal infections. Clin. Microbiol. Rev. 1996, 9, 499–511. [Google Scholar] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Campion, E.W.; Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar]
- Celebi, S.; Hacimustafaoglu, M.; Ozdemir, O.; Ozkaya, G. Nosocomial candidaemia in children: Results of a 9-year study. Mycoses 2008, 51, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Festekjian, A.; Neely, M. Incidence and predictors of invasive candidiasis associated with candidaemia in children. Mycoses 2011, 54, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gamaletsou, M.N.; Walsh, T.J.; Zaoutis, T.; Pagoni, M.; Kotsopoulou, M.; Voulgarelis, M.; Panayiotidis, P.; Vassilakopoulos, T.; Angelopoulou, M.K.; Marangos, M.; et al. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin. Microbiol. Infect. 2014, 20, O50–O57. [Google Scholar] [CrossRef] [PubMed]
- Zaoutis, T. Candidemia in children. Curr. Med. Res. Opin. 2010, 26, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Sood, P.; Rudramurthy, S.M.; Chen, S.; Kaur, H.; Capoor, M.; Chhina, D.; Rao, R.; Eshwara, V.K.; Xess, I.; et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensiv. Care Med. 2014, 41, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Chuang, Y.C.; Wang, J.T.; Sheng, W.H.; Yu, C.J.; Chu, C.C.; Hsueh, P.R.; Chang, S.C.; Chen, Y.C. Comparison of epidemiology and treatment outcome of patients with candidemia at a teaching hospital in Northern Taiwan, in 2002 and 2010. J. Microbiol. Immunol. Infect. 2014, 47, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Akova, M.; Herbrecht, R.; Viscoli, C.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. 2012, 18, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control. 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Hii, I.M.; Chang, H.L.; Lin, L.C.; Lee, Y.L.; Liu, Y.M.; Liu, C.E.; Chen, C.H.; Cheng, Y.R.; Chang, C.Y. Changing epidemiology of candidemia in a medical center in middle Taiwan. J. Microbiol. Immunol. Infect. 2015, 48, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Al Thaqafi, A.H.O.; Farahat, F.M.; Al Harbi, M.I.; Al Amri, A.F.W.; Perfect, J.R. Predictors and outcomes of Candida bloodstream infection: Eight-year surveillance, western Saudi Arabia. Int. J. Infect. Dis. 2014, 21, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Dzajic, E.; Jensen, R.H.; Johansen, H.K.; Kjældgaard, P.; Knudsen, J.D.; Kristensen, L.; Leitz, C.; Lemming, L.E.; Nielsen, L.; et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: Data from a nationwide fungaemia surveillance programme. Clin. Microbiol. Infect. 2013, 19. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 2010, 16, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Chakrabarti, A. Strategies to Reduce Mortality in Adult and Neonatal Candidemia in Developing Countries. J. Fungi 2017, 3, 41. [Google Scholar] [CrossRef]
- Montero, A.; Gilsanz, F.; Maseda, E. [Diagnostic and therapeutic approach of intraabdominal candidiasis]. Rev. Esp. Quimioter. Publ. Soc. Esp. Quimioter. 2016, 29, 52–55. [Google Scholar]
- Hasibeder, W.; Halabi, M. Candida peritonitis. Minerva Anestesiol. 2014, 80, 470–481. [Google Scholar] [PubMed]
- Levallois, J.; Nadeau-Fredette, A.C.; Labbé, A.C.; Laverdière, M.; Ouimet, D.; Vallée, M. Ten-year experience with fungal peritonitis in peritoneal dialysis patients: Antifungal susceptibility patterns in a North-American center. Int. J. Infect. Dis. 2012, 16. [Google Scholar] [CrossRef] [PubMed]
- Vergidis, P.; Clancy, C.J.; Shields, R.K.; Park, S.Y.; Wildfeuer, B.N.; Simmons, R.L.; Nguyen, M.H. Intra-abdominal candidiasis: The importance of early source control and antifungal treatment. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Giacomazzi, J.; Baethgen, L.; Carneiro, L.C.; Millington, M.A.; Denning, D.W.; Colombo, A.L.; Pasqualotto, A.C.; Association With The LIFE Program. The burden of serious human fungal infections in Brazil. Mycoses 2016, 59, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, K.; Farooqi, J.; Mirza, S.; Denning, D.; Zafar, A. Serious fungal infections in Pakistan. Eur. J. Clin. Microbiol. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Taj-Aldeen, S.J.; Chandra, P.; Denning, D.W. Burden of fungal infections in Qatar. Mycoses 2015, 58, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Chayakulkeeree, M.; Denning, D.W. Serious fungal infections in Thailand. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Sinkó, J.; Sulyok, M.; Denning, D.W. Burden of serious fungal diseases in Hungary. Mycoses 2015, 58, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Denning, D.W. Estimating the burden of fungal diseases in Israel. Isr. Med. Assoc. J. 2015, 17, 374–380. [Google Scholar]
- Mortensen, K.L.; Denning, D.W.; Arendrup, M.C. The burden of fungal disease in Denmark. Mycoses 2015, 58, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Klimko, N.; Kozlova, Y.; Khostelidi, S.; Shadrivova, O.; Borzova, Y.; Burygina, E.; Vasilieva, N.; Denning, D.W. The burden of serious fungal diseases in Russia. Mycoses 2015, 58, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tudela, J.L.; Alastruey-Izquierdo, A.; Gago, S.; Cuenca-Estrella, M.; León, C.; Miro, J.M.; Nuñez Boluda, A.; Ruiz Camps, I.; Sole, A.; Denning, D.W.; et al. Burden of serious fungal infections in Spain. Clin. Microbiol. Infect. 2015, 21, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Dorgan, E.; Denning, D.W.; McMullan, R. Burden of fungal disease in Ireland. J. Med. Microbiol. 2015, 64, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Oladele, R.O.; Denning, D.W. Burden of serious fungal infection in Nigeria. West Afr. J. Med. 2014, 33, 107–114. [Google Scholar] [PubMed]
- Tilavberdiev, S.A.; Denning, D.W.; Klimko, N.N. Serious fungal diseases in the Republic of Uzbekistan. Eur. J. Clin. Microbiol. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chekiri-Talbi, M.; Denning, D.W. Burden of fungal infections in Algeria. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, H.C.; Denning, D.W.; Rahim, R.; Sadat, A.; Belal, M.; Mahbub, M.S. Burden of serious fungal infections in Bangladesh. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Lagrou, K.; Maertens, J.; Van Even, E.; Denning, D.W. Burden of serious fungal infections in Belgium. Mycoses 2015, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Duarte, E.; Denning, D.W. Serious fungal infections in Chile. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Chrdle, A.; Mallátová, N.; Vašáková, M.; Haber, J.; Denning, D.W. Burden of serious fungal infections in the Czech Republic. Mycoses 2015, 58, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, H.C.; Denning, D.W. Burden of serious fungal infections in the Dominican Republic. J. Infect. Public Health 2016, 9, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zurita, J.; Denning, D.W.; Paz-y-Miño, A.; Solís, M.B.; Arias, L.M. Serious fungal infections in Ecuador. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.M.; Denning, D.W. Serious fungal infections in Egypt. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Gamaletsou, M.N.; Drogari-Apiranthitou, M.; Denning, D.W.; Sipsas, N.V. An estimate of the burden of serious fungal diseases in Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Medina, N.; Samayoa, B.; Lau-Bonilla, D.; Denning, D.W.; Herrera, R.; Mercado, D.; Guzmán, B.; Pérez, J.C.; Arathoon, E. Burden of serious fungal infections in Guatemala. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, H.C.; Denning, D.W. Estimated Burden of Serious Fungal Infections in Jamaica by Literature Review and Modelling. West Ind. Med. J. 2015, 64, 245–249. [Google Scholar]
- Guto, J.A.; Bii, C.C.; Denning, D.W. Estimated burden of fungal infections in Kenya. J. Infect. Dev. Ctries 2016, 10, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Corzo-León, D.E.; Armstrong-James, D.; Denning, D.W. Burden of serious fungal infections in Mexico. Mycoses 2015, 58, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, B.; Denning, D.W.; Campos, P.E. Serious fungal infections in Peru. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Faini, D.; Maokola, W.; Furrer, H.; Hatz, C.; Battegay, M.; Tanner, M.; Denning, D.W.; Letang, E. Burden of serious fungal infections in Tanzania. Mycoses 2015, 58, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Gugnani, H.C. Burden of serious fungal infections in Trinidad and Tobago. Mycoses 2015, 58, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Osmanov, A.; Denning, D.W. Burden of serious fungal infections in Ukraine. Mycoses 2015, 58, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.; Nguyen, T.A.; Ha, H.T.T.; Thang, P.H.; Thuy, C.; Xuan Lien, T.; Bui, H.T.; Le, T.H.; Struminger, B.; McConnell, M.S.; et al. Prevalence of Cryptococcal Antigenemia and Cost-Effectiveness of a Cryptococcal Antigen Screening Program–Vietnam. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, M.; Groll, A.; Mayser, P.; Schaller, M.; Mendling, W.; Ullmann, A.; Denning, D.W.; The University of Manchester in association with the LIFE program. Burden of serious fungal infections in Germany. Int. J. Infect. Dis. 2014, 21, 281. [Google Scholar] [CrossRef]
- Statistisches Bundesamt. Wiesbaden. 2015. Available online: https://www.destatis.de/EN/Homepage.html (accessed on 21 September 2017).
- Huh, K.; Ha, Y.E.; Denning, D.W.; Peck, K.R. Serious fungal infections in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Gangneux, J.-P.; Bougnoux, M.-E.; Hennequin, C.; Godet, C.; Chandenier, J.; Denning, D.W.; Dupont, B.; LIFE program; the Société française de mycologie médicale SFMM-study group. An estimation of burden of serious fungal infections in France. J. Mycol. Méd. J. Med. Mycol. 2016, 26, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, S.F.; Cole, D.C.; Denning, D.W.; Sheppard, D.C. Serious fungal infections in Canada. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Gregson, D.B.; Church, D.L.; Ross, T.; Elsayed, S. Invasive Candida species infections: A 5 year population-based assessment. J. Antimicrob. Chemother. 2005, 56, 532–537. [Google Scholar] [CrossRef] [PubMed]
- St-Germain, G.; Laverdière, M.; Pelletier, R.; René, P.; Bourgault, A.M.; Lemieux, C.; Libman, M. Epidemiology and antifungal susceptibility of bloodstream Candida isolates in Quebec: Report on 453 cases between 2003 and 2005. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 55–62. [Google Scholar] [PubMed]
- Lass-Flörl, C.; Denning, D.W. “Fungal Burden” weltweit und in Österreich—LIFE Project. 2016. Available online: http://www.gaffi.org/wp-content/uploads/Fungal-Burden.pdf (accessed on 1 August 2017).
- Jayasekera, P.I.; Denning, D.; Perera, P.; Fernando, A.; Kudavidanage, S. The burden of serious fungal infections in Sri Lanka. Mycoses 2013, 56, 103. [Google Scholar]
- Sabino, R.; Verissímo, C.; Brandão, J.; Martins, C.; Alves, D.; Pais, C.; Denning, D.W. Serious fungal infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Faria-Ramos, I.; Neves-Maia, J.; Ricardo, E.; Santos-Antunes, J.; Silva, A.T.; Costa-de-Oliveira, S.; Cantón, E.; Rodrigues, A.G.; Pina-Vaz, C. Species distribution and in vitro antifungal susceptibility profiles of yeast isolates from invasive infections during a Portuguese multicenter survey. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Batac, M.C.R.; Denning, D. Serious fungal infections in the Philippines. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Bories, P.; Moulin, J.-C.; Ledoux, M.-P.; Letscher-Bru, V. Risk stratification for invasive aspergillosis in immunocompromised patients. Ann. N. Y. Acad. Sci. 2012, 1272, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Iversen, M.; Burton, C.M.; Vand, S.; Skovfoged, L.; Carlsen, J.; Milman, N.; Andersen, C.B.; Rasmussen, M.; Tvede, M. Aspergillus infection in lung transplant patients: Incidence and prognosis. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierichini, A.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W.; Walsh, T.J. Special considerations for the diagnosis and treatment of invasive pulmonary aspergillosis. Expert Rev. Respir. Med. 2017, 11, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Gavaldà, J.; Meije, Y.; Fortún, J.; Roilides, E.; Saliba, F.; Lortholary, O.; Muñoz, P.; Grossi, P.; Cuenca-Estrella, M.; ESCMID Study Group for Infections in Compromised Hosts. Invasive fungal infections in solid organ transplant recipients. Clin. Microbiol. Infect. 2014, 20, 27–48. [Google Scholar]
- Kimura, S.-I. Invasive Aspergillosis in Hematological Patients. Med. Mycol. J. 2016, 57, J77–J88. [Google Scholar] [CrossRef] [PubMed]
- Garbino, J.; Fluckiger, U.; Elzi, L.; Imhof, A.; Bille, J.; Zimmerli, S. Survey of aspergillosis in non-neutropenic patients in Swiss teaching hospitals. Clin. Microbiol. Infect. 2011, 17, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, M.; Jiang, M.; Zou, L.-Q.; Luo, F.; Jiang, Y. Clinical characteristics of 45 patients with invasive pulmonary aspergillosis: Retrospective analysis of 1711 lung cancer cases. Cancer 2009, 115, 5018–5025. [Google Scholar] [CrossRef] [PubMed]
- Prattes, J.; Hoenigl, M.; Krause, R.; Buzina, W.; Valentin, T.; Reischies, F.; Koidl, C.; Zollner-Schwetz, I. Invasive aspergillosis in patients with underlying liver cirrhosis: A prospective cohort study. Med. Mycol. 2017, 55, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Massetti, A.P.; Russo, A.; Vullo, V.; Venditti, M. Invasive aspergillosis in patients with liver disease. Med. Mycol. 2011, 49, 406–413. [Google Scholar] [CrossRef] [PubMed]
- López-Medrano, F.; Fernández-Ruiz, M.; Silva, J.T.; Carver, P.L.; van Delden, C.; Merino, E.; Pérez-Saez, M.J.; Montero, M.; Coussement, J.; de Abreu Mazzolin, M.; et al. Multinational retrospective case-control study of risk factors for the development of late invasive pulmonary aspergillosis following kidney transplantation. Clin. Microbiol. Infect. 2017. [Google Scholar] [CrossRef] [PubMed]
- Komase, Y.; Kunishima, H.; Yamaguchi, H.; Ikehara, M.; Yamamoto, T.; Shinagawa, T. Rapidly progressive invasive pulmonary aspergillosis in a diabetic man. J. Infect. Chemother. 2007. [Google Scholar] [CrossRef] [PubMed]
- Tartiere, D.; Seguin, P.; Malledant, Y.; Gangneux, J.P. Sepsis may be a new risk factor for invasive aspergillosis in immunocompetent patients. Int. J. Tuberc. Lung Dis. 2012, 16, 1135–1136. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Andes, D.R.; Marr, K.A.; Kontoyiannis, D.P.; Alexander, B.D.; Kauffman, C.A.; Oster, R.A.; Anaissie, E.J.; Walsh, T.J.; Schuster, M.G.; et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin. Infect. Dis. 2010, 50, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, L.; Huang, W.-J.; Wang, L.-X.; Li, W.-F.; Yuan, W.-F. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: A case control study from China. Clin. Microbiol. Infect. 2012, 18, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Aquino, V.R.; Nagel, F.; Andreolla, H.F.; de-Paris, F.; Xavier, M.O.; Goldani, L.Z.; Denning, D.W.; Pasqualotto, A.C. The Performance of Real-Time, P.C.R.; Galactomannan, and Fungal Culture in the Diagnosis of Invasive Aspergillosis in Ventilated Patients with Chronic Obstructive Pulmonary Disease (COPD). Mycopathologia 2012, 174, 163–169. [Google Scholar] [CrossRef] [PubMed]
- OECD. Avoidable admissions: Respiratory diseases. In Health at a Glance 2011: OECD Indicators; OECD Publishing: Paris, France, 2011. [Google Scholar]
- López-Campos, J.L.; Hartl, S.; Pozo-Rodriguez, F.; Roberts, C.M. Antibiotic Prescription for COPD Exacerbations Admitted to Hospital: European COPD Audit. PLoS ONE 2015, 10, e0124374. [Google Scholar] [CrossRef]
- Tan, W.C.; Seale, P.; Ip, M.; Shim, Y.S.; Chiang, C.H.; Ng, T.P.; Abisheganadan, J.; Charoenratanakul, S.; DeGuia, T.; Mahayiddin, A.; et al. Trends in COPD mortality and hospitalizations in countries and regions of Asia-Pacific. Respirology 2009, 14, 90–97. [Google Scholar] [CrossRef] [PubMed]
- IARC. Globocan Registry 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 12 September 2017).
- Beardsley, J.; Denning, D.; Chau, N.; Crump, J.; Day, J. Estimating the burden of fungal diseases in Vietnam. Eur. Conf. Clin. Microbiol. Infect. Dis. Published Online First: 2014. Available online: http://eccmid.meetingxpert.net/ECCMID_699/poster_108496/program.aspx/anchor108496 (accessed on 31 August 2017).
- Lim, S.; Lam, D.C.L.; Muttalif, A.R.; Yunus, F.; Wongtim, S.; Lan le, T.T.; Shetty, V.; Chu, R.; Zheng, J.; Perng, D.W.; et al. Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region: The EPIC Asia population-based survey. Asia Pac. Fam. Med. 2015, 14, e4. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Murio, C.; Tirado-Conde, G.; Levy, G.; Muellerova, H.; Soriano, J.B.; Ramirez-Venegas, A.; Ko, F.W.S.; Canelos-Estrella, B.; Giugno, E.; et al. Geographic differences in clinical characteristics and management of COPD: The EPOCA study. Int. J. Chron. Obstruct. Pulmon. Dis. 2008, 3, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Goncer, I. Aspergillosis in AIDS. Aspergillus & Aspergillosis Website. 2016. Available online: http://www.aspergillus.org.uk/content/aspergillosis-aids-0 (accessed on 12 August 2017).
- Denis, B.; Guiguet, M.; de Castro, N.; Mechaï, F.; Revest, M.; Melica, G.; Costagliola, D.; Lortholary, O. French Hospital Database on HIV National Agency for Research on AIDS and Viral Hepatitis, France CO4. Relevance of EORTC Criteria for the Diagnosis of Invasive Aspergillosis in HIV-Infected Patients, and Survival Trends Over a 20-Year Period in France. Clin. Infect. Dis. 2015, 61, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, J.; Denning, D.W.; Chau, N.V.; Yen, N.T.B.; Crump, J.A.; Day, J.N. Estimating the burden of fungal disease in Vietnam. Mycoses 2015, 58, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Khwakhali, U.S.; Denning, D.W. Burden of serious fungal infections in Nepal. Mycoses 2015, 58, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Parkes-Ratanshi, R.; Achan, B.; Kwizera, R.; Kambugu, A.; Meya, D.; Denning, D.W. Cryptococcal disease and the burden of other fungal diseases in Uganda; Where are the knowledge gaps and how can we fill them? Mycoses 2015, 58, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, H.C.; Denning, D.W.; Rahim, R.; Sadat, A.; Belal, M.; Mahbub, M.S.; Medina, N.; Samayoa, B.; Lau-Bonilla, D.; Denning, D.W.; et al. Burden of serious fungal infections in Bangladesh. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 965–969. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Report on the Global AIDS Epidemic. Geneva, Switzerland: Joint UN Program on HIV/AIDS. AIDSInfo. 2016. Available online: http://aidsinfo.unaids.org/ (accessed on 7 July 2017).
- Samayoa, B.; Isern, V.; Serra, L.; Rivera, B.E.; Nikiforov, M.; Arathoon, E.; Samayoa, C.; Vallès, X.; Prieto, L.; Álvarez, C.; et al. Preliminary results of a prospective cohort of HIV+ patients in Guatemala. In Proceedings of the XVIII International AIDS Conference, Vienna, Austria, 18–23 July 2010. [Google Scholar]
- Krajicek, B.J.; Thomas, C.F.; Limper, A.H. Pneumocystis Pneumonia: Current Concepts in Pathogenesis, Diagnosis, and Treatment. Clin. Chest Med. 2009, 30, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Cooley, L.; Dendle, C.; Wolf, J.; Teh, B.W.; Chen, S.C.; Boutlis, C.; Thursky, K.A. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern. Med. J. 2014, 44, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, S.; Tokuda, H. Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J. Infect. Chemother. 2012, 18, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, A.-L.; Traore, K.; Plekhanova, I.; Bouchrik, M.; Bossard, C.; Picot, S. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int. J. Infect. Dis. 2016, 46, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cattamanchi, A.; Davis, J.L.; den Boon, S.; Kovacs, J.; Meshnick, S.; Miller, R.F.; Walzer, P.D.; Worodria, W.; Masur, H.; et al. HIV-associated Pneumocystis pneumonia. Proc. Am. Thorac. Soc. 2011, 8, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ren, Y.; Wang, X.; Li, R. Recent Advances in the Diagnosis of Pneumocystis Pneumonia. Med. Mycol. J. 2016, 57, E111–E116. [Google Scholar] [CrossRef] [PubMed]
- Oladele, R.O.; Otu, A.A.; Richardson, M.D.; Denning, D.W. Diagnosis and management of pneumocystis pneumonia (PCP) in resource poor settings. J. Health Care Poor Underserved 2017, in press. [Google Scholar]
- Badiane, A.S.; Ndiaye, D.; Denning, D.W. Burden of fungal infections in Senegal. Mycoses 2015, 58, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2011, 37, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Lowes, D.; Al-Shair, K.; Newton, P.J.; Morris, J.; Harris, C.; Rautemaa-Richardson, R.; Denning, D.W. Predictors of mortality in chronic pulmonary aspergillosis. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Denning, D.W.; Chakrabarti, A. Estimation of the burden of chronic and allergic pulmonary Aspergillosis in India. PLoS ONE 2014, 9, e114745. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pashley, C.; Hartl, D.; Wardlaw, A.; Godet, C.; Del Giacco, S.; Delhaes, L.; Sergejeva, S. Fungal allergy in asthma-state of the art and research needs. Clin. Transl. Allergy 2014, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; Greenberger, P.A.; Radin, R.C.; Roberts, M. Allergic bronchopulmonary aspergillosis: Staging as an aid to management. Ann. Intern. Med. 1982, 96, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Moss, R.B.; Kurup, V.P.; Knutsen, A.P.; Greenberger, P.; Judson, M.A.; Denning, D.W.; Crameri, R.; Brody, A.S.; Light, M.; et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis—State of the art: Cystic Fibrosis Foundation Consensus Conference. Clin. Infect. Dis. 2003, 37, S225–S264. [Google Scholar] [CrossRef] [PubMed]
- Al-Mobeireek, A.F.; Gad El-Rab, M.O.; Al-Hedaithy, S.S.A.; Alasali, K.; Al-Majed, S.; Joharjy, I. Allergic bronchopulmonary mycosis in patients with asthma: Period prevalence at a university hospital in Saudi Arabia. Respir. Med. 2001, 95, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; Coggeshall, M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.P. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health 2012. [Google Scholar] [CrossRef] [PubMed]
- Anandan, C.; Gupta, R.; Simpson, C.; Fischbacher, C.; Sheikh, A. Epidemiology and disease burden from allergic disease in Scotland: Analyses of national databases. J. R. Soc. Med. 2009, 102, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Kala, J.; Sahay, S.; Panjabi, C. Frequency of familial occurrence in 164 patients with allergic bronchopulmonary aspergillosis. Ann. Allergy Asthma Immunol. 2008, 101, 363–369. [Google Scholar] [CrossRef]
- Denning, D.W.; O’Driscoll, B.R.; Hogaboam, C.M.; Bowyer, P.; Niven, R.M. The link between fungi and severe asthma: A summary of the evidence. Eur. Respir. J. 2006, 27, 615–626. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, B.R.; Hopkinson, L.C.; Denning, D.W. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm. Med. 2005, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, B.R.; Powell, G.; Chew, F.; Niven, R.M.; Miles, J.F.; Vyas, A.; Denning, D.W. Comparison of skin prick tests with specific serum immunoglobulin E in the diagnosis of fungal sensitization in patients with severe asthma. Clin. Exp. Allergy 2009, 39, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. Severe asthma with fungal sensitization. Curr. Allergy Asthma Rep. 2011, 11, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Appendix 3: Burden of Fungal Diseases. Glob. Action Fund Fungal Infect. 2015. Available online: http://www.gaffi.org/wp-content/uploads/Appendices-3-V3.pdf (accessed on 31 August 2017).
- Cao, J.; Yang, Y.; Yang, W.; Wu, R.; Xiao, X.; Yuan, J.; Xing, Y.; Tan, X. Prevalence of infectious keratitis in Central China. BMC Ophthalmol. 2014, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vanzzini Zago, V.; Manzano-Gayosso, P.; Hernández-Hernández, F.; Méndez-Tovar, L.J.; Gómez-Leal, A.; López Martínez, R. Mycotic keratitis in an eye care hospital in Mexico city./Queratomicosis en un centro de atención oftalmológica en la Ciudad de México. Rev. Iberoam. Micol. 2010, 27, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, U.; Ph, D.; Garg, P.; Fernandes, M.; Sharma, S.; Athmanathan, S.; Rao, G.N. The Epidemiological Features and Laboratory Results of Fungal Keratitis. Eye 2002, 21, 555–559. [Google Scholar] [CrossRef]
- Green, M.D.; Jg, A.; Franzco, A.; Naduvilath, T.; Stapleton, F.J. Clinical outcomes of keratitis. Clin. Exp. Ophthalmol. 2007, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ali Shah, S.I.; Shah, S.A.; Rai, P.; Katpar, N.A.; Abbasi, S.A.; Soomro, A.A. Visual outcome in patients of keratomycosis, at a tertiary care centre in Larkana, Pakistan. J. Pak. Med. Assoc. 2017, 67, 1035–1038. [Google Scholar] [PubMed]
- Farrell, S.; Mcelnea, E.; Moran, S.; Knowles, S.; Murphy, C.C. Fungal keratitis in the Republic of Ireland. Eye 2017. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Walken, A.; Au, L.; Fullwood, C.; Hamilton, A.; Qamruddin, A.; Armstrong, M.; Brahma, A.K.; Carley, F. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye 2017, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.P.; Karmacharya, P.C.; Koirala, S.; Tuladhar, N.R.; Bryan, L.E.; Smolin, G.; Whitcher, J.P. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am. J. Ophthalmol. 1991, 111, 92–99. [Google Scholar] [CrossRef]
- Gonzales, C.A.; Srinivasan, M.; Whitcher, J.P.; Smolin, G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996, 3, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Erie, J.C.; Nevitt, M.P.; Hodge, D.O.; Ballard, D.J. Incidence of Ulcerative Keratitis in a Defined Population From 1950 Through 1988. Arch. Ophthalmol. 1993, 111, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Hong Nhung, P. Epidemiology of Fungal Keratitis in North Vietnam. J. Clin. Exp. Ophthalmol. 2012, 3. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Y.; Feng, X.; Liu, Y.; Wang, S.; Zhu, X.; Chen, Q.; Pan, S. Candidemia: Incidence rates, type of species, and risk factors at a tertiary care academic hospital in China. Int. J. Infect. Dis. 2014, 22, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Nielsen, E.; Julian, H.O.; Lindegaard, J.; Højgaard, K.; Ivarsen, A.; Hjortdal, J.; Heegaard, S. Incidence and clinical characteristics of fungal keratitis in a Danish population from 2000 to 2013. Acta Ophthalmol. 2015, 93, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Sobel, J.D. Genital candidiasis. Medicine (Baltimore) 2014, 42, 364–368. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Sobel, J.D. Mucosal candidiasis. Infect. Dis. Clin. North Am. 2002, 16, 793–820. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Muraglia, R.; Dietz, J.-P.; Sobel, J.D.; Wagner, J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: Results from an internet panel survey. J. Low. Genit. Tract Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Adefemi, S.A.; Odeigah, L.O.; Alabi, K.M. Prevalence of dermatophytosis among primary school children in Oke-oyi community of Kwara state. Niger. J. Clin. Pract. 2011, 14, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Oke, O.O.; Onayemi, O.; Olasode, O.A.; Omisore, A.G.; Oninla, O.A. The prevalence and pattern of superficial fungal infections among school children in ile-ife, south-western nigeria. Dermatol. Res. Pract. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Dogo, J.; Afegbua, S.L.; Dung, E.C. Prevalence of Tinea Capitis among School Children in Nok Community of Kaduna State, Nigeria. J. Pathog. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nweze, E.I.; Eke, I.E. Dermatophytes and dermatophytosis in the eastern and southern parts of Africa. Med. Mycol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J. Tinea Capitis: Current Status. Mycopathologia 2017, 182, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kieliger, S.; Glatz, M.; Cozzio, A.; Bosshard, P.P. Tinea capitis and tinea faciei in the Zurich area—An 8-year survey of trends in the epidemiology and treatment patterns. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Moto, J.N.; Maingi, J.M.; Nyamache, A.K. Prevalence of Tinea capitis in school going children from Mathare, informal settlement in Nairobi, Kenya. BMC Res. Notes 2015, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Buginco, C. Dermatophytic Infection of the Scalp in the Region of Butare (Rwanda). Int. J. Dermatol. 1983, 22, 107–109. [Google Scholar] [CrossRef]
- Diongue, K.; Diallo, M.A.; Ndiaye, M.; Badiane, A.S.; Seck, M.C.; Diop, A.; Ndiaye, Y.D.; Ndiaye, D. Champignons agents de mycoses superficielles isolés à Dakar (Sénégal): Une étude rétrospective de 2011 à 2015. J. Mycol. Med. 2016, 26, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, O.; Kone, A.K.; Niaré-Doumbo, S.; Goïta, S.; Gaudart, J.; Djimdé, A.A.; Piarroux, R.; Doumbo, O.K.; Thera, M.A.; Ranque, S. Dermatophytosis among Schoolchildren in Three Eco-climatic Zones of Mali. PLoS Negl. Trop. Dis. 2016, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Salinas, M.; Marin-Cabanas, I.; Betlloch, I.; Tesfasmariam, A.; Reyes, F.; Belinchon, I.; Ramos, J.M. Tinea capitis in schoolchildren in a rural area in southern Ethiopia. Int. J. Dermatol. 2015, 54, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Kalu, E.I.; Wagbatsoma, V.; Ogbaini-Emovon, E.; Nwadike, V.U.; Ojide, C.K. Age and sex prevalence of infectious dermatoses among primary school children in a rural South-Eastern Nigerian community. Pan. Afr. Med. J. 2015, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ayaya, S.O.; Kamar, K.K.; Kakai, R. Aetiology of tinea capitis in school children. East Afr. Med. J. 2001, 78, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Ayanbimpe, G.M.; Taghir, H.; Diya, A.; Wapwera, S. Tinea capitis among primary school children in some parts of central Nigeria. Mycoses 2008, 51, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, A.A.; Adegnika, A.A.; Bouwes Bavinck, J.N.; Yazdanbakhsh, M.; Kremsner, P.G.; van der Raaij-Helmer, E.M.H.; Staats, C.C.; Willemze, R.; Lavrijsen, A.P. Prevalence and causative fungal species of tinea capitis among schoolchildren in Gabon. Mycoses 2011, 54. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, A.; Amoah, A.; Bavinck, J.N.B.; Boakye, D.; Yazdanbakhsh, M.; Adegnika, A.; De Smedt, S.; Fonteyne, Y.; Willemze, R.; Lavrijsen, A.; et al. Skin diseases among schoolchildren in Ghana, Gabon, and Rwanda. Int. J. Dermatol. 2013, 52, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Frederick, T.; Satimia, A.; Sandra, R.M.; Barbara, L. Prevalence of skin diseases in rural tanzania and factors influencing the choice of health care, mordern or traditional. Arch. Dermatol. 1998, 134, 1363–1366. [Google Scholar]
- Ayanlowo, O.; Akinkugbe, A.; Oladele, R.; Balogun, M. Prevalence of Tinea capitis infection among primary school children in a rural setting in south-west Nigeria. J. Public Health Afr. 2014, 5, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Fulgence, K.K.; Abibatou, K.; Vincent, D.; Henriette, V.; Etienne, A.K.; Kiki-Barro, P.C.; Yavo, W.; Koné, M.; Hervé Menan, E.I. Tinea capitis in schoolchildren in southern Ivory Coast. Int. J. Dermatol. 2013, 52, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Chepchirchir, A.; Bii, C.; Ndinya-Achola, J.O. Dermatophyte infections in primary school children in kibera slums of nairobi. East Afr. Med. J. 2009, 86, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Emele, F.E.; Oyeka, C.A. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses 2008, 51, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Kechia, F.A.; Kouoto, E.A.; Nkoa, T.; Nweze, E.I.; Fokoua, D.C.M.; Fosso, S.; Somo, M.R. Epidemiology of tinea capitis among school-age children in Meiganga, Cameroon. J. Mycol. Med. 2014, 24, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Schmeller, W.; Baumgartner, S.; Dzikus, A. Dermatophytomycoses in children in rural Kenya: The impact of primary health care. Mycoses 1997, 40, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Komba, E.V.; Mgonda, Y.M. The spectrum of dermatological disorders among primary school children in Dar es Salaam. BMC Public Health 2010, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Sidat, M.M.; Correia, D.; Buene, T.P. Tinea capitis among children at one suburban primary school in the City of Maputo, Mozambique. Rev. Soc. Bras. Med. Trop. 2007, 40, 473–475. [Google Scholar] [CrossRef] [PubMed]
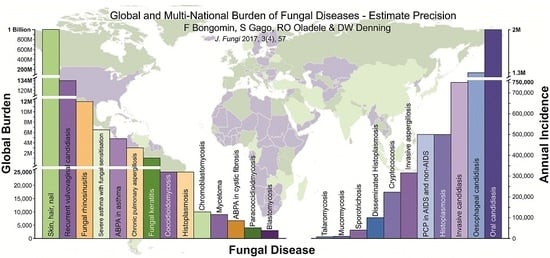
| Fungal Disease | Annual Incidence | Global Burden | Comments |
|---|---|---|---|
| Superficial | |||
| Skin, hair, nail | ~1,000,000,000 | ||
| Fungal keratitis | ~1,000,000 | ||
| Mucosal | |||
| Oral candidiasis | ~2,000,000 | HIV only, 90% of those not on ARVs | |
| Oesophageal candidiasis | ~1,300,000 | HIV only, 20% on those with CD4 counts <200 and 5% of those on ARVs | |
| Vulvovaginal candidiasis episode | 70% affected in their lifetime | ||
| Recurrent vulvovaginal candidiasis | ~134,000,000 | Annual prevalence. Nearly 500 million lifetime experience | |
| Allergic | |||
| Allergic bronchopulmonary aspergillosis in asthma | ~4,800,000 | Adults only, rare in children | |
| Allergic bronchopulmonary aspergillosis in cystic fibrosis | ~6675 | Adults only, starts from age 4 | |
| Severe asthma with fungal sensitisation | ~6,500,000 | Adults only, probably uncommon in children | |
| Fungal rhinosinusitis | ~12,000,000 | ||
| Chronic severe | |||
| Chronic pulmonary aspergillosis | ~3,000,000 | ||
| Mycetoma | ~9000 | 1950–2013 case reports, NTD | |
| Chromoblastomycosis | >10,000 | Limited data and uncommon, NTD | |
| Coccidioidomycosis | ~25,000 | ||
| Paracoccidioidmycosis | ~4000 | ||
| Blastomycosis | ~3000 | ||
| Histoplasma infection | ~500,000 | ~25,000 | Most of the new infections are asymptomatic based on skin testing |
| Sporotrichosis | >40,000 | Very limited global data. Very common in hyper endemic regions of Peru, Brazil and Mexico | |
| Acute invasive | |||
| Invasive candidiasis | ~750,000 | Includes 60,000–100,000 cases of intra-abdominal candidiasis | |
| Invasive aspergillosis | >300,000 | From about 10 million at risk annually | |
| Pneumocystis jirovecii pneumonia in AIDS and non-AIDS | ~500,000 | ||
| Cryptococcosis in AIDS | ~223,000 | HIV-related, up to another 10% non-HIV | |
| Mucormycosis | >10,000 | Based on French data = 4200. | |
| Based on Indian data = 910,000 | |||
| Disseminated histoplasmosis | ~100,000 | No reliable estimates | |
| Talaromycosis * | ~8000 | SE Asia only; |
| Country (Reference) | Burden | Rate/100,000 | Comments |
|---|---|---|---|
| Brazil [52] | 28,991 | 14.9 | No local incidence data |
| Pakistan [53] | 38,795 | 21 | 1.6 per 100,000 + 50% of candida peritonitis |
| Qatar [54] | 288 | 15.4 | 15.4 per 100,000 from previous studies |
| Thailand [55] | 8650 | 13.3 | 94% of non-neutropenic patients with fungaemia + 4.5% of neutropenic patients |
| Hungary [56] | 1110 | 11 | 3–10 per 100,000 from previous studies + 780 cases in chronic ambulatory peritoneal dialysis |
| Israel [57] | 664 | 11 | Incidence rate obtained from a nationwide surveillance between 2005 and 2007 and adjusted to the number of ICU hospital days in 2012 |
| Denmark [58] | 527 | 9.4 | Data from Arendrup et al. [30] |
| Russia [59] | 11,840 | 8.3 | 0.37 per 1000 hospitalised patients based on previous report |
| Spain [60] | 3807 | 8.1 | 8.1 per 100,000 from previous study |
| United Kingdom [19] | 5142 | 8.1 | 38% of probable or proven invasive candidiasis tested by blood culture techniques in England, wales and northern Ireland, plus 4.8 cases per 100,000 in Scotland |
| Ireland [61] | 403 | 6.3 | Statistics and Research records office voluntary laboratory reporting programme |
| Nigeria [62] | 9284 | 6 | 6 per 100,000 + 50% of candida peritonitis |
| Uzbekistan [63] | 1825 | 5.9 | Data from Ministry of Health |
| Algeria [64] | 2020 | 5 | No local incidence data |
| Bangladesh [65] | 8100 | 5 | No local incidence data |
| Belgium [66] | 555 | 5 | No local incidence data |
| Chile [67] | 878 | 5 | No local incidence data |
| Czech Republic [68] | 526 | 5 | No local incidence data |
| Dominican Republic [69] | 504 | 5 | No local incidence data |
| Ecuador [70] | 1037 | 5 | No local incidence data |
| Egypt [71] | 4127 | 5 | 5 per 100,000 and 0.98 per 100,000 of intra-abdominal peritonitis |
| Greece [72] | 541 | 5 | No local incidence data |
| Guatemala [73] | 772 | 5 | No local incidence data |
| Jamaica [74] | 136 | 5 | 5.5 per 100,000 |
| Kenya [75] | 1990 | 5 | No local incidence data |
| Mexico [76] | 5617 | 5 | 5 per 100,000 + 285 in candida peritonitis |
| Peru [77] | 1557 | 5 | 2–11 per 100,000 + 50% of candida peritonitis |
| Tanzania [78] | 2181 | 5 | No local incidence data |
| Trinidad and Tobago [79] | 87 | 5 | 5 per 100,000 + 285 in candida peritonitis |
| Ukraine [80] | 752 | 5 | 5 per 100,000 + 285 in candida peritonitis |
| Vietnam [81] | 4540 | 5 | No local incidence data |
| Germany [82] | 3712 | 4.6 | Data from Statistisches Bundesamt Wiesbaden 2015 [83] |
| Korea [84] | 1976 | 4.1 | 0.22 per 1000 patients |
| France [85] | 2370 | 3.6 | No local incidence data |
| Canada [86] | 1034 | 2.9 | Average from 2 studies, 2.8 cases/100,000 [87] and 3.3 cases/100,000 [88] over a 5 year period |
| Austria [89] | 209 | 2.6 | - |
| Sri Lanka [90] | 507 | 2.5 | unpublished data from the department of mycology |
| Portugal [91] | 231 | 2.2 | Data from Portuguese multicentre survey [92] |
| Philippines [93] | 1968 | 2 | 2 per 100,000 + 50% candida peritonitis |
| Country (Reference) | Burden | Rate/100,000 | Assumptions |
|---|---|---|---|
| Vietnam [120] | 14,523 | 16.0 | 10% of, A.M.L.; 10% of non-AML, 0.5% of renal, T.X.; 4% of lung and liver Tx, 6% of heart Tx, 3.9% of COPD admissions |
| Egypt [71] | 9001 | 10.7 | 2.6% of COPD admissions, 10% of, A.M.L.; 10% of all other predisposing haematological malignancies, 1% of renal Tx, 4% of liver Tx, and 2.6% of lung Tx |
| Greece [72] | 1125 | 10.4 | 10% of, A.M.L.; 8% of HSCT, 6% of heart Tx, 4% of lung and liver Tx, 1% of kidney, 1.3% of COPD admissions |
| Algeria [64] | 2865 | 7.1 | 1.3% of COPD admissions and 2.6% lung cancer, 7.7% neutropenic patients |
| Ireland [61] | 445 | 7.0 | 10% of, A.M.L.; 8% of HSCT, 0.5% of renal Tx, 2% of heart Tx, 0.9% of liver Tx, 9.1% of lung Tx, 1.3% of COPD admissions |
| Israel [57] | 574 | 6.8 | 10% of, A.M.L.; 10% of HSCT, 0.5% of renal Tx, 4% of lung, T.X.; 6% of heart Tx, 4% of liver Tx, 1.3% of COPD admissions |
| Belgium [66] | 675 | 6.1 | 10% of, A.M.L.; 0.5% of renal Tx 4% of lung and liver Tx, 6% of heart Tx, 1.3% of COPD admissions |
| Pakistan [53] | 10,949 | 5.9 | 3.9% of COPD admissions, 2.6% of lung cancer Tx, 10% of, A.M.L.; 10% of non-AML |
| Ecuador [70] | 748 | 5.5 | 10% of, A.M.L.; 8% of HSCT, 1% of Liver Tx, 0.5% renal of Tx, 2% of heart Tx, 9% of lung Tx, 1.3% of COPD admissions |
| Denmark [58] | 294 | 5.3 | 10% of HSCT, 6% of heart and lung Tx, 4% of liver, T.X.; 1% in renal, T.X.; 1.3% of COPD admission |
| Bangladesh [65] | 5166 | 5.1 | 1.3% of COPD (only COPD included in the assumptions) |
| Germany [82] | 4280 | 5.1 | 5% of, A.M.L.; 5% of non-AML haematological malignancies, 1% of renal Tx, 20% of lung Tx, 6% of heart Tx, 4% of liver Tx and 1.3% of COPD admissions |
| Peru [77] | 1621 | 5.0 | 10% of, A.M.L.; 10% of non-AML haematological malignancies, 1.3% of COPD admissions, 0.3% of renal Tx, 0.8% of liver Tx, 4.8% of heart Tx, 42% of HSCT |
| Uzbekistan [63] | 1521 | 4.8 | 50% of, A.M.L.; 1.3% of COPD admissions |
| United Kingdom [19] | 2912 | 4.6 | 9% of HSCT, 10% of, S.O.T.; 0.6% of, H.I.V.; 15% in haematological disease, 1.3% of COPD admissions |
| Brazil [52] | 1854 | 4.5 | 13.4% of, A.M.L.; 2.3% of HSCT receipients, 0.5% of renal Tx, 13.3% of lung Tx (no COPD data) |
| Korea [84] | 2150 | 4.5 | 14.7% of HSCT, 0.76% of liver Tx, 0.24% of renal Tx, 8.8% of lung Tx, 0.8% of heart Tx, and 5% of AML or non-AML haematological malignancy, 1.6% of COPD admissions |
| Guatemala [73] | 671 | 4.3 | 10% of, A.M.L.; 10% in Non-AML haematological malignancies, 1.3% of COPD admissions |
| Austria [89] | 333 | 4.1 | - |
| Mexico [76] | 4510 | 4.0 | 1.3% of COPD admissions, 8% of haematological conditions, 0.7% of solid organ Tx |
| Nepal [121] | 1119 | 4.0 | 3.9% of COPD admissions, 10% of, A.M.L.; 10% of non-AML haematological malignancies |
| Uganda [122] | 389 | 3.8 | 7% in haematological malignancy, 1.3% of COPD admissions |
| Hungary [56] | 319 | 3.2 | 12% of, A.M.L.; 6–8 % of HSCT, 8.6% of lung Tx, 3.4% of heart Tx, 4.7% of Liver Tx, 1.3% of renal Tx, 1.35% of COPD admissions |
| Philippines [93] | 3085 | 3.0 | 10% of, A.M.L.; 10% of non-AML, 0.5% of renal Tx, 4% of liver Tx, 1.3% of COPD admissions |
| Czech Republic [68] | 297 | 2.8 | 1.3% of COPD admissions, 10% of, A.M.L.; 1% of renal Tx, 5% of lung Tx, 5.5% of heart Tx, 4% in liver Tx |
| Spain [60] | 1293 | 2.8 | 10% of HSCT, 6% of heart Tx, 4% of lung and liver Tx, 1% of kidney Tx, 7% of, A.M.L.; 7.7% of non-AML haematological disease, 1.3% of admitted COPD cases |
| Ukraine [80] | 1233 | 2.7 | 10% of, A.M.L.; 10% of non-AML, 3.6% of COPD admissions |
| Portugal [91] | 243 | 2.3 | 1.3% of COPD admissions, 2.63% of lung cancers, 31% of HSCT, 6% of heart Tx, 4% of lung and liver Tx, 1% of kidney Tx |
| Russia [59] | 3238 | 2.3 | 10% of, A.M.L.; 20% of HSCT, 1% of renal Tx, 4% of liver Tx, 6% of heart Tx, 1.3% of admitted COPD patients |
| France [85] | 1185 | 1.8 | 1.8 per100,000 from previous studies and 1300 cases a year in COPD. |
| Chile [67] | 296 | 1.7 | 10% of Leukaemia, 1.3% of COPD admission, 2.6% of lung cancers |
| Canada [86] | 566 | 1.6 | 8.9% of, A.M.L.; 0.2% of kidney Tx, 0.5% of liver, 3.8% of lung Tx, 0.8% of heart Tx and 0.2% in pancreas Tx, and 7.5% in HSCT, 3.6/1000 COPD admissions |
| Thailand [55] | 941 | 1.4 | 13.5% of, A.M.L.; 3% of renal Tx, 4% of lung and liver Tx, 1.3% of COPD |
| Sri Lanka [90] | 229 | 1.1 | 10% of, A.M.L.; 100% of non-AML, 0.5% of renal Tx, 4% of liver Tx |
| Dominican Republic [69] | 61 | 0.8 | 10% of, A.M.L.; 10% of non-AML haematological patients, 13.4% of COPD over 40 years |
| Kenya [75] | 239 | 0.6 | 10% of, A.M.L.; 10% of non-AML haematological patients (COPD was not included) |
| Nigeria [62] | 928 | 0.6 | 10% of, A.M.L.; 10% of non-AML haematological malignancies (COPD was not includesd) |
| Qatar [54] | 11 | 0.6 | 11 cases recorded in the reference laboratory |
| Trinidad and Tobago [79] | 8 | 0.6 | 10% of AML (COPD population was not included) |
| Tanzania [78] | 20 | 0.1 | 10% of AML (COPD population was not included) |
| Country (Reference) | Burden | Rate/100,000 | Assumptions |
|---|---|---|---|
| Nigeria [62] | 74,595 | 48.2 | 40% of new AIDS cases in children and 10% in adults |
| Kenya [75] | 17,000 | 43.0 | 10% in HIV with CD4 < 200 |
| Trinidad and Tobago [79] | 400 | 30.0 | 80% of HIV patients with CD < 200 |
| Tanzania [78] | 9600 | 22.0 | 10.4% of adults living with HIV |
| Ukraine [80] | 6152 | 13.5 | 60% of HIV patients |
| Jamaica [74] | 350 | 13.0 | 255 in HIV HAART naïve |
| Senegal [133] | 1149 | 8.2 | 22% of new AIDS |
| Uzbekistan [63] | 165 | 5.37 | 60% of HIV with CD < 200 |
| Guatemala [73] | 722 | 4.7 | 4.7% in HIV patients |
| Peru [77] | 1447 | 4.6 | 13% in AIDS |
| Mexico [76] | 5130 | 4.5 | 24% in HIV |
| Chile [67] | 766 | 4.3 | 35% of new HIV cases |
| Nepal [121] | 990 | 3.6 | 16.7% of new AIDS case in children and 22.4% of adults |
| Spain [60] | 305 | 3.4 | 3.4/100,000 from previously published studies |
| Ecuador [70] | 535 | 3.28 | 10.7% of HIV with CD4 < 200 from previous study |
| Thailand [55] | 1708 | 2.6 | 21% of new AIDS cases |
| Dominican Republic [69] | 234 | 2.31 | 80% of HIV HAART naïve partients with CD4 < 200 |
| Brazil [52] | 4115 | 2.1 | 4.7% of AIDS patients with CD4 < 350 |
| Germany [82] | 1013 | 1.3 | Epidemiological data from Robert Koch institute 2012 |
| Pakistan [53] | 2200 | 1.2 | 16% in HIV |
| Belgium [66] | 120 | 1.1 | From unpublished data from the an AIDS reference from Leuven, Belgium |
| France [85] | 658 | 1.0 | Data from retrospective study in 2014 |
| Greece [72] | 112 | 1.0 | 26.2% of the new AIDS cases |
| Uganda [122] | 412 | 1.0 | 2.6% of non-HIV pneumonia deaths in children below 15 years + 36.8% of HIV adults HIV patients with CD4 < 100 + 10–49% of pneumonia admissions in children with HIV |
| Ireland [61] | 50 | 0.8 | 25% in HIV |
| Qatar [54] | 15 | 0.8 | 15 cases reported to the reference laboratory |
| Canada [86] | 252 | 0.71 | Cases from a single tertiary centre in Montreal |
| Czech Republic [68] | 72 | 0.7 | Internal registry of the Department of tropical and infectious diseases in Prague |
| Vietnam [120] | 608 | 0.67 | 13% of new AIDS diagnosis |
| Portugal [91] | 65 | 0.62 | 0.61/100,000 on a previous study |
| Korea [84] | 245 | 0.51 | 20% of new HIV patients with low CD4 |
| Philippines [93] | 391 | 0.4 | 31% of new AIDS diagnosis |
| United Kingdom [19] | 207 | 0.33 | Incidence rates in HIV and solid organ transplant recipients from previous studies. |
| Israel [57] | 26 | 0.3 | Records from Clinical Microbiological lab at Tel Aviv |
| Algeria [64] | 74 | 0.18 | 15% in HIV CD4 < 200 from various studies |
| Russia [59] | 1414 | 0.16 | 2.1% of new HIV |
| Egypt [71] | 125 | 0.15 | 1.9% of HIV based on previous study |
| Hungary [56] | 5 | 0.1 | Records from clinical laboratory |
| Bangladesh [65] | 58 | 0.04 | 17% of HIV |
| Denmark [58] | 2 | 0.04 | 35% in new AIDS patient from data of 1997–2009 and 255 in Non-AIDS based on previous study |
| Country (Reference) | Burden | Rate/100,000 |
|---|---|---|
| Russia [59] | 52,311 | 126.2 |
| Nigeria [62] | 120,747 | 78 |
| Philippines [93] | 77,172 | 78 |
| Pakistan [53] | 72,438 | 70 |
| Vietnam [120] | 55,509 | 61 |
| Dominican Republic [69] | 1374 | 55 |
| Uganda [122] | 18,000 | 46 |
| Bangladesh [65] | 20,720 | 41 |
| Kenya [75] | 12,927 | 32 |
| Thailand [55] | 19,044 | 29.2 |
| Belgium [66] | 662 | 27.7 |
| Quatar [54] | 176 | 26.8 |
| Nepal [121] | 6611 | 24.2 |
| India [136] | 209,147 | 24 |
| Tanzania [78] | 10,437 | 24 |
| Korea [84] | 10,754 | 22.4 |
| Ukranie [80] | 10,054 | 22 |
| Senegal [133] | 2700 | 19 |
| Mexico [76] | 18,246 | 15.9 |
| Sri Lanka [90] | 2886 | 14.4 |
| Egypt [71] | 3015 | 13.8 |
| Peru [77] | 3593 | 11 |
| Guatemala [73] | 1484 | 9.6 |
| Spain [60] | 4318 | 9.19 |
| Trinidad and Tobago [79] | 110 | 8.2 |
| Chile [67] | 1212 | 6.9 |
| Uzbekistan [63] | 1941 | 6.3 |
| Brazil [52] | 12,032 | 6.2 |
| Hungary [56] | 504 | 6 |
| United Kingdom [19] | 3600 | 5.7 |
| France [85] | 3450 | 5.2 |
| Denmark [58] | 270 | 4.8 |
| Austria [89] | 328 | 4.7 |
| Greece [72] | 386 | 3.7 |
| Czech Republic [68] | 365 | 3.5 |
| Ecuador [70] | 2100 | 3.28 |
| Ireland [61] | 196 | 3.1 |
| Portugal [91] | 776 | 3.1 |
| Jamaica [74] | 82 | 3 |
| Germany [82] | 2320 | 2.9 |
| Israel [57] | 200 | 2.5 |
| Algeria [64] | 897 | 2.2 |
| Canada [86] | 492 | 1.4 |
| Country (Reference) | Burden | Rate/100,000 | Comments |
|---|---|---|---|
| United Kingdom [19] | 235,070 | 372 | 2.5% of asthma + 12.5% of adult CF and 7.5% of children with CF |
| Trinidad and Tobago [79] | 3491 | 260 | 2.5%of asthma |
| Dominican Republic [69] | 25,149 | 249 | 2.5% of asthma |
| Belgium [66] | 23,119 | 208.3 | 2.5% of asthma patients and 15% in CF |
| Brazil [52] | 390,486 | 201.3 | 2.1% adult asthmatics and 22% adult with CF |
| Greece [72] | 20,843 | 193 | 2.5% of asthma and 17.7% of CF |
| Jamaica [74] | 5116 | 188 | 2.5% of asthma |
| Ecuador [70] | 26,642 | 185 | 2.5% of asthma |
| Canada [86] | 61,854 | 174 | 2.5% in Asthma and 18% in CF |
| Egypt [71] | 133,834 | 162 | 2.5% of asthma |
| Spain [60] | 59,210 | 156 | 2.5% of asthma |
| Germany [82] | 123,960 | 154 | 2.5% of asthma and 4.8% of CF |
| France [85] | 95,331 | 145 | 2.5% of asthma |
| Ireland [61] | 8960 | 140 | 2.5% of asthma and 17.75% in CF |
| Hungary [56] | 13129 | 132.5 | 2.5%of asthma and 15% in CF |
| Denmark [58] | 7328 | 131 | 2.5% of asthma and 5% CF |
| Philippines [93] | 121,113 | 123 | 2.5% of asthma |
| Russia [59] | 175,082 | 122.5 | 2.5% of asthma |
| Portugal [91] | 12,600 | 119 | 2.5% of asthma |
| India [136] | 1,380,000 | 114 | 2.5% (0.7–3.5%) of asthma |
| Israel [57] | 8297 | 101 | 2.5% of asthma and 6.6% in CF |
| Chile [67] | 17,183 | 97.9 | 2.5% of asthma |
| Austria [89] | 7537 | 91.7 | - |
| Algeria [64] | 31,310 | 77 | 2.5% of asthma patients |
| Peru [77] | 22,453 | 72 | 2.5% of asthma |
| Senegal [133] | 9976 | 71 | 2.5% of asthma |
| Ukranie [80] | 28,447 | 62.4 | 2.5%of asthma |
| Nigeria [62] | 93,649 | 60.5 | 2.5% of asthma |
| Qatar [54] | 1126 | 60.2 | 2.5% of asthma |
| Mexico [76] | 47,855 | 60 | 2.5% of asthma |
| Thailand [55] | 38,009 | 58.4 | 2.5% of asthma |
| Korea [84] | 27,312 | 56.9 | 2.5% of asthma |
| Bangladesh [123] | 90,262 | 56 | 2.5% of asthma patients |
| Pakistan [53] | 94,358 | 51 | 2.5% of asthma |
| Sri Lanka [90] | 10,344 | 49 | 2.5% of asthma |
| Uganda [122] | 18,700 | 47.9 | 2.5% of asthma |
| Czech Republic [68] | 4739 | 45 | 2.5% of asthma and 18% CF |
| Kenya [75] | 17,696 | 44 | 2.5% of asthma |
| Tanzania [78] | 18,987 | 44 | 2.5% of asthma |
| Guatemala [73] | 5568 | 36.1 | 2.5%of asthma |
| Nepal [121] | 9546 | 35 | 2.5% of asthma |
| Vietnam [120] | 23,607 | 23 | 2.5% of asthma |
| Uzbekistan [63] | 879 | 2.9 | 2.5% of asthma + 15% of CF |
| Country (Reference) | Burden | Rate/100,000 |
|---|---|---|
| United Kingdom [19] | 413,724 | 654 |
| Trinidad and Tobago [79] | 4608 | 344 |
| Dominican Republic [69] | 33,197 | 329 |
| Ecuador [70] | 45,183 | 311 |
| Brazil [52] | 599,283 | 288 |
| Belgium [66] | 30,402 | 273 |
| Greece [72] | 27,744 | 256 |
| Jamaica [74] | 6753 | 248 |
| Egypt [71] | 176,661 | 214 |
| Canada [86] | 73,344 | 206 |
| Germany [82] | 163,131 | 203 |
| Spain [60] | 93,044 | 198 |
| France [85] | 124,678 | 189 |
| Ireland [61] | 111,675 | 182 |
| Hungary [56] | 17,330 | 175 |
| Philippines [93] | 159,869 | 162 |
| Russia [59] | 231,000 | 161 |
| Portugal [91] | 16,614 | 159 |
| Denmark [58] | 7793 | 139 |
| Chile [67] | 22,300 | 127 |
| Austria [89] | 9949 | 121 |
| Algeria [64] | 41,329 | 102 |
| Peru [77] | 29,638 | 95 |
| Senegal [133] | 13,168 | 93 |
| Ukraine [80] | 37,491 | 82 |
| India [136] | 960,000 | 80 |
| Qatar [54] | 1486 | 80 |
| Nigeria [62] | 120,753 | 78 |
| Thailand [55] | 50,172 | 77 |
| Korea [84] | 36,052 | 75 |
| Bangladesh [65] | 119,146 | 74 |
| Pakistan [53] | 129,776 | 70 |
| Israel [57] | 5540 | 68 |
| Sri Lanka [90] | 13,654 | 65 |
| Czech Republic [68] | 6581 | 62 |
| Uganda [122] | 24,684 | 62 |
| Kenya [75] | 23,359 | 58 |
| Tanzania [78] | 25,063 | 57 |
| Mexico [76] | 66,997 | 53 |
| Guatemala [73] | 7349 | 48 |
| Nepal [121] | 12,600 | 46 |
| Vietnam [120] | 31,161 | 34 |
| Uzbekistan [63] | 1147 | 3.7 |
| Country | Burden | Rate/100,000 | Proportion of Microbial Keratitis that is Fungal |
|---|---|---|---|
| Nepal [121] | 19,938 | 73.00 | 27–62% of microbial keratitis |
| Pakistan [53] | 80,553 | 44.00 | 0.15% of general population based on the Chinese study [150] |
| Thailand [55] | 9765 | 15.00 | 15% of microbial keratitis |
| Egypt [71] | 11,550 | 14.00 | 40% of microbial keratitis |
| Mexico [76] | 11,638 | 10.40 | 0.15% of general population based on the Chinese study [150] |
| Vietnam [120] | 6356 | 7.00 | Based on previous Vietnamese study reporting a rate of 7 per 100,000 [160] |
| Sri Lanka [90] | 100,000 | 6.30 | 40% direct microscopy positivity rate for fungal elements in corneal buttons and scrapings |
| Qatar [54] | 6 | 1.68 | 6 cases recorded in the mycology reference laboratory |
| China [161] | 17,038 | 1.30 | 0.15% of general population [150] |
| Philippines [93] | 358 | 0.36 | Based on cases seen at a tertiary government hospital in the national capital region in 2015 |
| Korea [84] | 29 | 0.06 | Based on a previous Danish study [162] |
| Denmark [58] | 3 | 0.05 | Based on a previous Danish study [162] |
| Germany [82] | 32 | 0.04 | Based on a previous Danish study [162] |
| Prevalence (%) | Country | Year of Publication | Reference |
|---|---|---|---|
| 76.1 | Nigeria | 2011 | Adefemi et al. [169] |
| 68.0 | Kenya | 2015 | Moto et al. [175] |
| 49.5 | Rwanda | 1983 | Buginco et al. [176] |
| 45.0 | Nigeria | 2016 | Dogo et al. [171] |
| 44.8 | Senegal | 2016 | Diongue et al. [177] |
| 39.3 | Mali | 2016 | Coulibaly et al. [178] |
| 36.5 | Ethiopia | 2015 | Leiva-Salinas et al. [179] |
| 35.2 | Nigeria | 2015 | Kalu et al. [180] |
| 33.3 | Kenya | 2001 | Ayaya et al. [181] |
| 31.2 | Nigeria | 2008 | Ayanbimpe et al. [182] |
| 26.9 | Nigeria | 2014 | Oke et al. [170] |
| 23.1 | Gabon | 2011 | Hogewoning et al. [183], |
| 23.1 | Gabon | 2013 | Hogewoning et al. [184] |
| 22.5 | Tanzania | 1998 | Frederick et al. [185] |
| 20.6 | Kenya | 2013 | Hogewoning et al. [184] |
| 15.4 | Nigeria | 2014 | Ayanlowo [186] |
| 13.9 | Ivory Coast | 2013 | Fulgence et al. [187] |
| 11.2 | Kenya | 2009 | Chepchirchir et al. [188] |
| 9.4 | Nigeria | 2008 | Emele et al. [189] |
| 8.7 | Ghana | 2013 | Hogewoning et al. [184] |
| 8.4 | Ghana | 2013 | Hogewoning et al. [184] |
| 8.1 | Cameroon | 2014 | Kechia et al. [190] |
| 7.8 | Kenya | 1997 | Schmeller et al. [191] |
| 7.1 | Kenya | 2010 | Komba et al. [192] |
| 3.6–9.6 | Mozambique | 2007 | Sidat et al. [193] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. https://doi.org/10.3390/jof3040057
Bongomin F, Gago S, Oladele RO, Denning DW. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. Journal of Fungi. 2017; 3(4):57. https://doi.org/10.3390/jof3040057
Chicago/Turabian StyleBongomin, Felix, Sara Gago, Rita O. Oladele, and David W. Denning. 2017. "Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision" Journal of Fungi 3, no. 4: 57. https://doi.org/10.3390/jof3040057
APA StyleBongomin, F., Gago, S., Oladele, R. O., & Denning, D. W. (2017). Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. Journal of Fungi, 3(4), 57. https://doi.org/10.3390/jof3040057